Introduction
Phlyctenular keratoconjunctivitis is a nodular inflammation of perilimbal tissue that is probably a local immune-mediated response to a systemic sensitization.1,2 Although Mycobacterium tuberculosis and Staphylococcus aureus have been most frequently associated with phlyctenular eye disease,1,2 diverse microbes such as Chlamydia sp.3 and intestinal parasites such as Hymenolepsis nana 4 have also been implicated. Phlyctenules can also be seen in patients with blepharitis and ocular rosacea.5 In this report, we describe a case of bilateral phlyctenular keratoconjunctivitis associated with Dolosigranulum pigrum in a pediatric patient with asthma and environmental allergies. This newly recognized bacterial species has not been previously isolated in cases of phlyctenulosis. We discuss the pathogenesis of phlyctenular keratoconjunctivitis in our patient along with considerations for future diagnosis and management.
Case Report
A 2-year-old female was referred for evaluation of bilateral corneal opacities of 2 months duration. Her parents reported no apparent visual impairment but noted epiphora, eye rubbing, rhinorrhea, and intermittent photophobia. She had recently been diagnosed with environmental allergies and asthma and was managed with fexofenadine 60 mg and montelukast 5 mg, which had led to some decrease in the epiphora, rhinorrhea, and eye rubbing.
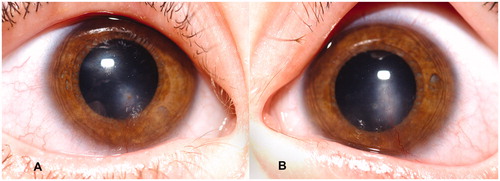
Examination revealed erythema of the lids and margins, mild papillary changes of the conjunctivae, and bilateral corneal opacities just inferior to the visual axes, with vessels extending toward the opacities from the 6 o'clock limbus (). The anterior chambers were quiet. Retinoscopy revealed minimal refractive error but significant optical interference from the opacities. Conjunctival swabs were obtained for smears and cultures for aerobic and anaerobic bacteria and viruses. Neomycin–polymyxin–dexamethasone 0.1% ophthalmic suspension and ointment were prescribed empirically for treatment of phlyctenular keratoconjunctivitis, which was presumed to be secondary to gram-positive cocci.
Figure 1. Clinical photographs: (A) right eye; (B) left eye. Bilateral corneal opacities can be seen just inferior to the visual axis with narrow superficial vessels extending from the 6 o'clock limbus superiorly toward the opacities.
Initial Gram stains were negative but aerobic cultures revealed gram-positive cocci. Although some of the initial biochemical testing suggested alpha hemolytic streptococcus, subsequent testing (positive pyrrolidonyl arylamidase and leucine aminopeptidase hydrolysis, growth in 6.5% NaCl broth, negative bile esculin hydrolysis) did not confirm this, and it was determined that sequencing would be needed to properly identify the organism. The specimen was sent to the New York State Department of Health for sequencing and Dolosigranulum pigrum was identified via 16S rRNA sequencing. Antibiotic susceptibility testing revealed sensitivity to moxifloxacin and vancomycin. Susceptibility testing to erythromycin could not be performed as the organism was unable grow without elevated carbon dioxide levels, which produced an acidic milieu (pH of ∼5.5) that rendered erythromycin inactive.
One month later, the patient's parents noted fading opacities and resolved epiphora and eye rubbing. They denied recent allergic exacerbations. Slit-lamp examination revealed blepharitis, conjunctival hyperemia, and fading of the corneal opacities and associated vessels. Neomycin–polymyxin–dexamethasone 0.1% ophthalmic suspension and ointment were continued based on initial favorable response to therapy.
Discussion
Phlyctenular keratoconjunctivitis is a common cause of pediatric referrals to corneal specialists and is seen predominantly in children of ages 6 months to 16 years. Without treatment, it is a potentially blinding disease from permanent corneal scarring or perforation.Citation2
Proposed Pathogenesis of Phlyctenulosis
Present evidence suggests that phlyctenular keratoconjunctivitis results from systemic sensitization followed by local exposure to microbial antigens.Citation1,Citation2 Animal models substantiate an immunologic pathogenesis for phlyctenular eye disease, but it is unclear if phlyctenules arise from a delayed cell-mediated or humoral hypersensitivity reaction, or a combination thereof.Citation6 Several microbes have been implicated in the development of phlyctenulosis. The inciting antigen could be specific to one microbe or shared by several.Citation1–5, 8–10
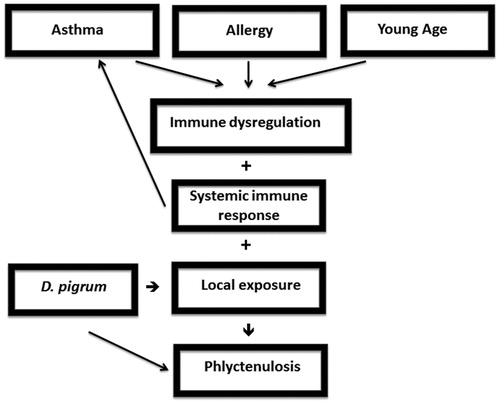
In this patient, phlyctenular keratoconjunctivitis arose against the backdrop of asthma and environmental allergies. Asthma, allergies, and phlyctenulosis have immunologic components that are central to their pathogeneses. We hypothesize that the immune dysregulation from our patient's underlying asthma and allergies may have contributed to the immune response of phlyctenulosis (). Immune dysregulation as a factor in the pathogenesis of phlyctenules is supported by previous reports of phlyctenular keratoconjunctivitis with blepharitis and rosacea,Citation5 as well as with the predominance of phlyctenular keratoconjunctivitis in young childrenCitation2 in whom the immune system is not yet mature.
Figure 2. Schematic diagram: pathogenesis of phlyctenular eye disease in our patient with asthma, allergy, and Dolosigranulum pigrum.
D. pigrum was isolated from the conjunctivae of this patient, who had no exposures that put her at risk of contracting tuberculous or parasitic infections that have been associated with phlyctenulosis. D. pigrum has been found to be part of the core upper respiratory microbiota,Citation7 but evidence regarding whether it is commensal conjunctival flora is currently lacking. The organism has emerged as a potential pathogen in humans and has been identified in a broad spectrum of diseases, including nosocomial pneumonia and septicemia, ventilator-associated pneumonia, synovitis, acute cholecystitis and pancreatitis,Citation8 biomaterial-associated arthritis,Citation9 and cystic fibrosis pneumonia.Citation10 Ocular involvement of D. pigrum has been reported in cases of a neurotrophic cornea,Citation11 blepharitis,Citation12 keratitis,Citation13 and bacterial conjunctivitis.Citation14 However, we are not aware of any prior reports of D. pigrum associated with phlyctenular keratoconjunctivitis.
D. pigrum is a relatively newly identified microbe. It was first described in 1993 by Aguirre et al. as a lactic acid, gram-positive, catalase-negative organism arranged in pairs and clusters.Citation11 It was previously misclassified as part of the Gemella species and closely resembles Streptococci viridans on blood agar cultures. It is phenotypically distinguished from other catalase-negative, gram-positive cocci by positive vancomycin susceptibility, pyrrolidonyl arylamidase and leucine aminopeptidase hydrolysis, growth in 6.5% NaCl broth, and esculin hydrolysis.Citation12
Identifying D. pigrum with culture is difficult as multiple tests are needed, some of which require lengthy incubation times before results are obtained. Additionally, further sequencing analysis is often required, and thus time to diagnosis can be prolonged. Furthermore, D. pigrum may be missed either as a member of the normal flora or as a causative agent if it is not suspected in the pathogenesis of the disease process.
Treatment Options for D. pigrum-associated Ocular Infections
Treatment of phlyctenular keratoconjunctivitis typically involves the use of appropriate therapy directed toward eradicating the inciting agent along with steroids to dampen the inflammatory response.Citation2,Citation3 Laclaire et al. tested antimicrobial susceptibilities of six clinical isolates of D. pigrum obtained from different sources in the eye. All isolates were sensitive to beta-lactam agents, while resistance was noted to trimethoprim–sulfamethoxazole in one isolate and erythromycin in all six of the ocular isolates.Citation12 A recent multicenter study identified D. pigrum as an emerging pathogen in conjunctivitis, which is sensitive to fourth-generation fluoroquinolones.Citation14 Susceptibility testing of the D. pigrum isolates from our patient demonstrated resistance to macrolides and sulfa drugs and sensitivity to beta-lactams consistent with Laclaire's findings,Citation12 as well as sensitivity to fluoroquinolones and vancomycin.
Given the antimicrobial susceptibility profile for D. pigrum, treatment of ocular infections from this organism raises important questions. Oral erythromycin is frequently used for pediatric phlyctenular keratoconjunctivitisCitation3 and ocular rosacea,Citation15 where it is known to produce long-lasting remission. In addition to its antibiotic activity, erythromycin is thought to improve meibomian gland function and consequently lengthen tear breakup time and resolve punctate kerathopathy.Citation15 Although D. pigrum isolates show high resistance to macrolides, their additional effects on meibomian gland function and tear film breakup may still warrant their use. When used in conjunction with steroids, macrolides may help dampen the underlying ocular inflammation that is central to the pathogenesis of phlyctenulosis. Our patient also responded favorably to empiric neomycin and steroid therapy prior to final speciation. However, susceptibility of D. pigrum to aminoglycoside therapy has not been previously reported. As such, it is difficult to determine if our patient's improvement is due to the antibiotic or due to the efficacy of the steroid in dampening the immune response.
D. pigrum isolates from our patient have also shown susceptibility to fluoroquinolones and vancomycin. Widespread and frequent use of vancomycin raises concern for the development of resistant bacterial species. In addition, topical vancomycin preparations are not commercially available, unlike fluoroquinolones, and need to be obtained from compounding pharmacies. Commercially available topical fourth-generation fluoroquinolones, in conjunction with topical steroids, may be a good choice for treatment to provide coverage of D. pigrum as well as other gram-positive and mycobacterial pathogens traditionally implicated in phlyctenulosis. Topical fluoroquinolones have been FDA approved for the treatment of acute conjunctivitis in children older than 12 months, and there are no concerns of systemic toxicity with topical therapy.Citation16 Analysis from a recent study found 100% microbial eradication in all identified cases of D. pigrum-associated bacterial conjunctivitis (n = 14) with besifloxacin ophthalmic suspension 0.6%.Citation17 However, chronic and frequent use of fluoroquinolones can also contribute to the emergence of resistant strains, which is worrisome given that they are one of the few agents to which D. pigrum is susceptible. As such, we recommend oral macrolide therapy and longer-term topical steroids for initial management of pediatric phlyctenular keratoconjunctivitis. Acute use of topical fourth-generation fluoroquinolones can be implemented if testing confirms D. pigrum as the causative agent or if conventional therapy fails. Long-term topical corticosteroid therapy must also be used judiciously, given the risks of elevated intraocular pressure, cataract formation, and corneal melting.Citation15 Vancomycin can then be considered for treatment failures.
Considerations for Future Diagnosis and Management
In our case, the identification of D. pigrum as a possible inciting agent for phlyctenular keratoconjunctivitis is novel and previously undocumented. It is important to consider D. pigrum in the differential diagnosis of bacterial conjunctivitis and phlyctenular eye disease given its recent emergence and identification as a pathogen, as well as its unique antibiotic susceptibility profile. An assessment of immunologic comorbidities that might contribute to the pathogenesis of phlyctenulosis, such as asthma, may prove helpful in identifying patients susceptible to phlyctenular keratoconjunctivitis.
We recommend obtaining conjunctival swabs to culture and sequence organisms and performing antibiotic susceptibility tests to identify effective therapeutic agents. Many facilities do not have the capability to sequence D. pigrum; thus, outsource testing may need to be considered when D. pigrum is on the differential diagnosis. Based on our findings and review of the literature, topical steroid therapy and oral macrolides can be used initially to manage cases of pediatric phlyctenular keratoconjunctivitis. Acute treatment with topical fourth-generation fluoroquinolones, to which D. pigrum is largely sensitive, can be instituted if this organism is identified or if conventional therapy fails. Topical vancomycin can be reserved as an alternative option for phlyctenular keratoconjunctivitis unresponsive to first-line treatment.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by NIH/NEI K23EY019353 (HBH), Research to Prevent Blindness.
References
- Thygeson P. The etiology and treatment of phlyctenular keratoconjunctivitis. Am J Ophthalmol. 1951;34:1217–1236
- Zaidman GW. The pediatric corneal infiltrate. Curr Opin Ophthalmol. 2011;22:261–266
- Culbertson WW, Huang AJ, Mandelbaum SH, et al. Effective treatment of phlyctenular keratoconjunctivitis with oral tetracycline. Ophthalmology. 1993;100:1358–1366
- Al-Amry MA, Al-Amri A, Khan AO. Resolution of childhood recurrent corneal phlyctenulosis following eradication of an intestinal parasite. J AAPOS. 2008;12:89–90
- Neiberg MN, Sowka J. Phlyctenular keratoconjunctivitis in a patient with staphylococcal blepharitis and ocular rosacea. Optometry. 2008;79:133–137
- Mondino BJ, Kowalski R, Ratajczak HV, et al. Rabbit model of phlyctenulosis and catarrhal infiltrates. Arch Ophthalmol. 1981;99:891–895
- Bogaert D, Keijser B, Huse S, et al. Variability and diversity of nasopharyngeal microbiota in children: a metagenomic analysis. PLoS One. 2011;6:e17035
- Lecuyer H, Audibert J, Bobigny A, et al. Dolosigranulum pigrum causing nosocomial pneumonia and septicemia. J Clin Microbio.l. 2007;45:3474–3475
- Johnsen BO, Ronning EJ, Onken A, et al. Dolosigranulum pigrum causing biomaterial-associated arthritis. APMIS. 2011;119:85–87
- Bittar F, Richet H, Dubus JC, et al. Molecular detection of multiple emerging pathogens in sputa from cystic fibrosis. PLoS One. 2008;3:e2908
- Aguirre M, Morrison D, Cookson BD, et al. Phenotypic and phylogenetic characterization of some Gemella-like organisms from human infections: description of Dolosigranulum pigrum gen. nov., sp. nov. J Appl Bacteriol. 1993;75(6): 608–612
- Laclaire L, Facklam R. Antimicrobial susceptibility and clinical sources of Dolosigranulum pigrum cultures. Antimicrob Agents Chemother. 2000;44:2001–2003
- Sampo M, Ghazouani O, Cadiou D, et al. Dolosigranulum pigrum keratitis: a three-case series. BMC Ophthalmol. 2013;13: 31
- Haas W, Gearinger LS, Hesje CK, et al. Microbiological etiology and susceptibility of bacterial conjunctivitis isolates from clinical trials with ophthalmic, twice-daily besifloxacin. Adv Ther. 2012;29:442–455
- Cetinkaya A, Akova YA. Pediatric ocular acne rosacea: long-term treatment with systemic antibiotics. Am J Ophthalmol. 2006;142:816–821
- Bradley JS, Jackson MA, Committee on Infectious Diseases, American Academy of Pediatrics. The use of systemic and topical fluoroquinolones. Pediatrics. 2011;128:e1034–e1045
- Gearinger LS, Sanfilippo CM, Zhang L, et al. Atypical pathogens observed in recent clinical studies of bacterial conjunctivitis. ARVO E-Abstract 2013
