Abstract
Purpose: To report, in vivo, on the quasi-histologic characteristics of a sarcoid choroidal granuloma as visualized by Enhanced Depth Imaging Spectral Domain Optical Coherence Tomography (EDI SD-OCT).
Methods: A 49 year-old woman showing a choroidal granuloma secondary to sarcoidosis was imaged by EDI OCT.
Result: On EDI SD-OCT examination, sarcoid choroidal granuloma appears as a localized hyporeflectivechoroidalthickening. Two weeks after systemic corticosteroids, the thickness of the granuloma decreased from 568 μm to 356 μm. Five months later, it reached 274 μm, and after eleven months, it decreased to 150 μm.
Conclusion: EDI SD-OCT allows direct visualization of choroidal granuloma secondary to sarcoidosis and evaluation of lesion regression after treatment.
Sarcoidosis is a systemic granulomatous disease of unknown etiology characterized by the formation of granulomas in multiple organs (such as lung, lymph node, skin, liver, and eye).Citation1 The isolated posterior segment manifestations are observed in 5% of cases.Citation2,Citation3 A new approach to improve depth imaging by spectral domain optical coherence tomography (SD-OCT), named enhanced depth imaging (EDI) OCT, reliably images the full thickness of the choroid.Citation4 Here we report the quasi-histologic characteristics of a presumed sarcoid choroidal granuloma, as visualized by EDI SD-OCT.
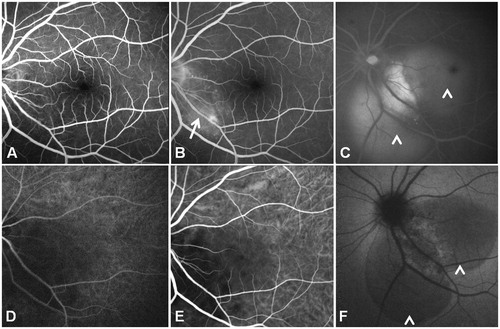
A 49-year-old woman presented with blurred vision in her left eye (LE) associated with chronic cough, shortness of breath, and a weight loss of 6 kg in 6 months. Visual acuity (VA) was 20/20 in the right eye (RE) and 20/50 in the LE. On slit-lamp biomicroscopy, RE and LE anterior segments showed small mutton-fat keratic precipitates. Fundus biomicroscopy revealed normal findings in the RE. A large juxtapapillary yellowish lesion was located along the inferior temporal arcade of the LE, with subretinal fluid accumulated on either side of the lesion (). The LE juxtapapillary lesion showed an early hyperfluorescence on fluorescein angiography and was characterized by late leakage (). Also, a decrease in the caliber of the lower temporal artery characterized the area occupied by the lesion. The lesion presented a hypofluorescent aspect in the early phase of indocyanine green angiography, and was characterized by inhomogeneous late staining on its borders. Ultrasonography (B-scan) showed a moderately elevated dome-shaped lesion.
FIGURE 1. Spectral domain optical coherence scan passing through the large juxtapapillary yellowish lesion (A, red free frame) shows a dome-shape appearance of the overlaying retina (arrowhead) and subretinal fluid accumulated on either sides of the lesion (B).
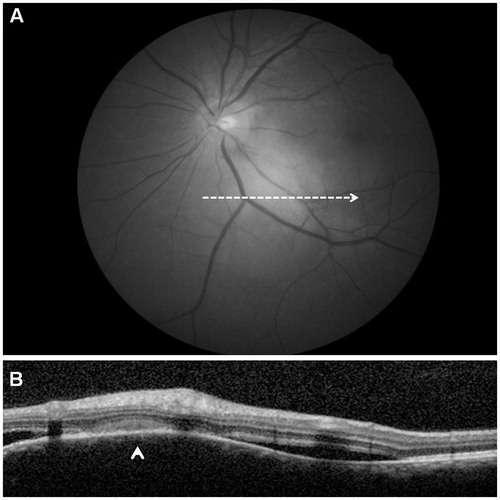
FIGURE 2. Fluorescein angiography of the left eye (A) shows a juxtapapillary hyperfluorescent lesion (arrow) with late (B, C) leakage (arrowheads) and a slight decrease in the caliber of the lower temporal artery (arrow). Indocyanine green angiography (bottom panel) shows the lesion as hypofluorescent in the early phase (D), characterized by inhomogeneous late (E, F) staining on its borders.
EDI SD-OCT revealed the choroidal lesion as a hyporeflective thickening of the choroid, responsible for a dome-shape appearance of the overlaying retina and retinal pigment epithelium. The adjacent choroid appeared of normal thickness and reflectivity. The outer retinal layers overlying the choroidal lesion were thickened and hyperreflective on OCT (probably due to an inflammatory reaction of the retina, or to intraretinal edema, even though we do not know for sure the exact nature of the fluid).
Weight loss, pulmonary symptoms, and the presence of small mutton-fat keratic precipitates in the anterior segment finding strongly suggested that the choroidal thickening represents one or more choroidal granulomas. A computerized tomography scan showed the presence of mediastinal lymphadenopathy. Lymph node biopsy has confirmed the diagnosis of sarcoidosis.
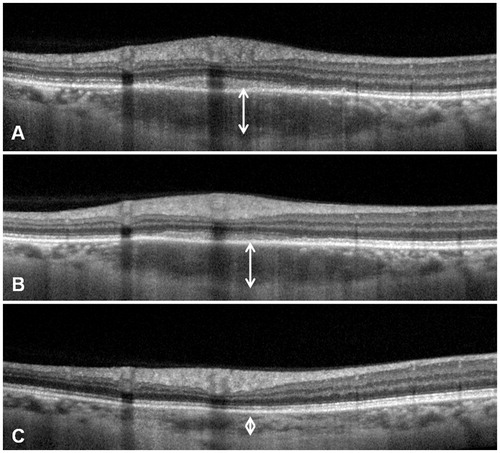
Two weeks after treatment with systemic corticosteroids, VA improved to 20/20, with resolution of the subretinal fluid and of the vasculitis associated with the choroidal granuloma. The thickness of the granuloma decreased from 568 to 356 μm (), although the choroidal lesion still appeared hyporeflective. Five months later, the thickness of the granuloma has reached 274 μm (). After 11 months, the choroidal thickness decreased to 150 μm (normal thickness compared to adjacent choroid).
FIGURE 3. Comparison between EDI SD-OCT before (A) and after (B) 2 weeks of systemic corticosteroids. The choroidal granulomatous lesions (a regional granulomatous reaction in the choroid) appear as a hyporeflective thickening of the choroid. The thickness of the granuloma decreased from 568 to 356 μm after treatment.
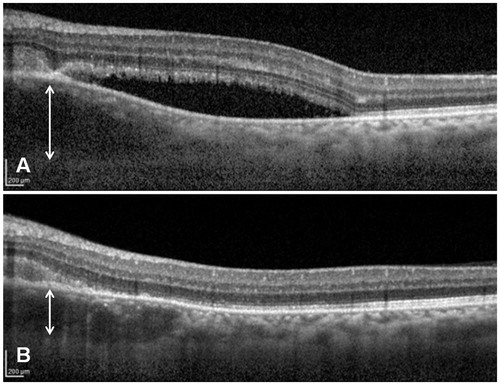
FIGURE 4. Comparison between EDI-OCT after 2 weeks of systemic corticosteroids (A) and 5 months later (B). The focal hyporeflective thickening of the choroid (arrowhead) decreased from 356 to 274 μm. After 11 months, the choroidal thickness decreased to 150 μm (C).
A solitary sarcoid choroidal granuloma is a rare entity in ocular sarcoidosis. EDI SD-OCT is a noninvasive technology that has transformed the exploration of the choroid.
In our patient, the presumed sarcoid choroidal granuloma appeared as a focal hyporeflective thickening of the choroid on EDI-OCT. The adjacent choroid appeared of normal thickness and reflectivity. To the best of our knowledge, this is the first quasi-histological description of a presumed sarcoid choroidal granuloma, although only a biopsy can confirm this diagnosis with certainty.
Of note, the decrease in choroidal thickness appeared as soon as 2 weeks after the beginning of corticosteroid therapy, with a quasi-normalization after 11 months. However, the rate of disappearance of a granuloma may be related to its size and location.
In conclusion, EDI-OCT allows direct visualization of choroidal granuloma secondary to sarcoidosis and evaluation of lesion regression after treatment.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper
References
- Sharma OP. Sarcoidosis. Dis Mon. 1990;36:469–535
- Obenauf DC, Shaw HE, Sydnor CF, et al. Sarcoidosis and its ophthalmic manifestations. Am J Ophthalmol. 1978;86:648–655
- Tingey DP, Gonder JR. Ocular sarcoidosis presenting as a solitary choroidal mass. Can J Ophthalmol. 1992;27:25–29
- Spaide RF, Koizumi H, Pozonni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500
