Abstract
Purpose: To report a case of successful treatment with infliximab for Behçet disease (BD) during pregnancy. Design: A case report.
Methods: A 30-year-old woman was diagnosed with BD at 12 weeks of pregnancy. Additional symptoms occurred, and infliximab at a dose of 5 mg/kg body weight was initiated at 18 weeks and repeated.
Results: All symptoms improved, and a 3950-g infant was delivered uneventfully without any abnormality at 39 weeks. A few weeks after delivery, uveitis and systemic symptoms relapsed. Infliximab was reinitiated and all symptoms were resolved.
Conclusions: Infliximab may be safe and effective for BD during pregnancy.
Behçet disease (BD) is a multisystem inflammatory disease characterized by recurrence of oral ulcers, uveitis, genital ulcers, and skin lesions. Systemic corticosteroids, immunosuppressants, and biologic agents are used for the treatment of severe uveitis or systemic symptoms.Citation[1] In pregnant women, potential adverse effects of immunosuppressants should be considered, and these agents should be used only if the potential benefit justifies the potential risk to the fetus. The U.S. Food and Drug Administration and the safety marked infliximab as a pregnancy category B, systemic corticosteroid; C, cyclosporin; C, colchicine; C, azathioprine; D, chloraminophen; D, cyclophosphamideb; D, methotrexate; X, thalidomide; X, tothalidmide.Citation[2],Citation[3]
The efficacy and safety of infliximab for Crohn disease and rheumatoid arthritis during pregnancy have been reported,Citation[2],Citation[4] but use of infliximab for BD during pregnancy remains poorly studied. We report a case of BD with severe uveitis during pregnancy, in which all symptoms remitted by infliximab treatment, without any problem during delivery.
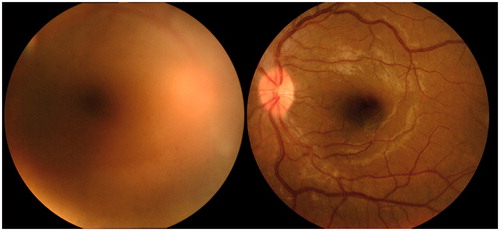
A 30-year-old woman who was 12 weeks pregnant presented at our hospital with blurred vision in her right eye since 3 weeks ago. Oral ulcers and genital ulcers appeared at 8 weeks of pregnancy and painful erythema nodosum appeared on her left leg at 10 weeks of pregnancy. At presentation, the best-corrected visual acuity was 20/20 in both eyes, and intraocular pressure was 18 mmHg in the right eye and 16 mmHg in the left eye. Severe iridocyclitis in right eye and mild cell infiltration in the anterior chamber of left eye were observed. Intensive vitreous opacities obscured fundus observation in the right eye, thus retinal vasculitis could not be detected (). Fluorescein angiography was contraindicated due to pregnancy. Uveitis and systemic symptoms led to a diagnosis of BD. Steroid eyedrops were prescribed, but no systemic agents. Additional symptoms of severe headache, paralysis of hands and legs and arthritis occurred, and systemic conditions, including uveitis, further worsened. Headache and paralysis were considered to be symptoms of neuro-BD, and effective systemic treatment was necessary.
Figure 1. Fundus photographs of both eyes at presentation: (A) right eye; (B) left eye. Intensive vitreous opacities in the right eye obscure fundus observation, thus retinal vasculitis was not detectable.
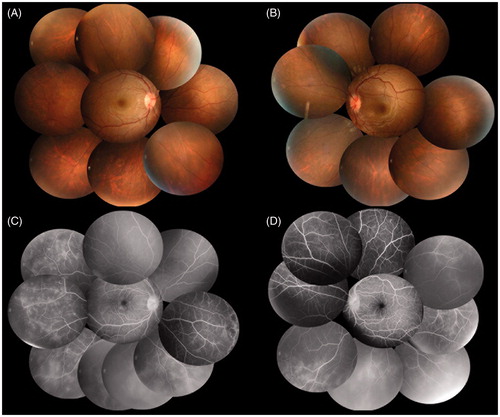
Efficacy of infliximab treatment for neuro-BDCitation[3] and safety of the drug during pregnancyCitation[2] were reported. Treatment options were discussed and the patient preferred infliximab therapy as a safer option. Therefore, after obtaining adequate informed consent regarding the risk to pregnancy, infliximab at a dose of 5 mg/kg body weight was initiated at 18 weeks of pregnancy and repeated at 20, 24, and 32 weeks of pregnancy, as per the Ministry of Health, Welfare and Labor treatment protocol. After infliximab administration, all systemic symptoms improved and cell infiltration in anterior chamber and vitreous opacities were reduced drastically. Considering the risk associated with breast-feeding,Citation[6] infliximab was discontinued after 32 weeks of pregnancy. At 39 weeks of pregnancy, a 3950-g infant was delivered uneventfully without any abnormality. A few weeks after delivery, uveitis recurred in both eyes and fluorescein angiography detected peripheral retinal vasculitis (). Oral ulceration, skin lesions, and severe headache also relapsed. Infliximab was reinitiated and all symptoms were immediately resolved and have remained controlled at the last follow-up.
Figure 2. Fundus photographs and fluorescence angiograms at recurrence a few weeks after delivery. (A, B) Fundus photographs of both eyes at a few weeks after delivery. Intensive vitreous opacities in the right eye were decreased and almost clear in both eyes. (C, D) Fluorescein angiography of both eyes. Fluorescene angiography detected peripheral retinal vasculitis and nonperfusion areas in both eyes.
Although this is a single case report, it suggests that treatment with infliximab may be safe and effective for BD during pregnancy.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Saadoun D, Wechsler B. Behcet's disease. Orphanet J Rare Dis. 2012;7:20
- Djokanovic N, Klieger-Grossmann C, Pupco A, Koren G. Safety of infliximab use during pregnancy. Reprod Toxicol. 2011;32:93–97
- Armenti VT, Moritz MJ, Cardonick EH, Davison JM. Immunosuppression in pregnancy: choices for infant and maternal health. Drugs. 2002;62:2361–2375
- Katz JA, Antoni C, Keenan GF, et al. Outcome of pregnancy in women receiving infliximab for the treatment of Crohn's disease and rheumatoid arthritis. Am J Gastroenterol. 2004;99:2385–2392
- Yamada Y, Sugita S, Tanaka H, et al. Timing of recurrent uveitis in patients with Behcet's disease receiving infliximab treatment. Br J Ophthalmol. 2011;95:205–208
- Stengel JZ, Arnold HL. Is infliximab safe to use while breastfeeding? World J Gastroenterol. 2008;14:3085–3087