Abstract
Purpose: The Central Retinal Enrichment Supplementation Trials (CREST) aim to investigate the potential impact of macular pigment (MP) enrichment, following supplementation with a formulation containing 10 mg lutein (L), 2 mg zeaxanthin (Z) and 10 mg meso-zeaxanthin (MZ), on visual function in normal subjects (Trial 1) and in subjects with early age-related macular degeneration (AMD; Trial 2).
Methods: CREST is a single center, double-blind, randomized clinical trial. Trial 1 (12-month follow-up) subjects are randomly assigned to a formulation containing 10 mg L, 10 mg MZ and 2 mg Z (n = 60) or placebo (n = 60). Trial 2 (24-month follow-up) subjects are randomly assigned to a formulation containing 10 mg L, 10 mg MZ, 2 mg Z plus 500 mg vitamin C, 400 IU vitamin E, 25 mg zinc and 2 mg copper (Intervention A; n = 75) or 10 mg L and 2 mg Z plus 500 mg vitamin C, 400 IU vitamin E, 25 mg zinc and 2 mg copper (Intervention B; n = 75). Contrast sensitivity (CS) at 6 cycles per degree represents the primary outcome measure in each trial. Secondary outcomes include: CS at other spatial frequencies, MP, best-corrected visual acuity, glare disability, photostress recovery, light scatter, cognitive function, foveal architecture, serum carotenoid concentrations, and subjective visual function. For Trial 2, AMD morphology, reading speed and reading acuity are also being recorded.
Conclusions: CREST is the first study to investigate the impact of supplementation with all three macular carotenoids in the context of a large, double-blind, randomized clinical trial.
INTRODUCTION
A yellow pigment composed of the carotenoids lutein (L), zeaxanthin (Z), and meso-zeaxanthin (MZ),Citation1 accumulates at the macula where it is known as macular pigment (MP). MP is a short-wavelength (blue) light filterCitation2 and a powerful antioxidant,Citation3 and is therefore believed to protect against age-related macular degeneration (AMD),Citation4 which is the commonest cause of blindness in the developed world.Citation5 In addition, MP is essential for optimal vision because of its optical properties.Citation6–8
In July 2011, the European Research Council (ERC) awarded funding of €1,493,342 to support and conduct the Central Retinal Enrichment Supplementation Trials (CREST). The CREST project is funded under the ERC “Ideas” Framework 7 program. These trials were designed to investigate the impact of supplementation with a combined carotenoid formulation of L, Z, and MZ on visual function in normal subjects (Trial 1) and in subjects with early AMD (Trial 2).
A novel and important feature of the CREST trials is the inclusion of MZ in the study intervention. Recent data from our laboratory show that optimal enrichment of MP is dependent upon inclusion of MZ (along with L and Z) in the supplement formulation.Citation7,Citation9,Citation10 MZ is believed to be particularly important for the following reasons. First, MZ is the dominant macular carotenoid at the foveal epicenter,Citation8 and is therefore ideally located to exert optimal antioxidant activity and short-wavelength light filtration at the central macula, the specialized part of the retina responsible for color vision and high spatial resolution.Citation11 Second, it has been shown (in vitro) that the antioxidant properties of the macular carotenoids (L, Z, and MZ) are enhanced when all three carotenoids are present.Citation12 Third, the absorbance spectrum of MZ extends the range of pre-receptoral short-wavelength (blue) light filtration, and its orientation (compared to L) in the Henle fiber layer likely confers beneficial effects related to light polarization at the macula.Citation13 Last, it has been shown that individuals at increased risk of AMD (i.e. older subjects and cigarette smokers) are more likely to display atypical and undesirable central dips in their MP spatial profiles,Citation14 and it has been shown that these central dips can only be normalized when MZ is included in a study intervention.Citation9,Citation15
A novel and distinctive feature of the CREST trials is the variety of methods and outcome measures selected to assess visual function in both the normal and AMD populations under investigation. These methods, which are described fully below, are appropriate and sensitive to detect change in visual function, if present.Citation16,Citation17 Indeed, previous clinical trials investigating the potential impact of carotenoid supplementation on visual function in normal subjectsCitation6 and in patients with early AMDCitation18 were limited by the outcome measures (e.g. typically best-corrected visual acuity; BCVA) and in terms of interventions used (i.e. an intervention without MZ).
The study hypothesis of CREST is that MP will be uniquely and best enriched using a supplement formulation containing all three macular carotenoids, and that enrichment of MP centrally, and across its spatial profile, will enhance visual function via the optical properties of this pigment, by reducing the deleterious effects of chromatic aberration, light scatter, and veiling luminance in normal subjects (non-diseased retina, Trial 1, see below) and in patients with early AMD (diseased retina, Trial 2, see below). This article outlines the design and methodology of the CREST trials.
MATERIALS AND METHODS
Management, Design and Registration
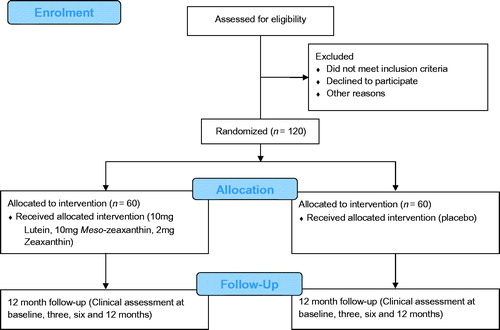
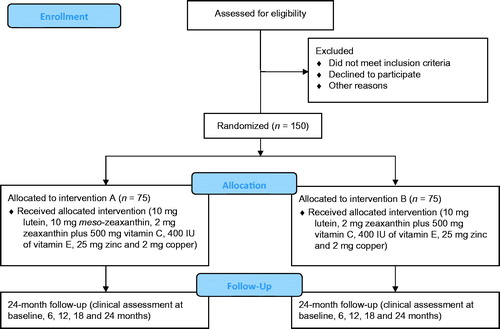
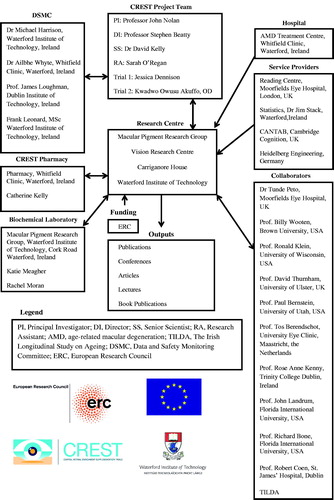
The management of CREST, including its research team, supporting service providers, and research collaborators is summarized in . CREST is a parallel group, double blind, randomized controlled trial studying two populations of interest. Trial 1 is investigating the impact of macular carotenoid nutrition on visual function in normal subjects, and Trial 2 is investigating the impact of macular carotenoid nutrition on visual function in patients with early AMD. These trials are registered on the current controlled trials register (Trial 1: ISRCTN68270512; Trial 2: ISRCTN13894787) and are being conducted as a single center study at the Macular Pigment Research Group (www.mprg.ie), Vision Research Centre, Waterford Institute of Technology, Ireland. and show the consolidated standards of reporting trials diagram,Citation19 explaining the flow of subjects through Trial 1 and Trial 2, respectively.
FIGURE 1. Structure of the Central Retinal Enrichment Supplementation Trials (CREST) management and collaboration.
Ethical Assessment and Approval
All subjects are required to provide written informed consent prior to enrolment into CREST. Ethical approval for the study was granted by the Research Ethics Committee of the Waterford Institute of Technology, Waterford, Ireland, and the Ethics Committee of the ERC. CREST adheres to the tenets of the Declaration of Helsinki, and will follow the full code of ethics with respect to subject recruitment, subject testing and data protection.
Research Questions
Trial 1: Does supplementation with all three macular carotenoids in a ratio (mg/day) of 10:10:2 (L:MZ:Z), for 12 months, enhance visual function in normal subjects (without retinal disease) when compared to placebo?
Trial 2: Does supplementation with all three macular carotenoids in a ratio (mg/day) of 10:10:2 (L:MZ:Z) plus 500 mg vitamin C, 400 IU vitamin E, 25 mg zinc and 2 mg copper for 24 months, enhance visual function in patients with early AMD when compared to 10:2 (L:Z) plus 500 mg vitamin C, 400 IU vitamin E, 25 mg zinc and 2 mg copper.
Primary Outcome Measure
Contrast sensitivity (CS) at 6 cycles per degree (cpd) is the primary outcome measure in both trials.
Secondary Outcome Measures
Secondary outcome measures include CS at other spatial frequencies, visual acuity, glare disability, photostress recovery, MP, light scatter, foveal architecture, serum carotenoid concentrations, subjective visual function, and cognitive function. In Trial 2, AMD morphology, reading acuity and reading speed are also being assessed.
Randomization and Intervention
Block randomization is used to assign subjects to intervention groups for both trials. The use of blocking is designed to ensure that an equal number of subjects are assigned to intervention groups. The random numbers were generated using Minitab 16 Statistical Software (Minitab Inc, State College, PA, USA) and the blocks generated by one of the methods described by Friedman and co-authors.Citation20 The randomization ratio is 1:1 with no stratification. The randomization code list was generated by the study statistician (JS) who has no contact with study subjects and no access to data until study completion. Random allocation is carried out by a pharmacist (CK) at Whitfield Clinic, Waterford, who has no contact with study subjects. The pharmacist tosses a coin to assign subjects to intervention groups based on the randomization code list. Study researchers only receive a box of tablets with subject identification label. The code is revealed only at study completion.
The intervention for Trial 1 is a softgel capsule containing 10 mg L, 10 mg MZ and 2 mg Z in a sunflower oil suspension (commercially available as Macushield™, provided by Macuvision Europe Limited, Solihull, UK, and prepared by EuroCaps Limited, Tredegar, South Wales, UK). The placebo is a softgel capsule containing sunflower oil (provided by EuroCaps Limited, Tredegar, South Wales, UK). Trial 1 subjects are instructed to take one capsule daily with a meal. The intervention and placebo supplements are identical in external appearance and therefore the two treatments are indistinguishable from each other.
The interventions for Trial 2 consist of a softgel capsule containing 10 mg L, 10 mg MZ and 2 mg Z in a sunflower oil suspension plus two multivitamin capsules each containing 250 mg vitamin C, 200 IU vitamin E, 12.5 mg zinc, and 1 mg copper (provided by Macuvision Europe Limited, Solihull, UK, prepared by EuroCaps Limited, Tredegar, South Wales, UK; Intervention A) or a softgel capsule containing 10 mg L, 2 mg Z in a sunflower oil suspension plus two multivitamin capsules each containing 250 mg vitamin C, 200 IU vitamin E, 12.5 mg zinc, and 1 mg copper (provided by Macuvision Europe Limited, Solihull, UK, prepared by EuroCaps Limited, Tredegar, South Wales, UK; Intervention B). The macular carotenoid capsules are also indistinguishable from each other in external appearance. Trial 2 subjects are instructed to take one macular carotenoid capsule and two multivitamin capsules daily with a meal.
Compliance
Frequent phone calls and reminder text messages are sent to subjects to ensure compliance with consumption of the study intervention. Capsule counting is implemented at follow-up visits. In addition, compliance will be assessed at the end of the study (after the randomization code is broken) by analyzing serum carotenoid concentrations using high performance liquid chromatography (see method below).
Sample Size Calculations
The primary outcome measure in CREST is change in CS at 6cpd over the course of the study: Y = CS2 – CS1, where CS1 is CS at baseline, CS2 is CS at the end of the study. The appropriate statistical test is the independent samples t-test, comparing the mean change in Y in treatment groups.
Pilot studies were conducted to inform CREST with respect to power and sample size (trial ISRCTN81595685). From this pilot work, estimates of standard deviation of CS, and the correlation between CS pre- and post-intervention were available and were used in the sample size calculations. There was strong evidence from the pilot work of a positive effect (i.e. improvement in mean CS) of treatment on CS relative to placebo and therefore a one-tailed rather than a two-tailed test was deemed more appropriate for Trial 1. For Trial 2, since there was no such evidence for the two treatment groups, a two-tailed test was used.
Using a clinically significant effect size of 0.15 logarithm (log) CS units (an improvement of one line on a Thomson logarithm of the minimum angle of resolution, LogMAR, chart) and on standard assumptions (5% level of significance, 80% power, equal group sizes), the required minimum sample size in Trial 1 is 90 subjects (45 per treatment group) and Trial 2 is 112 (56 per treatment group).Citation21
However, assuming a 25% dropout rate for both trials, we decided on a total sample size of 120 for Trial 1 and a total sample size of 150 for Trial 2.
Some power calculations for secondary outcome variables were also performed based on 45 subjects per group. These were based on Cohen’s suggested classificationCitation22 of effect sizes:
Interval variables (independent samples t-tests), e.g. for comparing changes in MP in the two groups, with 45 subjects in each group, there is very high power (0.98) for detecting a large effect size (0.8 standard deviations on Cohen’s definition) and close to acceptable power (0.76) for detecting a medium effect size (0.5 standard deviations). These power calculations, as with those for the principal outcome measure, assume a 5% level of significance and a one-tailed test.
Categorical variables, e.g. comparing numbers (at the end of the study) in terms of AMD severity grade categories for intervention groups. The power in this case depends on the dimensions of the contingency table. If there are two rows in the table (two intervention groups) and three columns (e.g. mild, moderate, and severe AMD) then the power is 0.99 for detecting a large effect (W = 0.5 on Cohen’s definition) and 0.72 for detecting a medium effect (W = 0.3). The W-statistic, devised by Cohen,Citation22 is derived from the χ2 statistic used to test for independence in the contingency table. We have assumed a 5% level of significance.
In summary, for analysis of secondary outcome variables, interval or categorical, the power of this study is inadequate for small effect sizes by Cohen’s definition (e.g. 0.2 standard deviations for an interval variable), but the power is adequate/strong for medium/large effect sizes. The results for power presented here were obtained from the PASS 2008 software (NCSS LLC, Kaysville, Utah, USA).Citation23
Eligibility Criteria
Trial 1
Inclusion criteria for Trial 1 include: (1) 18 years or older; (2) BCVA of 6/6 or better; (3) no more than five diopters spherical equivalence of refraction; (4) no previous consumption of supplements containing the macular carotenoids (L, Z and/or MZ); (5) no ocular pathology; and (6) MP at 0.25 degrees of eccentricity less than 0.5 optical density units. A subject is described as normal where he/she exhibits no vision-related abnormalities following a comprehensive battery of tests, including BCVA (better than or equal to 6/6), fundus photography (scrutinized by a retinal specialist), optical coherence tomography (OCT; scrutinized by a retinal specialist), and a general health questionnaire, with particular attention directed towards the possibility of diabetes mellitus or amblyopia.
Trial 2
Inclusion criteria for Trial 2 include: (1) early AMD in at least one eye, based on the grading of a fundus photograph from one (drusen absent or questionable or small hard drusen present, total drusen area <125 µm diameter, without retinal pigment abnormalities) to eight (drusen ≥0.5 disc area, DA, with retinal pigment epithelium depigmentation ≥350 µm to <0.5 DA or any drusen with ≥0.5 DA retinal pigment epithelium depigmentation) on the Age-Related Eye Disease Study (AREDS) severity scale;Citation24 (2) BCVA of 6/12 or better; (3) no more than five diopters spherical equivalence of refraction; (4) no previous consumption of supplements containing the macular carotenoids (L, Z and/or MZ); (5) no other retinal pathology beyond AMD; and (6) no diabetes mellitus.
Screening Visits to Assess and Confirm Eligibility
For both trials, efforts are made to ensure that subjects enrolled into the study meet the inclusion criteria. This is achieved by conducting a screening visit on all subjects prior to enrollment into either CREST trial.
Trial 1
Subjects are recruited into this trial through an organized advertising campaign. National and local media were informed of the trial and many mainstream Irish newspapers published the call for volunteers. Radio and online adverts have also been carried out. In addition, flyers have been developed for distribution to the general public. Educational events for general practitioners, optometrists and ophthalmologists are held regularly to create awareness of the trial and to solicit help with recruitment. Interested subjects attend our Vision Research Centre and an initial assessment is performed to determine if the subject meets the eligibility criteria for inclusion. Clinical examination consisting of ocular and medical history, BCVA, MP measurement, OCT and fundus photography is carried out. The screening visit is conducted to confirm absence of ocular pathology and to satisfy all other criteria for inclusion.
Trial 2
Subjects are recruited from hospitals in the Republic of Ireland. This has been facilitated by raising awareness of the trial at each hospital. Also, as above, educational events for general practitioners, optometrists and ophthalmologists are held regularly to create awareness of the trial and to solicit help with recruitment. Interested and potential volunteers are invited to attend our Vision Research Centre for assessment to confirm eligibility (with particular emphasis placed on presence of early AMD). During the screening visit, demographic information is collected. This is followed by measurement of BCVA. In addition, anterior and posterior segment examination using the Haag-Streit BM 900 Slit lamp biomicroscope (Haag-Streit AG, Switzerland) is carried out by a consultant ophthalmologist with a special interest in AMD (SB). Subjects who are deemed suitable following the ophthalmological examination by SB then have stereo fundus photographs taken. These stereo fundus photographs are then sent to the Reading Centre at Moorfields Eye Hospital, London, for confirmation that the patient has early AMD. Only patients who have such confirmation by the Reading Centre are invited to participate in the study.
Study Visits
In Trial 1, study visits are conducted at baseline, 3 months, 6 months, and 12 months. At each visit, subjects undergo a series of tests and procedures, which are described in detail below. summarizes the clinical procedures conducted in Trial 1 at each study visit. The duration of a typical study visit is approximately 120 minutes.
TABLE 1. Central Retinal Enrichment Supplementation Trials (CREST) study procedures in Trial 1.
In Trial 2, study visits are conducted at baseline, 6 months, 12 months, 18 months and 24 months. summarizes the clinical procedures conducted in Trial 2 at each study visit. A typical study visit in Trial 2 takes about 150 minutes.
TABLE 2. Central Retinal Enrichment Supplementation Trials (CREST) study procedures in Trial 2.
Statistical Analysis
Baseline analysis
Placebo and intervention groups will be investigated for statistically significant differences in outcome measures, demographic variables etc, at baseline. This will be done using standard statistical analyses, e.g. independent sample t-tests for interval variables and contingency table analysis for categorical variables. It is expected that the randomization process will result in the intervention groups being statistically comparable. However, any between-group differences in variables, which are identified at baseline, will be controlled for in subsequent analyses.
Analysis of changes over time
If there is no need to control for baseline differences between placebo and intervention groups, then a straightforward independent sample t-test will suffice for the analysis of change over time in the primary outcome measure (CS at 6cpd). However, linear mixed modelsCitation20 may also be used, to control (if necessary) for baseline differences between groups and to analyze data from multiple time points. The principle of intention to treatCitation25–27 will not, in general, be followed (but will nevertheless be performed) in the statistical analysis, but wherever intention to treat-based analysis is found to yield substantially different findings to the main analysis, such discrepancies will be reported.
Most of the secondary outcome measures in this study (contrast sensitivity at other frequencies, MP, serum concentrations, etc) are also interval variables, and will be analyzed using the same methods as for CS at 6cpd. However, some outcome variables (in particular, in Trial 2, change in AMD severity grade over time in the intervention groups), are ordinal rather than interval variables, and therefore logistic regression or contingency table analysis will be used. Statistical significance will be set at the standard p < 0.05 for all analyses. In order to reduce the risk of a type II error, there will be no adjustment for multiple comparisons, but this will be clearly stated when reporting study findings.
Questionnaires
Demographic and Lifestyle Questionnaire
The demographic and lifestyle questionnaire obtains the following details: contact details, ethnicity, education, occupation, smoking habits (history and frequency), alcohol intake (average consumption per week, frequency), exercise (number of sessions per week, duration of each session in minutes), light exposure (time spent outdoors, use of protective eyewear such as sunglasses, photochromic lenses), body mass index, blood pressure, medical history, and ocular medical history.
Subjective Visual Function Questionnaire
The subjective visual function questionnaire assesses visual function based on responses to closed-ended questions under four subscales, namely glare disability, acuity/spatial vision, light/dark adaptation and daily visual tasks. This questionnaire is administered in only Trial 1. All questions must be answered (forced-choice). In each of the four subscales, subjects respond to questions in three tiers. First, subjects rate their visual function in specified daily scenarios (situational analysis) using a five-point Likert scale (never, rarely, sometimes, often, always). Second, subjects compare their visual function to friends and family in a comparative analysis using a five-point Likert scale (significantly better than others, marginally better than others, equivalent to others, marginally worse than others, significantly worse than others). Last, subjects rate their overall visual performance on a scale from zero (worst) to 10 (best) known as subjective satisfaction score. Each tier analysis is computed to give a score out of 100 for each subscale. This questionnaire has been previously described.Citation6
National Eye Institute Visual Functioning Questionnaire 25
Subjective visual function is assessed in Trial 2 using the validatedCitation28,Citation29 National Eye Institute Visual Functioning Questionnaire 25.Citation30
Dietary Carotenoid Screener
The dietary carotenoid screener is a simplified questionnaire which assesses the dietary intake of four carotenoid-rich food substances (eggs, broccoli, corn and dark green leafy vegetables). Subjects indicate their serving size by ticking any of six categories (<1/week, 1/week, 2–3/week, 4–6/week, 1/day, >1/day) with respect to each of the food substances. Responses are entered into a computer program developed by Professor Elizabeth Johnson, Tufts University, USA, which weighs responses based on the frequency of food intake and the bioavailability of L and Z within these food substances, and calculates a dietary score. The dietary scores generated range from 0–75, and are further divided into three subgroups (low intake, category 1, 0–15: ≤2 mg/day; medium intake, category 2, 16–30: 3–13 mg/day; high intake, category 3, 31–75: >13 mg/day). This method has been used previously by our group.Citation9,Citation31
Cognitive Function Assessment
Cambridge Neuropsychological Test Automated BatteryCitation32–34 (CANTAB, Cambridge Cognition, Cambridge, UK) assesses cognition using a computerized software program. A battery of tests consisting of the motor screening task,Citation35,Citation36 verbal recognition memory,Citation37 attention switching taskCitation38,Citation39 and the paired associate learningCitation40 is used. The CANTAB protocolCitation41 is followed in the administration of these tests.
Best-corrected Visual Acuity
BCVA is measured with a computerized LogMAR Early Treatment Diabetic Retinopathy Study (ETDRS) test chart (Test Chart 2000 Xpert, Thomson Software Solutions, Hatfield, UK) viewed at 4 m. The Sloan ETDRS letterset is used for this test. At the first incompletely read line, the letters of the line are randomized three times using the testing software’s randomization function and an average of three scores is taken. BCVA is recorded in visual acuity rating.
Contrast Sensitivity
Letter Contrast Sensitivity
Letter CS is assessed using the computerized LogMAR ETDRS test chart (Test Chart 2000 PRO, Thomson Software Solutions) at five different spatial frequencies (1.2, 2.4, 6.0, 9.6, 15.15cpd).Citation42 The Sloan optotypes are chosen and subjects are asked to read the letters aloud while fixating on the chart at a distance of 4 m. The letter set is randomized during the test at each change of contrast. The percentage contrast of letter optotypes is decreased in 0.15 log CS steps until the lowest contrast value for which subjects see at least three letters is reached. The test is then repeated for the other spatial frequencies. Each letter has a nominal log CS value of 0.03. Missed letters at any contrast level are noted. The resultant log CS value for the subject at a particular spatial frequency is calculated by adding any extra letter(s) and/or subtracting missed letters from best log CS value corresponding to the lowest percentage contrast.
Contrast Sensitivity with Functional Acuity Contrast Test
The Optec Functional Vision AnalyzerCitation43 (Stereo Optical Co, Inc, Chicago, IL, USA) uses the functional acuity contrast testCitation44,Citation45 to assess contrast sensitivity at five different spatial frequencies (1.5, 3, 6, 12, 18cpd). A detailed description of the method has been reported previously.Citation6,Citation46
Light Scatter
Using the compensation comparison method, the C-Quant Straylight Meter (Oculus GmbH, Wetzler, Germany)Citation48–50 measures light scatter by objectively determining the amount of intraocular straylight on the retina. Straylight measurements are reported in logarithmic form and judged reliable when standard deviation is ≤0.08, and the reliability coefficient is ≥1.
Photostress Recovery
Photostress recovery time is measured by assessing CS and investigating the impact of a light stress using a 300 watt tungsten spotlight (ARRI 300 Plus lamp, ARRI Lighting Solutions GmbH, Berlin, Germany) with a low-pass glass dichroic filter. Subjects view the lamp directly with the study eye (the other eye is covered with an eye patch) at a distance of 1 m for 10 seconds while limiting blinking. After 10 seconds, the lamp is extinguished and removed from the subject’s field of view. A letter size of 6/24 (LogMAR 0.6) is displayed on the LogMAR test chart (Test Chart 2000 PRO, Thomson Software Solutions), and viewed at 4 m. A CS value of 0.30 log units (i.e. two lines) above the individual’s contrast threshold, is used. The time taken for the subject’s eye to recover and see all five letters on the chart after the 10-second exposure is taken as the photostress recovery time.
Macular Pigment Measurement by Customized Heterochromatic Flicker Photometry
Using the Macular Densitometer (Macular Metrics Corp, Providence, RI, USA),Citation51,Citation52 MP is measured by customized heterochromatic flicker photometry. The spatial profile of MP is assessed by measuring MP at 0.25°, 0.5°, and 1.75° of retinal eccentricity, with a reference point at 7°. A detailed description of the protocol is reported elsewhere.Citation53,Citation54
Pupillary Dilation
Subjects’ pupils are dilated prior to performing stereo fundus photography, OCT and MP measurement using dual-wavelength autofluorescence. A drop each of 0.5% proxymetacaine hydrochloride, 2.5% phenylephrine hydrochloride, and 1% tropicamide is used.
Macular Pigment Measurement by Dual-wavelength Autofluorescence
Using the Spectralis HRA + OCT MultiColor (Heidelberg Engineering GmbH, Heidelberg, Germany), MP is measured by dual-wavelength (488 nm and 518 nm) autofluorescence.Citation55–57 Subject details are input into the Heidelberg Eye Explorer (HEYEX version 1.7.1.0) software. Assessment is performed with the room lights off. The following acquisition parameters are used: high speed scan resolution, two seconds cyclic buffer size, internal fixation, 30 seconds movie and manual brightness control. Alignment, focus and illumination are first adjusted in infrared mode. Once the image is evenly illuminated, the laser mode is switched from infrared to blue plus green laser light autofluorescence. Focus and illumination are re-adjusted for optimal acquisition. A 30-second movie of the macula is acquired for subsequent MP analysis using the HEYEX software.
Optical Coherence Tomography
Using the Spectralis HRA + OCT MultiColor (software version 5.6, Heidelberg Engineering GmbH),Citation58 foveal architecture is assessed using OCT. The device produces non-invasive retinal histological tomographs by integrating spectral (Fourier) domain OCT technology with confocal scanning laser ophthalmoscopy. The following scan acquisition protocol is used for Trial 1: compact volume scan (20° × 20°) of the macular area, 97 B-scans each spaced 60 µm apart at high speed with automatic real-time mean (ART) of 9/frame rate; cross scan (20° × 20°) at high resolution with an ART of 10/frame rate. The following scan acquisition protocol is used for Trial 2: volume scan (20° × 20°) of the macular area, 193 B-scans each spaced 30 µm apart at high speed with ART of 5/frame rate; cross scan (20° × 20°) at high resolution with an ART of 10/frame rate.
Fundus Photography and Grading
All photography is performed by trained and certified photographers. For subjects in Trial 1, standard color fundus photographs centered on the macula are taken using the Zeiss Visucam 200 (Carl Zeiss Meditec AG, Jena, Germany) at a 45° magnification setting. These fundus photographs are reviewed by SB in order to exclude any other ocular pathology.
For subjects in Trial 2, stereo color fundus photographs are taken using the Zeiss Visucam 200 (Carl Zeiss Meditec AG) at a 45° magnification setting. The stereo photography technique used is the modified 3-standard stereoscopic fields (Field 1: optic disc, Field 2: macula, Field 3: temporal to macula). In addition, fundus reflex photographs of the external eye are taken in order to document any media opacities. The anonymized photographs are then sent for grading at the Reading Centre, Moorfields Eye Hospital, London, UK, using a secure file transfer protocol. Fundus grading follows the AREDS 11-step severity scale.Citation24
Reading Acuity and Reading Speed
This test is only being performed in Trial 2. Reading acuity and reading speed are assessed with the English version of the standardized Radner reading chartCitation59 at 40 cm. Reading acuity is recorded in logarithm of the reading acuity determination (LogRAD). Reading speed (the time taken to read the number of words in a sentence) is measured in words per minute.
Serum Carotenoid Analysis
Non-fasting blood samples are collected at each study visit by standard venepunture techniques in 9 mL vacuette tubes (BD Vacutainer SST Serum Separation Tubes, Becton, Dickinson and Company, Plymouth, United Kingdom) containing a “Z Serum Sep Clot Activator.” All collection tubes are inverted a minimum of five times to ensure appropriate mixing of the clot activator. The blood samples are allowed to clot at room temperature for 30 minutes and then centrifuged for 10 minutes at 2700 rpm in a Gruppe GC 12 centrifuge (Desaga Sarstedt, Hampshire, UK) to separate the serum from the whole blood. After centrifugation, serum is transferred to light-resistant microtubes and stored at −80 °C until the time of analysis. Carotenoid analysis is carried out using a procedure described elsewhere.Citation31
Data and Safety Monitoring Committee
An independent data and safety monitoring committee (DSMC) has been appointed to examine and review data collected during the CREST project. This committee scrutinizes the data for evidence of safety and efficacy each year. The CREST DSMC consists of a statistician, a medical ophthalmologist, a health science researcher and a vision scientist. The DSMC has full access to the randomization code for both trials and the authority to break the code if needed. The DSMC has the authority to recommend any of the following: continuation of the study uninterrupted, alteration of either trial or any arm of either trial, or termination of either trial or any arm of either trial.
DISCUSSION
CREST has been designed to investigate the impact of supplementation with a combined carotenoid formulation of L, Z and MZ on visual function in normal subjects (Trial 1) and in subjects with early AMD (Trial 2). Enhancement of visual function as a result of MP augmentation, if present, is likely the result of its attenuation of both chromatic aberration and veiling luminance, with consequential benefits in terms of CS and glare disability,Citation7 and rests on its anatomical (pre-receptoral and central retinal)Citation1 and optical (short wavelength-filtering) properties.Citation2 The hypothesis that MP confers protection against AMD is premised on these same attributes of this pigment, as well as its antioxidant capacity,Citation3,Citation12 as (photo)-oxidative stress is believed to be important in the pathogenesis of this condition.Citation60
The landmark AREDS provided level 1 evidence that supplementation with a formulation of antioxidants and zinc, but which was devoid of the macular carotenoids, was associated with risk reduction for visual loss and disease progression in subjects with at least intermediate AMD.Citation61 The AREDS, therefore, furnished the scientific community with proof of principle that supplemental dietary antioxidants are of benefit in AMD, and somewhat paradoxically, generated interest in the role that MP might play, given its exquisite biological relevance to the tissue affected by this condition. As a consequence, studies were designed to investigate the putative benefits of supplementation with MP’s constituent carotenoids on the course of AMD.
Indeed, the subsequent AREDS2 study which was recently published, was designed to assess the impact of supplemental L, Z and omega-3 fatty acids plus co-antioxidants on progression to advanced AMD in eyes with at least intermediate AMD.Citation62,Citation63 In brief, AREDS2 has found that, controlling for baseline AMD status, none of the treatments were shown to significantly reduce risk of AMD progression relative to the group who received the “placebo” AREDS1 supplement only, although the trend was in favor of the treatments including L and Z (primary analysis). Also, there are many important secondary outcome variables available from this study. For example, there was a statistically significant (p = 0.01) reduction of 9% in risk of progression to advanced AMD for subjects receiving L and Z when compared with subjects not receiving L and Z; participants with the lowest dietary intake of L and Z showed a statistically significant (p = 0.01) reduction of 26% in risk of progression to advanced AMD, when compared with subjects not receiving L and Z; and, there was a statistically significant (p = 0.02) reduction of 18% in risk of progression to advanced AMD for subjects receiving L and Z in the absence of beta carotene when compared with subjects receiving an AREDS formulation with beta carotene (and not receiving L and Z). However, it is important to point out the major differences between CREST and the AREDS (1 and 2) studies. AREDS was designed and powered to investigate change in AMD morphology following supplementation with antioxidants, whereas CREST (which is a much smaller sample) is designed and powered to investigate change in visual function (i.e. CS) following supplementation with the macular carotenoids. These fundamental differences between two randomized controlled trials need to be appreciated when either of these studies is under discussion.
Another published clinical trial that deserves mention is the Carotenoids in Age-Related Maculopathy (CARMA) study. CARMA was a randomized, double-blind, placebo-controlled clinical trial of L (12 mg) and Z (0.6 mg) supplementation with co-antioxidants versus placebo in patients with AMD.Citation64 The primary outcome measure, corrected distance visual acuity (CDVA) at one year, did not differ significantly between the placebo and the intervention arms of the study.Citation65 It was noted, however, that CDVA was significantly better in the intervention arm of the study at 36 months follow-up.Citation18 In addition, an increase in serum L was associated with significantly improved CDVA and slowing of progression along the AMD severity scale.Citation18 However, one clear difference between CARMA and CREST was the absence of MZ in the CARMA study intervention, the importance of which has been discussed.
In addition, as a secondary outcome measure, the impact of macular carotenoid supplementation on cognitive function is being assessed. Indeed, a recent study has shown that MP is related to brain carotenoid levels,Citation66 and there is a growing body of evidence that poor antioxidant status represents risk for age-related loss of cognitive function.Citation67–69 Therefore, as MP represents a readily accessible and a non-invasive biomarker of antioxidant status within the central nervous system (i.e. retina), we believe that it is important to investigate the relationship, if any, between MP and cognitive function in CREST.
CREST will ascertain, through sufficiently powered, double-blind, randomized controlled clinical trials, the impact of supplementation with all three macular carotenoids (uniquely including the centrally dominant macular carotenoid, MZ) on vision in normal subjects and subjects with AMD. CREST will inform and advance our understanding of the protective and optical hypotheses of MP, and potentially identify ways to optimize vision in the absence of ocular disease and prevent or delay blindness attributable to AMD.
Declaration of interest
Kwadwo Owusu Akuffo, OD: None; Jessica Dennison, BSc: None; Sarah O’Regan: None; Jim Stack, PhD: None; Katherine A. Meagher, BSc: None; Tunde Peto, PhD: None; John Nolan, PhD and Stephen Beatty, MD do consultancy work for nutraceutrical companies in a personal capacity and as directors of Nutrasight Consultancy Limited.
This study was funded by the European Research Council (ERC); reference number: 281096.
Acknowledgements
CREST Research Group
Professor John Nolan, Principal Investigator
Professor Stephen Beatty, Director
Sarah O’Regan, Research Assistant
Jessica Dennison, Trial 1 Researcher
Kwadwo Owusu Akuffo, OD, Trial 2 Researcher
Dr David Kelly, Senior Scientist
DSMC Members
Dr Ailbhe Whyte, Medical Ophthalmologist
Professor James Loughman, Vision Scientist
Dr Michael Harrison, Research Ethics Committee member
Frank Leonard, MSc, Statistician
CREST Pharmacist
Catherine Kelly
References
- Bone RA, Landrum JT, Friedes LM, et al. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exper Eye Res 1997;64:211–218
- Snodderly DM, Brown PK, Delori FC, Auran JD. The Macular Pigment.1. Absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Invest Ophthalmol Vis Sci 1984;25:660–673
- Khachik F, Bernstein PS, Garland DL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci 1997;38:1802–1811
- Sabour-Pickett S, Nolan JM, Loughman J, Beatty S. A review of the evidence germane to the putative protective role of the macular carotenoids for age-related macular degeneration. Mol Nutr Food Res 2012;56:270–286
- Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA 2003;290:2057–2060
- Nolan JM, Loughman J, Akkali MC, et al. The impact of macular pigment augmentation on visual performance in normal subjects: COMPASS. Vision Res 2011;51:459–469
- Loughman J, Nolan JM, Howard AN, et al. The impact of macular pigment augmentation on visual performance using different carotenoid formulations. Invest Ophthalmol Vis Sci 2012;53:7871–7880
- Stringham JM, Garcia PV, Smith PA, et al. Macular pigment and visual performance in glare: benefits for photostress recovery, disability glare, and visual discomfort. Invest Ophthalmol Vis Sci 2011;52:7406–7415
- Nolan JM, Akkali MC, Loughman J, et al. Macular carotenoid supplementation in subjects with atypical spatial profiles of macular pigment. Exp Eye Res 2012;101:9–15
- Meagher KA, Thurnham DI, Beatty S, et al. Serum response to supplemental macular carotenoids in subjects with and without age-related macular degeneration. Br J Nutr 2013;110:289–300
- Hirsch J, Curcio CA. The spatial resolution capacity of human foveal retina. Vision Res 1989;29:1095–1101
- Li B, Ahmed F, Bernstein PS. Studies on the singlet oxygen scavenging mechanism of human macular pigment. Arch Biochem Biophys 2010;504:56–60
- Billsten HH, Bhosale P, Yemelyanov A, et al. Photophysical properties of xanthophylls in carotenoproteins from human retinas. Photochem Photobiol 2003;78:138–145
- Kirby ML, Beatty S, Loane E, et al. A central dip in the macular pigment spatial profile is associated with age and smoking. Invest Ophthalmol Vis Sci 2010;51:6722–6728
- Connolly EE, Beatty S, Thurnham DI, et al. Augmentation of macular pigment following supplementation with all three macular carotenoids: an exploratory study. Curr Eye Res 2010;35:335–351
- Charalampidou S, Loughman J, Nolan J, et al. Prognostic indicators and outcome measures for surgical removal of symptomatic nonadvanced cataract. Arch Ophthalmol 2011;129:1155–1161
- Neelam K, Nolan J, Chakravarthy U, Beatty S. Psychophysical function in age-related maculopathy. Surv Ophthalmol 2009;54:167–210
- Beatty S, Chakravarthy U, Nolan JM, et al. Secondary outcomes in a clinical trial of carotenoids with coantioxidants versus placebo in early age-related macular degeneration. Ophthalmology 2013;120:600–606
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Trials 2010;11:32
- Friedman LM, Furberg CD, Demets DL. Fundamentals of clinical trials. 4th ed. New York: Springer; 2010
- Kramer CFL, Theimann J. How many subjects?: Statistical power analysis in research. Thousand Oaks, CA: Sage Publications; 1987
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988
- Hintze J. PASS 2008. In: NCSS LLC. Kaysville, Utah; 2008
- Davis MD, Gangnon RE, Lee LY, et al. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol 2005;123:1484–1498
- Feinman RD. Intention-to-treat. What is the question? Nutr Metab (Lond) 2009;6:1
- Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res 2011;2:109–112
- Newell DJ. Intention-to-treat analysis: implications for quantitative and qualitative research. Int J Epidemiol 1992;21:837–841
- Orr P, Rentz AM, Margolis MK, et al. Validation of the National Eye Institute Visual Function Questionnaire-25 (NEI VFQ-25) in age-related macular degeneration. Invest Ophthalmol Vis Sci 2011;52:3354–3359
- Revicki DA, Rentz AM, Harnam N, et al. Reliability and validity of the National Eye Institute Visual Function Questionnaire-25 in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci 2010;51:712–717
- Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 2001;119:1050–1058
- Meagher KA, Thurnham DI, Beatty S, et al. Serum response to supplemental macular carotenoids in subjects with and without age-related macular degeneration. Br J Nutr 2013;110:289–300
- Lawrence AD, Sahakian BJ. The neuropsychology of frontostriatal dementias. In: Woods RT, editor. Handbook of the clinical psychology of aging. New York: Wiley; 1996:243–265
- Robbins TW, James M, Owen AM, et al. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia 1994;5:266–281
- Wild K, Howieson D, Webbe F, et al. Status of computerized cognitive testing in aging: a systematic review. Alzheimers Dement 2008;4:428–437
- Lawrence AD, Sahakian BJ, Hodges JR, et al. Executive and mnemonic functions in early Huntington’s disease. Brain 1996;119:1633–1645
- Owen AM, Downes JJ, Sahakian BJ, et al. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia 1990;28:1021–1034
- Fried R, Hirshfeld-Becker D, Petty C, et al. How informative is the CANTAB to assess executive functioning in children with ADHD? A controlled study. J Atten Disord 2012, Aug 24 (Epub ahead of print)
- Sahakian BJ, Downes JJ, Eagger S, et al. Sparing of attentional relative to mnemonic function in a subgroup of patients with dementia of the Alzheimer type. Neuropsychologia 1990;28:1197–1213
- Robbins TW, James M, Owen AM, et al. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia 1994;5:266–281
- Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biol Psychiatry 2000;48:674–684
- Cognition Limited. CANTAB Eclipse Test Administration Guide. Cambridge, UK: Cambridge Cognition Limited; 2012
- Charalampidou S, Nolan J, Loughman J, et al. Psychophysical impact and optical and morphological characteristics of symptomatic non-advanced cataract. Eye (Lond) 2011;25:1147–1154
- Hohberger B, Laemmer R, Adler W, et al. Measuring contrast sensitivity in normal subjects with OPTEC 6500: influence of age and glare. Graefes Arch Clin Exp Ophthalmol 2007;245:1805–1814
- Hitchcock EM, Dick RB, Krieg EF. Visual contrast sensitivity testing: a comparison of two F.A.C.T. test types. Neurotoxicol Teratol 2004;26:271–277
- Terzi E, Buhren J, Wesemann W, Kohnen T. [Frankfurt-Freiburg Contrast and Acuity Test System (FF-CATS). A new test to determine contrast sensitivity under variable ambient and glare luminance levels]. Ophthalmologe 2005;102:507–513
- Loughman J, Akkali MC, Beatty S, et al. The relationship between macular pigment and visual performance. Vision Res 2010;50:1249–1256
- van Gaalen KW, Jansonius NM, Koopmans SA, et al. Relationship between contrast sensitivity and spherical aberration: comparison of 7 contrast sensitivity tests with natural and artificial pupils in healthy eyes. J Cataract Refract Surg 2009;35:47–56
- Coppens JE, Franssen L, Van Rijn LJ, van den Berg TJ. Reliability of the compensation comparison stray-light measurement method. J Biomed Opt 2006;11:34027
- Franssen L, Coppens JE, van den Berg TJ. Compensation comparison method for assessment of retinal straylight. Invest Ophthalmol Vis Sci 2006;47:768–776
- van den Berg TJ, Van Rijn LJ, Michael R, et al. Straylight effects with aging and lens extraction. Am J Ophthalmol 2007;144:358–363
- Wooten BR, Hammond BR. Spectral absorbance and spatial distribution of macular pigment using heterochromatic flicker photometry. Optometry Vis Sci 2005;82:378–386
- Wooten BR, Hammond BR, Land RI, Snodderly DM. A practical method for measuring macular pigment optical density. Invest Ophthalmol Vis Sci 1999;40:2481–2489
- Stringham JM, Hammond BR, Nolan JM, et al. The utility of using customized heterochromatic flicker photometry (cHFP) to measure macular pigment in patients with age-related macular degeneration. Exp Eye Res 2008;87:445–453
- Loane E, Stack J, Beatty S, Nolan JM. Measurement of macular pigment optical density using two different heterochromatic flicker photometers. Curr Eye Res 2007;32:555–564
- Delori FC, Goger DG, Hammond BR, et al. Macular pigment density measured by autofluorescence spectrometry: comparison with reflectometry and heterochromatic flicker photometry. J Optical Soc Am A-Optics Image Sci Vis 2001;18:1212–1230
- Wustemeyer H, Jahn C, Nestler A, et al. A new instrument for the quantification of macular pigment density: first results in patients with AMD and healthy subjects. Graefes Arch Clin Exp Ophthalmol 2002;240:666–671
- Trieschmann M, Heimes B, Hense H, Pauleikhoff D. Macular pigment optical density measurement in autofluorescence imaging: comparison of one- and two-wavelength methods. Graefes Arch Clin Exp Ophthalmol 2006;244:1565–1574
- Wolf-Schnurrbusch UE, Ceklic L, Brinkmann CK, et al. Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Invest Ophthalmol Vis Sci 2009;50:3432–3437
- Stifter E, Konig F, Lang T, et al. Reliability of a standardized reading chart system: variance component analysis, test-retest and inter-chart reliability. Graefes Arch Clin Exp Ophthalmol 2004;242:31–39
- Beatty S, Koh HH, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 2000;45:115–134
- Kassoff A, Kassoff J, Buehler J, et al. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss – AREDS Report No. 8. Arch Ophthalmol 2001;119:1417–1436
- Chew EY, Clemons TE, SanGiovanni JP, et al. Secondary Analyses of the Effects of Lutein/Zeaxanthin on Age-Related Macular Degeneration Progression: AREDS2 Report No. 3. JAMA Ophthalmol 2013
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–2015
- Neelam K, Hogg RE, Stevenson MR, et al. Carotenoids and co-antioxidants in age-related maculopathy: design and methods. Ophthalmic Epidemiol 2008;15:389–401
- Beatty S, Nolan JM, Muldrew KA, et al. Visual outcome after antioxidant supplementation. Ophthalmology 2013;120:645
- Vishwanathan R, Neuringer M, Snodderly DM, et al. Macular lutein and zeaxanthin are related to brain lutein and zeaxanthin in primates. Nutr Neurosci 2013;16:21–29
- Rafnsson SB, Dilis V, Trichopoulou A. Antioxidant nutrients and age-related cognitive decline: a systematic review of population-based cohort studies. Eur J Nutr 2013;52:1553–1567
- Gray SL, Hanlon JT, Landerman LR, et al. Is antioxidant use protective of cognitive function in the community-dwelling elderly? Am J Geriatr Pharmacother 2003;1:3–10
- Salerno-Kennedy R, Cashman KD. Relationship between dementia and nutrition-related factors and disorders: an overview. Int J Vitam Nutr Res 2005;75:83–95