To the Editor,
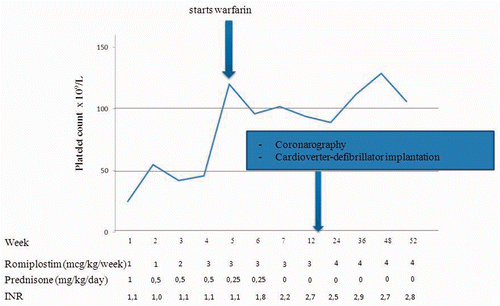
Thrombopoietin receptor agonists (TRAs) offer new opportunities for the treatment of chronic immune thrombocytopenia (ITP). However, little is known about their safety and efficacy in combination with vitamin K antagonists in patients who need chronic anticoagulation. In this study, we report of a 68-year-old man with chronic ITP and severe cardiac comorbidities who has achieved a 1-year follow-up of combined treatment with romiplostim and warfarin. He was affected by dilated-hypokinetic cardiopathy with chronic atrial fibrillation and was admitted to our hospital in February 2011 for progressive dyspnea and fatigue with diffuse mucocutaneous purpura. Coagulation laboratories were normal except for an international normalized ratio (INR) of 2.6, as he was on treatment with warfarin for the atrial fibrillation, and an ischemic stroke occurred 2 months before. Hematological data showed thrombocytopenia and mild leukocytosis (leukocytes 11.3 × 109/l with absolute neutrophil count 9.6 × 109/l; hemoglobin 13.5 g/dl; and platelet count 21 × 109/l). The patient was affected by ITP since 2008. Diagnosis of ITP was confirmed throughout by the exclusion of other causes of thrombocytopenia and with a bone marrow histological analysis showing an increased number of megakaryocytes with normal erythroid and myeloid lineages. He had received several cycles of prednisone and intravenous immunoglobulins (IVIG) for ITP. However, since about a year, IVIG were no longer effective. At the time of hospital admission, he was taking prednisone 1 mg/kg per day since 1 month, with poor tolerance due to an insulin-dependent type 2 diabetes mellitus. We stopped warfarin and administered intravenous vitamin K, achieving normalization of INR (1.1). An abdomen ultrasound scan showed a mild enlargement of the liver consistent with chronic heart failure but normal size of the spleen, whereas transthoracic echocardiography revealed a severe left ventricular systolic impairment (0.20 residual ejection fraction) and a moderate pulmonary hypertension. As thrombocytopenia prevented the use of warfarin, a transesophageal echocardiography was performed in order to evaluate the feasibility of percutaneous left atrial appendage transcatheter occlusion as a prophylaxis of cardioembolic stroke Citation[1], but a recent left atrial appendage thrombosis was found. Thus, our patient had a chronic ITP with the loss of response to prednisone Citation[2], but further treatment was needed to ensure a long-term control of platelet count in order to restore the anticoagulation. Splenectomy is a highly effective second-line treatment for chronic ITP but its complications also include thrombotic events and may be greater in patients older than 65 Citation[3]. Furthermore, our patient had severe comorbidities that increased the risk of surgical and anesthesiological complications. TRAs are licensed for the treatment of chronic ITP in patients who fail to respond to splenectomy or have contraindications to it. Long-term data with romiplostim show a sustained platelet response and a significant reduction in the use of steroids, with an acceptable safety profile Citation[4]. Nevertheless, patients treated with romiplostim in a multicenter phase III trial had a median weekly platelet count ≥50 × 109/l during quite all the treatment period, but the first quartile of the range was often below this threshold. Moreover, although non-splenectomized patients had a higher rate of durable platelet response (at least 6 weeks with platelet counts of 50 × 109/l or more during the last 8 weeks of treatment), no more than 61% of them achieved such end-point and the percentage only marginally improved by including patients who resorted to rescue therapies Citation[5]. Thus, we choose to restore the anticoagulation at a higher threshold of platelet count (100 × 109/l) to have a broader safety margin in the case of sudden drops. Besides, as it has been shown that variations of the dosage of romiplostim are more likely to be required during the first 24 weeks of treatment and are substantial in a small number of patients, both platelet count and INR were checked weekly for such period and then every 2 weeks Citation[4]. Romiplostim was started from 1 mcg/kg/week, adjusting dose on the basis of weekly platelet response according to the prescribing information Citation[6]. Despite the loss of response and the poor tolerance to prednisone, we continued it until the platelet count was above 50 × 109/l to avoid a further worsening of thrombocytopenia before romiplostim was effective. summarizes the behavior of platelet count during the first year of treatment. A week after the first administration of romiplostim, we reduced the dose of prednisone, which was definitely stopped few weeks later. Warfarin was restarted as platelet count overcame the threshold of 100 × 109/l, achieving a target INR between 2.5 and 3.0 during all 52 weeks. Sixteen weeks after the onset of treatment with romiplostim, both a follow-up coronarography and a biventricular implantable cardioverter-defibrillator implantation were successfully performed. Coronarography showed the absence of critical stenosis. Once the anticoagulation was restored, platelet count ranged between 88 and 137 × 109/l (median: 98) except in one case. Indeed, despite the patient was on stable dosage of romiplostim from week 7, an increase of 1 mcg/kg was needed on week 19 because of a drop in platelet count slightly below 50 × 109/l. This drop was unexpected as was not related to an infection or to a delayed administration of romiplostim, but was promptly corrected by increasing the dosage. Smaller fluctuations of platelet count occurred, but did not require further dose adjustments. Mostly important, neither bleedings nor thrombotic complications occurred during this 1-year-long treatment. Concluding, in our single-case experience the combination of warfarin and romiplostim was feasible and effective for up to 1 year in a patient with chronic ITP and severe comorbidities. Both a high threshold and a frequent check of platelet count were adopted to maintain a broader safety margin in the case of sudden drops. Although TRAs might be useful in certain circumstances, the clinical management of such drugs is complex, and data to establish whether they allow a safe anticoagulation are lacking. Well-designed clinical trials are strongly required to define their role in patients with ITP who need anticoagulation.
Figure 1. Behavior of the platelet count up to the achievement of first stable dosage and at different time points during the one-year long follow-up. Warfarin was restarted as platelet count overcame the threshold of 100 × 109/l. Dosages of romiplostim and prednisone, INR values, and time-points of invasive procedures are also reported.
Declaration of interest: Alberto Bosi received lecture fees from Amgen Inc. The other authors report no declarations of interest.
References
- Bayal YL, Omran H, Neuzil P, Thuesen L, Pichler M, Rowland E, Ramondo A, Ruzyllo W, Budts W, Montalesco G, et al. PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) for prevention of cardioembolic stroke in non-anticoagulation eligible atrial fibrillation patients: Results from the European PLAATO study. EuroIntervention 2010; 6: 220–226
- Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, Cines D, Chong BH, Cooper N, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. Blood 2009; 113: 2386–2393
- Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, Chong BH, Cines DB, Gernsheimer TB, Godeau B, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010; 115: 168–186
- Bussel JB, Kuter DJ, Pullarkat V, Lyons RM, Guo M, Nichol JL. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood 2009; 113: 2161–2171
- Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, Aledort LM, George JN, Kessler CM, Sanz MA, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: A double-blind randomized controlled trial. Lancet 2008; 371: 395–403
- Amgen Inc. Nplate® (romiplostim) prescribing information [Internet]. Amgen Inc, Thousand Oaks, CA 2008, Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/125268lbl.pdf