We read with great interest the article from Baum et al. [Citation1] entitled “Does local injury to the endometrium before IVF cycle really affect treatment outcome? Results of a randomized placebo controlled trial” and published in Gynecological Endocrinology. The authors performed a controlled randomized trial evaluating the effect of endometrial injury on assisted reproductive technology (ART) outcomes in women with recurrent implantation failure. Contrary to other studies published so far, they did not observe a beneficial effect of endometrial injury performed in the month before the ART cycle; they actually observed a trend of worse reproductive outcomes in the group submitted to endometrial injury.
It is an interesting result as there is a growing body of evidence pointing to the opposite way. Since, a systematic review and meta-analysis addressing this question was published [Citation2], two other studies [Citation3,Citation4] were completed, and the updated pooled data are shown in . All other randomized controlled trials (RCTs) published so far consistently reported a beneficial effect on live birth: risk ratio (RR) = 1.97; 95% CI from 1.35 to 2.88 (); and on clinical pregnancy: RR = 1.86; 95% CI from 1.46 to 2.38 (); without heterogeneity (I2 = 0%) for both outcomes. However, when adding the study from Baum et al. [Citation1] to the analysis and applying the test for subgroup differences, inconsistency between the results is substantial: the I2 increased to 73.9% for live birth and to 77.8% for clinical pregnancy. Nevertheless, we would prefer not to pool these results together. While all other RCTs compared endometrial injury with either no injury or with a mock procedure; the study by Baum et al. compared two interventions: “endometrial pipelle” versus “cervical pipelle”. The latter cannot be simply considered as a “placebo”. Cervical pipelle causes disturbance to the endocervix, and the procedure may also cause some degree of endometrial injury in some of the participants: the pipelle might be easily inserted beyond internal cervical os unnoticed.
Figure 1. Forest-plot of live birth per allocated woman in randomized controlled trials evaluating the effect of endometrial injury.
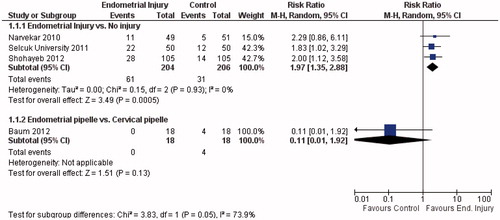
Figure 2. Forest-plot of clinical pregnancy per allocated woman in randomized controlled trials evaluating the effect of endometrial injury.
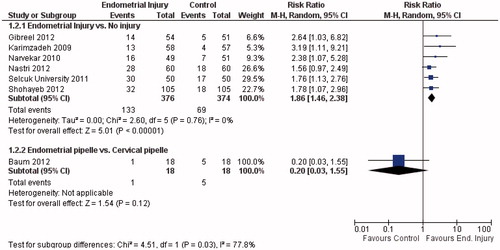
There are some other issues to be clarified. As defined in the revised glossary of ART terminology [Citation5], clinical pregnancy is “a pregnancy diagnosed by ultrasonographic visualization of one or more gestational sacs or definitive clinical signs of pregnancy”. The authors stated that “with regard to pregnancy outcome, a total of five pregnancies and four deliveries of healthy babies were achieved in the control group compared to one pregnancy that ended in a miscarriage in the experimental group”; and also stated that implantation rate in the experimental group was not 0. So, clinical pregnancy rate could not be 0 in the “endometrial pipelle” group as reported in the graphs and comparisons. Considering this and the intention-to-treat principle, the clinical pregnancy rate would be 1/18 in the “endometrial pipelle” group, and 5/18 in the “cervical pipelle” group: p = 0.12; RR = 0.20; 95% CI from 0.03 to 1.55. There was no significant difference between the groups. Another issue is the lack of multiple pregnancy: it is very likely that at least one of the 6 pregnancies were multiple, since the mean number of embryos transferred was 2.9. Maybe a twin pregnancy was incorrectly accounted as two clinical pregnancies and two live births; if this had happened, the difference between the groups in clinical pregnancy and live birth would be even smaller.
We kindly ask the authors to clarify all these issues, as they are relevant for future systematic reviews.
Declaration of interest
The authors report no conflicts of interest.
References
- Baum M, Yerushalmi GM, Maman E, et al. Does local injury to the endometrium before IVF cycle really affect treatment outcome? Results of a randomized placebo controlled trial. Gynecol Endocrinol 2012;28:933–6
- Nastri CO, Gibreel A, Raine-Fenning N, et al. Endometrial injury in women undergoing assisted reproductive techniques. Cochrane Database Syst Rev 2012;7:CD009517
- Shohayeb A, El-Khayat W. Does a single endometrial biopsy regimen (S-EBR) improve ICSI outcome in patients with repeated implantation failure? A randomised controlled trial. Eur J Obstet Gynecol Reprod Biol 2012;164:176–9
- Gibreel A, Badawy A, El-Refai W, El-Adawi N. Endometrial scratching to improve pregnancy rate in couples with unexplained subfertility: a randomized controlled trial. J Obstet Gynaecol Res 2012 (in press) DOI: 10.1111/j.1447-0756.2012.02016.x
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology. Fertil Steril 2009;92:1520–4