To the editor
Bernard-Soulier syndrome (BSS) is an inherited macrothrombocytopenia induced by mutations in glycoprotein (GP) Ibα, GPIbβ or GPIX resulting in quantitative (classical BSS) or qualitative (variant BSS) abnormalities of the GPIb/IX/V complex that affect platelet-von Willebrand factor (vWF) interaction. Patients with homozygous or heterozygous biallelic mutations always present a severe form with greatly reduced platelet counts, giant platelets, defective ristocetin induced platelet aggregation and recurrent episodes of spontaneous bleeding, while subjects with monoallelic mutation have normal phenotypes Citation[1]. The Ala156Val mutation in the GPIbα (Bolzano mutation) represents an exception, in that in the monoallelic status induces a slightly reduced platelet count with platelet macrocytosis and mild bleeding tendency Citation[2].
While the altered GPIbα-vWF interaction explains the platelet functional defects in BSS, new insights need to be addressed to explain the mechanisms that lead to macrothrombocytopenia. However, we recently investigated in vitro megakaryopoiesis in six subjects with monoallelic Bolzano mutation and observed that megakaryocyte (Mk) differentiation and maturation were similar to controls, while proplatelet formation was severely affected. Thus, we concluded that the GPIbα functional defect induced by Ala156Val monoallelic mutation affects in vitro platelet release Citation[2].
We are now reporting that a severe defect of proplatelet formation is also responsible for thrombocytopenia in patients with classical forms of BSS deriving from homozygous mutations in GPIbβ or GPIX.
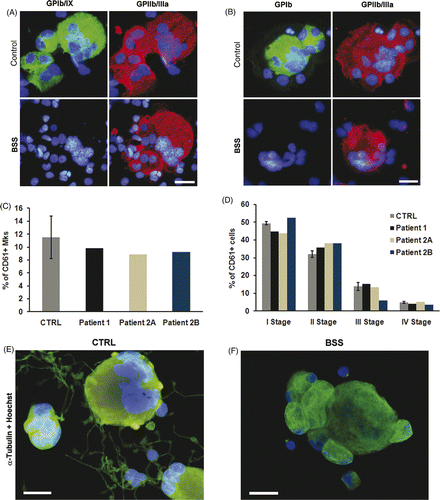
Three adult patients from two unrelated families have been investigated. Patient 1 is a 30 year old woman who suffered from recurrent epistaxis, ecchymosis, and gum bleedings since childhood. She received transfusions of both red blood cells and platelets for menorrhagia until estroprogestinic therapy was started. Platelet concentrates were also given at each dental prophylaxis and extractions. Patients 2A and 2B are brothers born from parents who are second cousins. Thrombocytopenia was incidentally detected in patient 2A when he was 2 years old and experienced a severe epistaxis after a mild nasal trauma. A complete blood cell count performed in the brother (patient 2B) revealed that he was similarly affected. Both had a mild bleeding tendency with easy bruising and sporadic gum bleedings. Patient 2A received prophylactic platelet transfusion before dental extraction. Laboratory investigations Citation[3], Citation[4] revealed manual platelet counts and mean platelet diameters ranging between 38 and 52 × 109/L and 3,7 and 4,1 µm, respectively. Platelet aggregation induced by ristocetin 3 mg/ml was absent in patient 1 and severely reduced in both patients 2A and 2B (7 and 11%) while it was normal after ADP 5 µm and collagen 4 µg/ml (data not shown). In all the patients the components of the GPIb/IX/V complex were lower than 10% of control while the expression of GPIIb-IIIa was normal (data not shown). Mutation screening of GP1BA, GP1BB and GP9 revealed the homozygous frameshift mutation c.491dupA in GP1BB of patient 1 and the homozygous c.72T > G substitution in the GP9 of patients 2A and 2B (Savoia A, personal communication). Fifteen milliliters of each patient's peripheral blood were collected in parallel with control samples and processed within 3 hours. CD45+ cells were separated using the immunomagnetic beads method (Miltenyi-Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions, then cultured as described elsewhere Citation[2]. The Mk yield and proplatelet formation were evaluated at the end of the culture as reported in detail elsewhere Citation[2], Citation[5]. Briefly, Mks were identified as large CD61+ cells and assigned to distinct stages of maturity according to standard morphological criteria Citation[6]. To evaluate proplatelet formation, large Mks were separated after 14 days of culture by a Bovine serum albumin (BSA) gradient (3–4%) and plated in fresh medium supplemented with Thrombopoietin (TPO) in 24-well plates (1 × 105 cells per well). After 16 h, proplatelets were counted by phase-contrast microscopy and than cytospun onto glass coverslips and stained with antibodies against CD61 (clone SZ21, Immunotech, Marseille, France; clone C20, Santa Cruz Biotechnology, Heidelberg, Germany) and α-tubulin (Sigma-Aldrich, Milan, Italy). Mks forming proplatelets were identified as large CD61+ cells extending α-tubulin-positive, long, filament-like structures. The extent of proplatelet formation was calculated as the percentage of proplatelet-bearing CD61+ cells with respect to the total CD61+ cells. Glicoproteins of the GPIb/IX/V complex were stained using antibodies SZ1 and SZ2 (Immunotech). Immunofluorescence analysis was performed as previously described Citation[2], Citation[5].
The immunomorphological evaluation of cultured peripheral blood progenitors on day 14 showed that patients' Mks reproduced the severe deficiency of GPIbα and GPIX observed in their peripheral blood platelets (). Starting from the same number of seeded CD45+ cells, the percentage of CD61+ cells with Mk morphology was comparable to control (11.51% ± 3.26, median 1.5 × 105 mature Mks) (). Further, Mk classification, according to standard criteria Citation[6], showed no significant differences in the maturation profiles of patients with respect to controls (), indicating that the homozygous BSS mutations did not affect Mk differentiation or maturation. However, patients' Mks reseeded in fresh medium did not extend proplatelets, while 10.3 ± 2.1% of control Mks extended regular proplatelets (). This in vitro observation suggests that a defect of proplatelet formation could be involved in the thrombocytopenia of BSS patients.
Figure 1. Analysis of megakaryocytes. Immunofluorescence analysis failed to identify GPIbα (A) and GPIb/IX (B) complex on the membrane of Mks derived from peripheral blood of BSS patients. Patient's and control's Mks were stained with anti-CD61 (red) and anti-GPIbα or GPIb/IX complex (green) antibodies. The GP defect did not affect either Mk differentiation from hemopoietic progenitors (C) or their maturation (D) with respect to relative controls (E–F). Representative immunofluorescence images of control Mks and patient derived Mks stained with anti-α tubulin antibody (green). Nuclei were always counterstained with Hoechst 33288 (blue) (scale bars, 20 µm).
Previously, we performed in vitro studies Citation[5] where we showed that proplatelet formation is prevented by the blockade of GPIbα, or upon its cleavage by the metalloproteinase mocarhagin. Further, proplatelet-forming Mks showed membrane-bound vWF, indicating that release of α-granules occurred during this process. We concluded that GPIb/IX/V mediates a sort of autocrine stimulation through secreted vWF, essential for proplatelet formation. Accordingly, Ware et al. observed that a GPIb alpha-/- mouse model reproduced the phenotype of classical BSS Citation[7]. The exact role of GPIbα-vWF in Mk development is still a matter of debate since minor defects in a chimeric (I-L4R-GPIbα) mouse model lacking a GPIbα-vWF interaction have been observed Citation[8]. However, GPIbα-vWF interaction could be restricted to proplatelet bearing Mks. Interestingly recent studies have demonstrated that altered vWF-GPIbα interactions hamper proplatelet formation in patients with von Willebrand disease type 2B that presented variable numbers of giant platelets Citation[9]. Moreover, other studies have demonstrated that vWF promotes proplatelet formation both in bone marrow environment Citation[10] and in the presence of high shear stress Citation[11]. Altogether these results indicate that a correct binding of vWF to GPIb/IX/V could serve as one of the regulators of proplatelet formation and platelet release Citation[12].
The present study further supports this hypothesis showing that the almost total absence of the GPIb/IX/V complex observed in our patients made their Mks unable to produce proplatelets in vitro consistent with similar findings of impaired proplatelet formation in a mouse model of BSS Citation[13]. Thus, we hypothesize that the functional defect of GPIbα deriving from monoallelic Bolzano mutation hampers in vitro proplatelet formation and results in mild thrombocytopenia, while the drastic reduction of the GPIb/IX/V complex, which is typical of the classic forms of biallelic BSS, completely inhibits in vitro platelet release and results in more severe thrombocytopenia. The reason why large and giant platelets characterize monoallelic and biallelic BSS, respectively, is unclear although it seems reasonable to hypothesize that the severity of the GPIbα defect also modulates the degree of platelet macrocytosis.
Although in vitro studies showed that Mks cultured from the peripheral blood of homozygous BSS subjects completely lost their ability to extend proplatelets, in vivo characterization of our patients revealed that they had 38–52 × 109 circulating platelets/L of blood. The most obvious explanation for this discrepancy is that in vitro experiments did not closely reproduce in vivo megakaryopoiesis. To support this hypothesis, recently Eckly et al. demonstrated the different behavior in proplatelet formation of Myh9Δ megakaryocytes in their native environment and in in vitro cultures Citation[14]. These results underlined the importance of a bone marrow microenvironment in regulating platelet production. Another possibility is that platelet release in humans does not occur only by the proplatelet model, but also by the fragmentation model Citation[15]. This theory predicts that Mks travel from the bone marrow to the lung, where they are broken up into platelets in the microvasculature. Thus, it could be that BSS Mks lose the ability to form platelets in bone marrow, but maintain the capacity for releasing them by fragmentation in lung vessels Citation[16].
In conclusion this work suggests that the severe deficiency of the GPIb/IX/V complex that characterizes the classical form of biallelic BSS induces thrombocytopenia by hampering Mk proplatelet formation.
Acknowledgments
The authors thank Valeria Bozzi and Alessandro Pecci for helping with slide preparation. This work was supported by grants from Cariplo Foundation (2006.0596/10.8485) and from Istituto Superiore di Sanità (Italy-USA project on rare diseases).
Declaration of interest: The authors declare no conflicts of interest.
References
- Ware J, Russell SR, Marchese P, Murata M, De Marco L, Ruggeri ZM. Point mutation in a leucine-rich repeat of platelet glycoprotein Iβα resulting in the Bernard-Soulier syndrome. J Clin Invest 1993; 92: 1213–1218
- Balduini A, Malara A, Pecci A, Badalucco S, Bozzi V, Pallotta I, et al. Proplatelet formation in heterozygous Bernard-Soulier syndrome type Bolzano. J Thromb Haemost 2009; 7: 478–484
- Savoia A, Balduini CL, Savino M, Noris P, Del Vecchio M, Perrotta S, et al. Autosomal dominant macrothrombocytopenia in Italy is most frequently a type of heterozygous Bernard-Soulier syndrome. Blood 2001; 97: 1330–1335
- Noris P, Klersy C, Zecca M, Arcaini L, Pecci A, Melazzini F, et al. Platelet size distinguishes between inherited macrothrombocytopenias and immune thrombocytopenia. J Thromb Haemost 2009; 7: 2131–2136
- Balduini A, Pallotta I, Malara A, Lova P, Pecci A, Viarengo G, et al. Adhesive receptors, extracellular proteins and myosin IIA orchestrate proplatelet formation by human megakaryocytes. J Thromb Haemost 2008; 6: 1900–1907
- Williams N, Levine RF. The origin, development and regulation of megacaryocytes. Br J Haematol 1982; 52: 173–180
- Ware J, Russell S, Ruggeri ZM. Generation and rescue of a murine model of platelet dysfunction: The Bernard-Soulier syndrome. Proc Natl Acad Sci USA 2000; 97: 2803–2808
- Kanaji T, Russell S, Ware J. Amelioration of the macrothrombocytopenia associated with the murine Bernard-Soulier syndrome. Blood 2002; 100: 2102–2107
- Nurden P, Gobbi G, Nurden A, Enouf J, Youlyouz-Marfak I, Carubbi C, La Marca S, et al. Abnormal vWF modifies megakaryocytopoiesis: Studies of platelets and megakaryocyte cultures from patients with von Willebrand disease type 2B. Blood 2010; 115: 2649–2656
- Larson MK, Watson SP. Regulation of proplatelet formation and platelet release by integrin αIIbβ3. Blood 2006; 108: 1509–1514
- Dunois-Lardé C, Capron C, Fichelson S, Bauer T, Cramer-Bordé E, Baruch D. Exposure of human megakaryocytes to high shear rates accelerates platelet production. Blood 2009; 114: 1875–1883
- Takahashi R, Sekine N, Nakatake T. Influence of monoclonal antiplatelet glycoprotein antibodies on in vitro human megakaryocyte colony formation and proplatelet formation. Blood 1999; 93: 1951–1958
- Strassel C, Eckly A, Leon C, Petitjean C, Freund M, Cazenave JP, et al. Intrinsic impaired proplatelet formation and microtubule coil assembly of megakaryocytes in a mouse model of Bernard-Soulier syndrome. Haematologica 2009; 94: 800–810
- Eckly A, Rinckel JY, Laeuffer P, Cazenave JP, Lanza F, Gachet C, Léon C. Proplatelet formation deficit and megakaryocyte death contribute to thrombocytopenia in Myh9 knockout mice. J Thromb Haemost 2010; 8: 2243–2251
- Geddis A. Megakaryopoiesis. Semin Hematol 2010; 47: 212–221
- Patel SR, Hartwig JH, Italiano JE, Jr. The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest 2005; 115: 3348–3354