Abstract
Purpose: To report a case of Acute Retinal Necrosis (ARN)-developed nephrotoxicity during intravenous acyclovir treatment and toxic hepatitis during oral valacyclovir treatment.
Design: Interventional case report.
Methods: Retrospective chart review.
Results: A 45-year-old male with ARN treated with intravenous acyclovir developed nephrotoxicity. After switching to oral valacyclovir, toxic hepatitis developed. Both renal and liver function tests returned to normal levels after drug cessation
Conclusions: Although rare, clinicians should be aware of the potential nephrotoxic and hepatotoxic side effects of antiviral therapy during ARN treatment.
Acute retinal necrosis (ARN) is an uncommon disorder caused by the herpes virus family.Citation1 The typical clinical characteristics are peripheral retinal necrosis with circumferential spread, occlusive arteriolar retinopathy, and varying degrees of intraocular inflammation.Citation2 It usually starts unilaterally, but the progression to bilateral involvement may occur in 10% of cases.Citation3 If left untreated, it can progress rapidly. First-line treatment is usually intravenous acyclovir followed by oral acyclovir, but in certain cases intravitreal injections of gancyclovir or foscarnet are needed.Citation4 In addition, due to oral enhanced bioavailability, newer antiviral agents such as valacyclovir or oral famciclovir are being used for ARN treatment.Citation5,Citation6 However, numerous adverse effects of these agents have been identified. Herein, we report a previously healthy case diagnosed with ARN. Initially, nephrotoxicity developed during intravenous acyclovir treatment. After switching to oral valacyclovir, toxic hepatitis developed.
Case Report
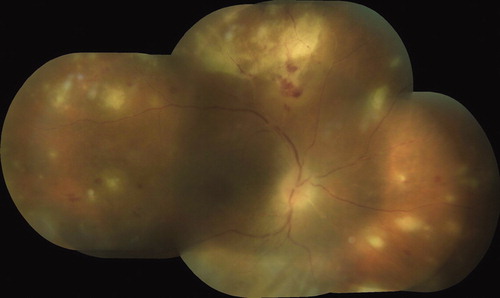
A 45-year old man was admitted to our hospital with blurred vision in his right eye, which had started 1 week earlier. The best-corrected visual acuity (BCVA) was 20/400 OD (right eye) and 20/20 OS (left eye). Slit-lamp examination of the right eye revealed granulomatous anterior uveitis with mild vitritis. The fundus examination revealed multiple foci of full-thickness retinitis, diffuse retinal arteritis associated with retinal hemorrhage, optic disc hyperemia, and 360-degree confluent full-thickness retinal necrosis at the peripheral retina (). The left eye examination was within normal limits. The clinical presentation was consistent with ARN. Complete blood count, erythrocyte sedimentation rate, and renal and liver function tests were all within normal limits. PCR analysis of aqueous humor was positive for HSV-2 (herpes simplex virus type 2). Intravenous acyclovir (10 mg/kg/day) treatment was started immediately. After 48 h, systemic corticosteroid was added to the treatment regime. Because of the possible side effects of antiviral medications such as acyclovir, complete blood count and liver and renal function tests were obtained on a daily basis. Serum creatinine level increased to 2.4 mg/dL on the second day of the iv acyclovir treatment. No pain or other physical symptom was reported. Despite vigorous hydration, serum creatinine level steadily increased to 5 mg/dL on the 8th day of treatment.
FIGURE 1. Fundus photograph shows full thickness retinal necrosis, diffuse retinal arteritis, multiple foci of retinitis and optic disc hyperemia in the right eye of the patient.
The patient consulted a nephrology clinic and received a diagnosis of acyclovir-induced nephrotoxicity. Acyclovir infusion was discontinued and oral valacyclovir, 2000 mg/day, was initiated. During this time, BCVA increased to 20/100 OD and retinitis foci resolved gradually. Serum creatinine started decreasing quickly and returned to normal levels 4 days after cessation of the acyclovir treatment. On the 17th day of oral valacyclovir treatment, liver enzymes were significantly elevated (alanine aminotransferase (ALT): 219, aspartate aminotransferase (AST): 74, lactate dehydrogenase (LDH): 721, γ-glutamyl transferase (GGT): 455. Urgent consultation was obtained from the gastrohepatology clinic and the patient was diagnosed with toxic hepatitis. Oral valacyclovir treatment was suspended and liver function tests returned to normal levels within 1 week.
In the meantime, BCVA was 20/30 in OD. A sectoral atrophy of iris had developed. Vitreous haze resolved and all retinitis foci turned into atrophic areas. Furthermore, retinal tears were detected in multiple peripheral sites involving areas of necrosis. Prophylactic argon laser photocoagulation therapy was applied to these areas. Ten days after cessation of valacyclovir treatment, noticeable vitreous haze recurred. Intravitreal injections of gancyclovir sodium were performed (2 mg/0.05 mL) twice per week. One week after the intravitreal gancyclovir therapy, retinal detachment developed in the affected eye. Pars plana vitrectomy and silicone oil tamponade were performed. Retinal reattachment was successful and BCVA was stabilized at 20/50 6 months after surgery.
Discussion
Intravenous acyclovir (500 mg/m2, 3 times per day) for 7–10 days followed by oral antiviral therapy for 14 weeks is the first-choice therapy for ARN.Citation7 A recognized side effect is nephrotoxicity, which can occur in 12–48% of patients.Citation8 The mechanism for this injury is crystalline-induced nephropathy, which is characterized by a rapid rise in serum creatinine.Citation8 There have been only a few case reports of acyclovir-induced nephrotoxicity during ARN treatment in the published literature.Citation9,Citation10 This nephrotoxicity is reversible in most cases with early diagnosis and discontinuation of the drug.Citation8 In our case, acyclovir nephrotoxicity developed despite adequate hydration and kidney improved after drug cessation.
Oral valacyclovir is a relatively new treatment modality for HSV infections and has a greater bioavailability than acyclovir.Citation10 Moreover, plasma concentrations of valacyclovir are higher than those obtained with oral acyclovir.Citation1 In our case, we initiated valacyclovir after cessation of acyclovir and followed the patient closely with complete blood count and renal and liver function tests. On the 17th day of therapy, alterations in the liver function tests were noticed. There are few data available regarding the reversibility of liver damage and toxic hepatitis after valacyclovir,Citation12 and the exact mechanism of this damage is still unknown. In our case, liver function tests returned to the normal range shortly after cessation of valacyclovir, supporting the possibility of reversing damage. On the other hand, we observed nephrotoxicity only after intravenous acyclovir treatment, whereas, during the oral valacyclovir usage, renal function tests were at normal levels. Rapid and bolus high-dose intravenous acyclovir treatment has been shown to be a risk factor for crystal-induced nephrotoxicity.Citation9 Similarly, our case emphasized nephrotoxicity following intravenous administration of acyclovir. This case demonstrated the necessity of closely monitoring patients on either acyclovir or valacyclovir treatment. Clinicians should be aware of the potential side effects of these antiviral drugs during ARN treatment.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Geux-Crozier Y, Rochat C, Herbort CP. Necrotizing herpetic retinopathies: a spectrum of herpes virus-induced diseases determined by the immune state of the host. Ocul Immunol Inflamm. 1997;5:259–265
- Holland GN; the Executive Committee of the American Uveitis Society. Standard diagnostic criteria for the acute retinal necrosis syndrome. Am J Ophthalmol. 1994;117:663–667
- Muthiah MN, Michaelides M, Child CS, et al. Acute retinal necrosis: a national population-based study to assess the incidence, methods, diagnosis, treatment strategies and outcomes in the UK. Br J Ophthalmol. 2007;91:1452–1455
- Luu KK, Scott IU, Caudrhy NA, Verm A, et al. Intravitreal antiviral injections as adjunctive therapy in the management of immunocompetent patients with necrotizing herpetic retinopathy. Am J Ophthalmol. 2000;129:811–813
- Emerson GG, Smith JR, Wilson DJ, et al. Primary treatment of acute retinal necrosis with antiviral therapy. Ophthalmology. 2006;113:2259–2261
- Figueroa MS, Garabito I, Gutierrez C, et al. Famciclovir for the treatment of acute retinal necrosis (ARN) syndrome. Am J Ophthalmol. 1997;123:255–257
- Palay DA, Sternberg P Jr, Davis J, et al. Decrease in the risk of bilateral acute retinal necrosis by acyclovir therapy. Am J Ophthalmol. 1991;112:250–255
- Perazella MA. Crystal induced acute renal failure. Am J Med. 1999;106:459–465
- Liu DTL, Lee WYW, Lam PTH, et al. Acyclovir-induced nephrotoxicity in a patient with acute retinal necrosis. Hong Kong Med J. 2007;13:155–156
- Minces RM, Gallagher DS, Shields RK. Acute retinal necrosis in a monocular patient complicated by acyclovir-induced nephrotoxicity. J Clin Virol. 2010;49:1–3
- Weller S, Blum R, Doucette M, et al. Pharmocokinetics of the acyclovir prodrug valaciclovir after escalating single and multiple dose administration to normal volunteers. Clin Pharmacol Ther. 1993;54:595–605
- Tyring SK, Baker D, Snowden W. Valacyclovir for herpes simplex virus infection: long-term safety and sustained efficacy after 20 years' experience with acyclovir. J Infect Dis. 2002;186:40–46
