To the Editor:
Churg-Strauss syndrome (CSS, eosinophilic granulomatosis with polyangiitis) is an autoimmune vasculitis of small and medium vessels leading to fibrinoid necrosis. It can involve the vasculature of a multitude of organs, including the lungs, nerves, kidneys, heart, and gastrointestinal system. The condition is slightly more common in males than females, with the age of onset varying from 15 to 70 years. The syndrome has three phases: allergic rhinitis and asthma; eosinophilic infiltrative disease; and systemic medium- and small-vessel eosinophilic vasculitis with granulomatous inflammation. Ophthalmic involvement is very rare and has been documented in only a handful of cases in the literature. We report of a case of Churg-Strauss syndrome who presented to an eye emergency department with bilateral bulbar subconjunctival masses whose biopsy of the lesions showed the presence of an eosinophilic vasculitis.
A 27-year-old female with a history of asthma and rhinitis since childhood presented to the eye emergency department with bilateral subconjunctival masses, bilateral cervical lymphadenopathy, and a swelling in the left forearm. Four months earlier, the patient had been investigated while abroad for symptoms including recurrent episodes of shortness of breath and swelling around the eyes, the left side of neck, and the extensor aspect of left forearm. These symptoms responded to a continuing course of oral steroids, oral antihistamine, and an oral mast cell stabilizer. Investigations performed at that time included a computerized tomography (CT) scan of the thorax, which was reported as normal, and venous Doppler imaging, which showed no evidence of an axillary vein thrombus. Routine blood tests showed an increased eosinophil count and raised IgE levels. Her CRP was also raised but her serum tryptase and kininase II levels were both within normal limits. Fecal microscopy was negative for any parasites. Her spirometry readings were consistent with nonobstructive small airways disease. Skin tests and a radioallergosorbent test (RAST) showed an IgE-mediated allergy for house dust mites, weeds, grass pollen, and cats and dogs.
She had no other past medical history of significance and was a nonsmoker. On this occasion, her presentation was due to an unusual appearance of her eyes in the mirror. On examination, she was found to have periorbital edema associated with bilateral subconjunctival multinodular swellings in the superior fornix of both eyes, with each nodule measuring 5–6 mm. There were no signs of inflammation in the surrounding tissue (). The exophthalmometry did not show any significant proptosis, and extraocular movements were restricted in up gaze in the right eye producing diplopia. The optic nerve functions and appearance were normal. General systemic examination was otherwise normal. However, she did have clear, rubbery diffuse submandibular lymphadenopathy. Multiple subcutaneous swellings were also noted in the left supraclavicular fossa and left forearm which were discrete, firm, and mobile. Her urine dipstick was negative for blood and protein.
Figure 1. (A) The patient at presentation with bilateral multinodular involvement of the bulbar conjunctiva. (B) The appearance of the nodules on closer examination. (C) Conjunctival stroma with mixed inflammatory infiltrate and numerous eosinophils within a vessel wall (×20 objective, hematoxylin & eosin stain). (D, E) Resolution of the multinodular masses of the conjunctiva on the right and left, respectively.
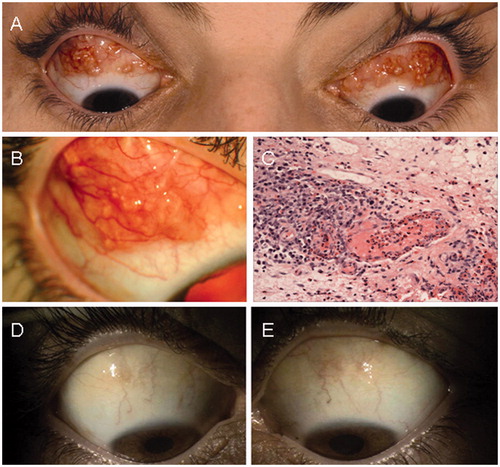
Parasitic infection, particularly of worms (e.g., Strongyloides stercoralis), was excluded by analysis of blood serology and stool samples. Her blood tests showed a slightly raised ESR and CRP associated with a marked eosinophilia, raised serum IgE levels, and a small IgG kappa paraprotein on serum electrophoresis. Anti-neutrophil cytoplasmic antibodies (ANCA) was negative. Her chest X-ray and electrocardiogram were normal. A CT scan of the orbits showed no posterior extension of the masses into the orbit. Following an incisional biopsy of these subconjunctival masses, the histopathology of the conjunctiva revealed a stromal chronic inflammatory infiltrate, consisting of lymphocytes, plasma cells, and eosinophils, with areas of eosinophilic vasculitis. Granulomata and necrosis were not seen ().
After consultation with the general physicians a diagnosis of Churg-Strauss syndrome was made, although the patient did not have any evidence of severe end organ damage. The patient was started on a course of topical steroids initially but this had a limited response. Subsequently she was started on oral steroids (prednisolone 30 mg/day) to induce a remission. A favorable response was seen with the decrease in size and eventual resolution of the conjunctival nodules (). After 3 months, her steroids were gradually reduced to a maintenance dose of prednisolone 5 mg daily.
Churg-Strauss syndrome, Wegener granulomatosis (granulomatosis with polyangiitis), and microscopic polyangiitis are three closely related vasculitic syndromes that affect medium- and small-sized vessels and are associated with antibodies to neutrophil cytoplasm antigens (ANCA). The American College of Rheumatology (ACR) has proposed six criteria for the diagnosis of CSS.Citation[1] The presence of four or more criteria yields a sensitivity of 85% and a specificity of 99.7%. These criteria include (1) asthma, (2) eosinophilia of more than 10% in the peripheral blood, (3) paranasal sinusitis, (4) pulmonary infiltrates (may be transient), (5) histological proof of vasculitis with extravascular eosinophils, and (6) mononeuritis multiplex or polyneuropathy. No causes of CSS are known,Citation[2] although possible causes include allergic or autoimmune reactions to an environmental agent or drug (e.g., mesalazine,Citation[3] propylthiouracilCitation[4]). HLA-DRB4 positivity may be a genetic risk factor for the development of CSS and may increase the likelihood of vasculitic manifestations of the disease.Citation[5] Although this patient met only three of the six proposed ACR criteria, the clinical signs of her condition were most distinctive and in keeping with a diagnosis of CSS on clinical grounds. Indeed, Lanham et al. have reported that although the classical histological picture comprises a necrotizing vasculitis, eosinophilic tissue infiltration and extravascular granulomas, it is only found in a minority of cases, and is not pathognomonic of the condition. On the other hand, they have identified a very distinct clinical pattern of the disorder that can allow CSS to be readily identified on clinical grounds alone.Citation[6] Additionally, her presentation fulfills the definition of CSS as outlined by the Chapel Hill Consensus Conference on the nomenclature of systemic vasculitis: eosinophil-rich and granulomatous inflammation involving the respiratory tract and necrotizing vasculitis affecting small to medium-sized vessels and associated with asthma and blood eosinophilia.Citation[7]
Investigations typically reveal an eosinophilia, raised erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels, raised immunoglobulin E (IgE) levels, and hypergammaglobulinemia. The patient may also be anemic and may have abnormalities in renal function tests if there is renal involvement. Anti-neutrophil cytoplasmic antibodies (ANCA) are present in approximately 40% of patients with CSS, with most of these patients being perinuclear-ANCA (P-ANCA) positive,Citation[8] compared with the closely related condition of Wegener granulomatosis (granulomatosis with polyangiitis), where patients are usually cytoplasmic (C-ANCA) positive. Patients positive for ANCA are more likely to have systemic vasculitis affecting several organs, so it is not surprising that our patient was ANCA negative.Citation[9]
Ocular involvement in CSS is unusual; there have been reported cases with varied ocular manifestations. To the best of our knowledge, following a review of the published literature using key databases that include Medline and PubMed, this is the first reported case where there is bilateral simultaneous nodular involvement of the bulbar conjunctiva as part of a complex in the initial presentation of CSS. No formal diagnosis of CSS had been made in this patient until the histological confirmation. The remaining cases cited in the literature all had unilateral involvementCitation[10–15] with either a single nodular elevation of the conjunctiva,Citation[10–12] progressive thickening of the bulbar and palpebral conjunctiva,Citation[13] or a nodular infiltration of the tarsal conjunctiva causing a fullnessCitation[14] and an irregular consistency of the upper eyelids.Citation[15] However, the nodular tarsal conjunctival lesions in this last case also showed amyloid deposits and were similar in appearance to that in other reported cases of conjunctival amyloidosis.Citation[15] Yaman et al. have previously reported on a patient with bilateral superior conjunctival involvement but this patient was already diagnosed with CSS and his ocular symptoms manifested 1 month after the cessation of antituberculosis therapy.Citation[16]
In 2001 Takanashi et al. classified ocular manifestations into two groups: orbital inflammatory pseudotumor and ischemic vasculitis.Citation[17] Orbital pseudotumor is an idiopathic inflammatory condition that usually involves the extraocular muscles, although in some cases there is inflammatory change involving the uvea, sclera, lacrimal gland, and retrobulbar soft tissues. The characteristics of the orbital inflammatory pseudotumor are chronic onset, positive conjunctival involvement, abnormalities in orbital imaging studies, negative ANCA, and good visual prognosis. It is assumed that this is the category that would be applicable to this patient. On histological examination, the characteristic changes seen in CSS, most notably in the lung, include small necrotizing granulomas and a necrotizing vasculitis involving small arteries and venules. In this case, no granulomas were seen on the histological specimen, although it has to be borne in mind that the biopsy had been taken when the patient was already on a course of oral steroids.
Because CSS is a multiorgan disease, the management of a CSS patient may require the input of several medical specialities, depending on the organ–system involvement. The mainstay of treatment is the use of glucocorticoids.Citation[18],Citation[19] In the 20% of patients where there is a poor or inadequate response to steroid treatment alone, cytotoxic drugs may be necessary. The ESR and the peripheral blood eosinophilia may be used to monitor the response to therapy, and raised ANCA titers may suggest disease activity. In this patient, the ANCA was negative from the outset of her presentation to our department; this is more likely to be explained by the fact that she falls into the category of the 40% ANCA-negative CSS cases, rather than being negative because of a lack of disease activity. Indeed, Cohen et al. have reported that the ANCA level does not relate well to disease activity.Citation[20]
In conclusion, this case describes a unique presentation of CSS in the conjunctiva as multinodular, bilateral masses causing diplopia. There was no systemic end organ damage at presentation and systemic steroids were used successfully to initiate symptom resolution and induce remission of the disease.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
We acknowledge support from the Moorfields and Imperial College NIHR Biomedical Research Centres.
References
- Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum. 1990;33:1094–1100
- Hellmich B, Ehlers S, Csernok E, et al. Update on the pathogenesis of Churg-Strauss syndrome. Clin Exp Rheumatol. 2003;21:S69–S77
- Sinico RA, Sabadini E, Maresca AM. Mesalazine-induced Churg-Strauss syndrome in a patient with Crohn's disease and sclerosing cholangitis. Clin Exp Rheumatol. 2006;24:S104
- Quax RA, Swaak AJ, Baggen MG. Churg-Strauss Syndrome following PTU treatment. Int J Rheumatol. 2009;2009:504105
- Vaglio A, Martorana D, Maggiore U, et al. HLA-DRB4 as a genetic risk factor for Churg-Strauss syndrome. Arthritis Rheum. 2007;56:3159–3166
- Lanham JG, Elkon KB, Pusey CD, et al. Systemic vasculitis with asthma and eosinophilia: a clinical approach to the Churg-Strauss syndrome. Medicine (Baltimore). 1984;63:65–81
- Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides: proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–192
- Sable-Fourtassou R, Cohen P, Mahr A, et al. Antineutrophil cytoplasmic antibodies and the Churg-Strauss syndrome. Ann Intern Med. 2005;143:632–638
- Pagnoux C, Guillevin L. Churg-Strauss syndrome: evidence for disease subtypes?. Curr Opin Rheumatol. 2010;22:21–28
- Nissim F, Von der Valde J, Czernobilsky B. A limited form of Churg-Strauss syndrome: ocular and cutaneous manifestations. Arch Pathol Lab Med. 1982;106:305–307
- Margolis R, Kosmorsky GS, Lowder CY, et al. Conjunctival involvement in Churg-Strauss syndrome. Ocul Immunol Inflamm. 2007;15:113–115
- Billing K, Malhotra R, Selva D, et al. Orbital myositis in Churg-Strauss syndrome. Arch Ophthalmol. 2004;122:393–396
- Shields CL, Shields JA, Rozanski TI. Conjunctival involvement in Churg-Strauss syndrome. Am J Ophthalmol. 1986;102:601–605
- Ameli F, Phang KS, Masir N. Churg-Strauss syndrome presenting with conjunctival and eyelid masses: a case report. Med J Malaysia. 2011;66:517–519
- Meisler DM, Stock EL, Wertz RD, et al. Conjunctival inflammation and amyloidosis in allergic granulomatosis and angiitis (Churg-Strauss syndrome). Am J Ophthalmol. 1981;91:216–219
- Yaman A, Ozbek Z, Saatci AO, et al. Topical steroids in the management of Churg-Strauss syndrome involving the conjunctiva. Cornea. 2007;26:498–500
- Takanashi T, Uchida S, Arita M, et al. Orbital inflammatory pseudotumor and ischemic vasculitis in Churg-Strauss syndrome: report of two cases and review of the literature. Ophthalmology. 2001;108:1129–1133
- Grau RG. Churg-Strauss syndrome: 2005–2008 update. Curr Rheumatol Rep. 2008;10:453–458
- Mukhtyar C, Flossmann O, Hellmich B, et al. Outcomes from studies of antineutrophil cytoplasm antibody associated vasculitis: a systematic review by the European League Against Rheumatism systemic vasculitis task force. Ann Rheum Dis. 2008;67:1004–1010
- Cohen P, Guillevin L, Baril L, et al. Persistence of antineutrophil cytoplasmic antibodies (ANCA) in asymptomatic patients with systemic polyarteritis nodosa or Churg-Strauss syndrome: follow-up of 53 patients. Clin Exp Rheumatol. 1995;13:193–198