To the editor,
Elevated plasma concentrations of cell-membrane-derived microparticles (MP) are found in many diseases such as sepsis, cancer, and diabetes mellitus Citation[1], Citation[2]. Various cell types are known as sources of MP, which play a crucial role in angiogenesis, endothelial cell function as well as coagulation and inflammation. A uniform characteristic of the various cell-specific MP is the surface exposure of negatively charged phospholipids. Due to these phospholipids and also due to the exposure of tissue factor (TF) and/or TF pathway inhibitor, MP are believed to be involved in the regulation of coagulation Citation[3], Citation[4]. Furthermore, MP bear cell-specific antigens enabling them to interact with a variety of target cells. Thus, MP can be considered as intercellular signal transferring entities Citation[5], Citation[6]. Low levels of circulating MP, mainly derived from platelets, are also present in healthy individuals but their functional relevance in haemostasis and inflammation is not well understood Citation[7].
Increased levels of circulating MP are known to be associated with an increased risk of cardiovascular disease and atherothrombotic events Citation[1], Citation[8]. Since moderate physical exercise is known to lower the risk of cardiovascular diseases Citation[9], Citation[10], we were interested to study the effect of moderate physical exercise on both concentration and pro-coagulant activity of circulating MP.
After approval by the local Ethics Committee and obtaining written informed consent, 16 healthy male volunteers (age 25 ± 3 years; mean ± SD) underwent a singular 90 min exercise test on a bicycle ergometer with a constant power of 80% of individual anaerobic threshold (IAT). IAT was determined one week prior to the test according to Stegmann et al. Citation[11]. Blood samples were taken before (rest), immediately post (post) and 45 min (45 min post) as well as 2 hours (2 h post) after exercise.
MP were identified by flow cytometry according to their size and the binding of FITC-labelled annexin V as well as by the binding of PE-labelled antibodies against cell-specific antigens: platelet-derived MP (PMP) by anti-CD42a, monocyte-derived MP (MMP) by anti-CD14 and endothelial cell-derived MP (EMP) by anti-CD62E. To estimate pro-coagulant activities of MP two different tests were performed: The ACTICHROME® test (American Diagnostica), which measures the pro-thrombinase activity of FXa/Va of MP bound to an annexin V-coated surface in absence of any other plasma constituents and a one-stage clotting test, in which the formation of a fibrin clot was monitored by measuring the optical density in MP-containing plasma samples after re-calcification and addition of a small amount of TF.
The 90 min exercise at 80% IAT (mean power 173 ± 73 W) increased heart rate to 156 ± 16 beats/min, the systolic blood pressure to 156 ± 25 mm Hg and plasma lactate concentration to 2.17 ± 0.81 mmoles/l. As shown in , the exercise resulted in a significant increase in the numbers of white blood cells and platelets. It has been shown elsewhere that the increase in platelet and leukocyte counts is not only due to a reduction in plasma volume but also due to a release of platelets and leukocytes from various storage sites (spleen, lung, bone marrow etc.) Citation[9]. With respect to MP, we also observed an increase in the number of all annexin-positive MP as well as PMP, whereas EMP and MMP remained unchanged. Maximum values in platelet number were found immediately post exercise and returned to basal level at least after 45 min post exercise. In contrast, white blood cells and MP counts peaked at 45 min post-exercise and did not return to basal levels within 2 h post exercise ().
Table I. Time-dependent changes in the numbers of blood cells and circulating MP after physical exercise. Data are given as mean ± SD. ANOVA for repeated measurements was used to test for significant time-dependent changes; t-test for paired samples was applied to test for significant effects of exercise in comparison to basal values (rest). ns, not significant; § p ≤ 0.05.
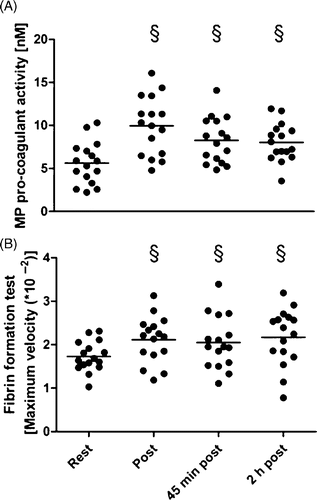
The procoagulant activity of annexin V-bound MP (Actichrome test) was nearly two-fold increased immediately post-exercise when compared to rest and remained elevated at least up to 2 h after exercise (). A similar time course was observed for the TF-initiated fibrin formation in the one-stage clotting test with MP-rich plasma ().
Figure 1. Functional activity tests at rest, immediately post-, 45 min post-, 2 h post exercise. A: MP pro-coagulant activity given as concentration of negatively charged phospholipids (nM). B: Maximum velocity (value*10−2) of fibrin formation (Δ optical density/time); § p ≤ 0.05 for differences to rest (t-test).
We could show in the present study that moderate exercise in young healthy subjects increased the number of circulating pro-coagulant MP. The increase was mainly due to a release of CD42a-positive PMP with a maximum at 45 min post-exercise, whereas the numbers of MMP and EMP remained unchanged. As PMP and platelet count had different time-course, the increase in PMP is probably caused by exercise-induced platelet activation rather then a simple consequence of the increase in platelet count. Exercise-induced platelet activation was recently reported Citation[12], Citation[13].
Interestingly, pro-coagulant activity of annexin V-positive MP and their absolute number in plasma have different time-course. Maximum of activity was found immediately post-exercise whereas maximum number was found at 45 min post-exercise. Thus one could speculate that the specific activity of the MP decreases with time whereas the number of MP still increases.
Two mechanisms might contribute to the release of PMP after physical exercise. Platelets that are present in the circulation at the beginning of exercise become stimulated by agonists and/or shear stress, release MP and afterwards will be removed by phagocytes. As another consequence of exercise, juvenile platelets from spleen, bone marrow and the intravascular pool of the lungs enter the circulation. These platelets have a high sensitivity to agonists such as epinephrine and nor-epinephrine and may release MP with other procoagulant activity then elderly platelets. Furthermore, juvenile platelets have high energetic capacity enabling them to reorganise their cytoskeleton after MP release, and continue to circulate and function Citation[14], Citation[15].
Moderate exercise in young as well as middle-aged, healthy subjects has been shown to activate coagulation Citation[16], Citation[17]. Our data on MP and their procoagulant activity after moderate exercise may support the phenomenon of an exercise-induced increase in the haemostatic potential. In a previous study with coronary artery diseased patients we observed positive correlations between the number of circulating PMP and thrombin generation Citation[18].
Numerous epidemiological and prospective cohort studies confirm an important beneficial role of moderate endurance exercise in the prevention and treatment of cardiovascular diseases Citation[9]. Thus, our data presented may support the hypothesis that MP are not only ‘bad’ but–at least in some conditions–also ‘good’ Citation[1].
References
- Freyssinet JM. Cellular microparticles: What are they bad or good for?. J Thromb Haemost 2003; 1: 1655–1662
- Morel O, Morel N, Freyssinet JM, Toti F. Platelet microparticles and vascular cells interactions: A checkpoint between the haemostatic and thrombotic responses. Platelets 2008; 19: 9–23
- Lechner D, Weltermann A. Circulating tissue factor-exposing microparticles. Thromb Res 2008; 122(Suppl 1)S47–54
- Lösche W. Platelets and tissue factor. Platelets 2005; 16: 313–319
- Burnier L, Fontana P, Kwak BR, Angelillo-Scherrer A. Cell-derived microparticles in haemostasis and vascular medicine. Thromb Haemost 2009; 101: 439–451
- Setzer F, Oberle V, Blaess M, Möller E, Russwurm S, Deigner HP, Claus RA, Bauer M, Reinhart K, Lösche W. Platelet-derived microvesicles induce differential gene expression in monocytic cells: A DNA microarray study. Platelets 2006; 17: 571–576
- Berckmans RJ, Neiuwland R, Boing AN, Romijn FP, Hack CE, Sturk A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost 2001; 85: 639–646
- Tan KT, Lip GY. The potential role of platelet microparticles in atherosclerosis. Thromb Haemost 2005; 94: 488–492
- El-Sayed MS, El-Sayed Ali Z, Ahmadizad S. Exercise and training effects on blood haemostasis in health and disease: An update. Sports Med 2004; 34: 181–200
- Lippi G, Maffulli N. Biological influence of physical exercise on hemostasis. Semin Thromb Hemost 2009; 35: 269–276
- Stegmann H, Kindermann W, Schnabel A. Lactate kinetics and individual anaerobic threshold. Int J Sports Med 1981; 2: 160–165
- Hilberg T, Menzel K, Glaser D, Zimmermann S, Gabriel HH. Exercise intensity: Platelet function and platelet-leukocyte conjugate formation in untrained subjects. Thromb Res 2008; 122: 77–84
- Claus RA, Bockmeyer CL, Budde U, Kentouche K, Sossdorf M, Hilberg T, Schneppenheim R, Reinhart K, Bauer M, Brunkhorst FM, et al. Variations in the ratio between von Willebrand factor and its cleaving protease during systemic inflammation and association with severity and prognosis of organ failure. Thromb Haemost 2009; 101: 239–247
- Michelson AD, Barnard MR, Hechtman HB, MacGregor H, Connolly RJ, Loscalzo J, Valeri CR. In vivo tracking of platelets: Circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc Natl Acad Sci USA 1996; 93: 11877–11882
- Horstman LL, Ahn YS. Platelet microparticles: A wide-angle perspective. Crit Rev Oncol Hematol 1999; 30: 111–142
- Hilberg T, Glaser D, Reckhart C, Prasa D, Stürzebecher J, Gabriel HH. Blood coagulation and fibrinolysis after long-duration treadmill exercise controlled by individual anaerobic threshold. Eur J Appl Physiol 2003; 90: 639–642
- Menzel K, Hilberg T. Coagulation and fibrinolysis are in balance after moderate exercise in middle-aged participants. Clin Appl Thromb Hemost 2009; 15: 348–355
- Sossdorf M, König V, Gummert J, Marx G, Lösche W. Correlations between platelet-derived microvesicles and thrombin generation in patients with coronary artery disease. Platelets 2008; 19: 476–477