To the Editor
We read with interest the study by Arméro el al. verifying large intra-individual variability in clopidogrel responsiveness assessed by the vasodilator-stimulated phosphoprotein (VASP) phosphorylation index in coronary artery disease patients on long-term clopidogrel therapy Citation[1]. Results of the VASP phosphorylation assay were well correlated with those of the VeryfiNow P2Y12 point-of-care device (Accumetrics, San Diego, CA, USA) in subjects with acute coronary syndromes Citation[2]. VASP phosphorylation assay is labor intensive and not routinely available. In contrast, the VerifyNow P2Y12 assay is rapid and easy to use. Thus, it has been increasingly used to measure residual platelet reactivity in patients taking clopidogrel Citation[3], Citation[4]. Individual variability of response to clopidogrel has been well recognized and there is growing evidence of an association of clopidogrel resistance with increased risk of thromboembolic events Citation[5–9]. However, there has been yet no study on intra-individual variability of the VerifyNow P2Y12 assay.
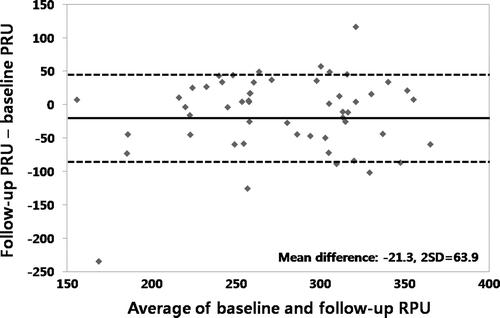
We conducted the present study to measure agreement of repeated VerifyNow P2Y12 reaction units (PRU) values over time in 50 (36 males, 14 females, 66 ± 11 years) patients with low response to clopidogrel. These patients were derived from 507 patients who were tested with the VerifyNow P2Y12 assay after percutaneous coronary intervention (PCI) from January 2006 to December 2007 at our hospital. Low response to clopidogrel was defined as percent platelet inhibition less than or equal to 20% or PRU more than or equal to 235 IU Citation[4]. The samples were collected at 1 month following PCI and 1 month after the initial measurement (mean time, 31 ± 28 days; range, 16 ∼ 62 days). Standard daily dual antiplatelet therapy (aspirin 100 mg and clopidogrel 75 mg) was maintained through the study. Agreement between the tests was assessed by the Spearman rank correlation and the Bland-Altman analyses. Baseline and follow-up PRU were 290.2 ± 49.2 and 268.9 ± 63.3, respectively. Initially, 43 of 50 (86%) patients were categorized into low response to clopidogrel in terms of both percentage platelet inhibition and PRU. At follow-up, only 36 of 50 patients (72%) were low responders fulfilling both definitions. There was a fair correlation between baseline and follow-up PRU values (Spearman's rho = 0.539, p < 0.001, ). We calculated the mean difference between two repeated measures and 95% limits of agreement as the mean difference (2 SD) using the Bland-Altman method Citation[10]. It is expected that the 95% limits include 95% of differences between the two repeated measures. The agreement between the two sets of measurements was not so high, with a mean difference of PRU of −21.3 ± 63.9 ().
Figure 1. Correlation of baseline and follow-up VerifyNow-P2Y12 reaction units (PRU) using the Spearman rank correlation analysis.
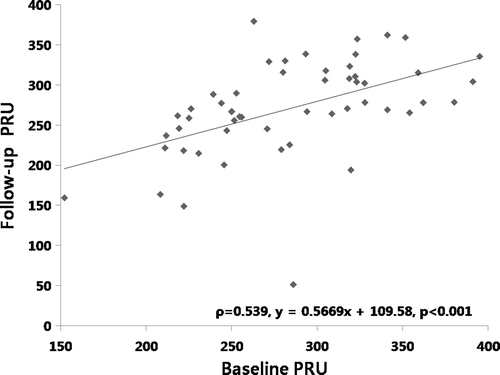
Figure 2. Bland-Altman analysis showing moderate agreement between baseline and follow-up VerifyNow-P2Y12 reaction units (PRU) with a mean difference of PRU of −21.3 ± 63.9.
These results indicate that there is a considerable intra-individual variation of the VerifyNow P2Y12 assay in patients receiving clopidogrel after PCI, and that low response to clopidogrel seems to improve over time in some patients. Low platelet inhibition after clopidogrel exposure is related to an increased number of adverse clinical events Citation[4–8]. However, there are various platelet function tests including light transmittance aggregometry, the platelet function analysis system (PFA-100), VASP phosphorylation, the VerifyNow P2Y12 assay, and multiple electrode platelet aggregometry. Breet et al. Citation[11] reported that the predictive accuracy of these tests for a composite of all-cause death, nonfatal acute myocardial infarction, stent thrombosis, and ischemic stroke was only modest. None of the tests provided accurate prognostic information to identify low-risk patients at higher risk of bleeding following stent implantation. It remains to be determined whether higher doses of clopidogrel are: (A) enough to overcome the resistance observed in some subjects on a standard dose of clopidogrel, and (B) have a comparable antiplatelet effect to novel potent antiplatelet agents, such as prasugrel and ticagrelor. To this end, the GRAVITAS study is currently underway to assess whether tailoring the dose of antiplatelet medication based on VerifyNow assay results improves clinical outcome Citation[12]. In this trial, additional loading doses and increased maintenance doses are being given to low responders and clinical outcomes investigated.
In summary, laboratory-determined clopidogrel resistance, assessed at a single time point, does not appear to be a stable phenomenon over time. The poor repeatability of the VerifyNow P2Y12 point-of-care assay might give rise to considerable confusion in risk stratification of patients and individualization of antiplatelet medication to improve outcome. Repeated measurements of platelet function might be necessary to define clopidogrel resistance in patients on long-term clopidogrel therapy.
References
- Arméro S, Camoin Jau L, Aït Mokhtar O, Mancini J, Burignat-Bonello C, Tahirou I, Arques S, Dignat-George F, Paganelli F, Bonello L. Intra-individual variability in clopidogrel responsiveness in coronary artery disease patients under long term therapy. Platelets 2010;21:503–507
- Bidet A, Jais C, Puymirat E, Coste P, Nurden A, Jakubowski J, Nurden P. VerifyNow and VASP phosphorylation assays give similar results for patients receiving clopidogrel, but they do not always correlate with platelet aggregation. Platelets 2010; 21: 94–100
- van Werkum JW, Harmsze AM, Elsenberg EH, Bouman HJ, ten Berg JM, Hackeng CM. The use of the VerifyNow system to monitor antiplatelet therapy: A review of the current evidence. Platelets 2008; 19: 479–488
- Price MJ, Endemann S, Gollapudi RR, Valencia R, Stinis CT, Levisay JP, Ernst A, Sawhney NS, Schatz RA, Teirstein PS. Prognostic significance of post-clopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drug-eluting stent implantation. Eur Heart J 2008; 29: 992–1000
- Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM. Clopidogrel for coronary stenting: Response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation 2003; 107: 2908–2913
- Matetzky S, Shenkman B, Guetta V, Shechter M, Bienart R, Goldenberg I, Novikov I, Pres H, Savion N, Varon D, Hod H. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 2004; 109: 3171–3175
- Gurbel PA, Bliden KP, Hayes KM, Yoho JA, Herzog WR, Tantry US. The relation of dosing to clopidogrel responsiveness and the incidence of high post-treatment platelet aggregation in patients undergoing coronary stenting. J Am Coll Cardiol 2005; 45: 1392–1396
- Cuisset T, Frere C, Quilici J, Barbou F, Morange PE, Hovasse T, Bonnet J-L, Alessi M-C. High post-treatment platelet reactivity identified low-responders to dual antiplatelet therapy at increased risk of recurrent cardiovascular events after stenting for acute coronary syndrome. J Thromb Haemost 2006;4:542–549
- Vila PM, Zafar MU, Badimon JJ. Platelet reactivity and nonresponse to dual antiplatelet therapy: a review. Platelets 2009; 20: 531–538
- Myles PS, Cui J. Using the Bland-Altman method to measure agreement with repeated measures. Br J Anaesth. 2007; 99: 309–311
- Breet NJ, van Werkum JW, Bouman HJ, Kelder JC, Ruven HJ, Bal ET, Deneer VH, Harmsze AM, van der Heyden JA, Rensing BJ, et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA. 2010; 303: 754–762
- Price MJ, Berger PB, Angiolillo DJ, Teirstein PS, Tanguay JF, Kandzari DE, Cannon CP, Topol EJ. Evaluation of individualized clopidogrel therapy after drug-eluting stent implantation in patients with high residual platelet reactivity: Design and rationale of the GRAVITAS trial. Am Heart J. 2009; 157: 818–824.e1