To the Editor
We have previously reported the expression of the TRPV1 ion channel in human platelets and have demonstrated a role for this channel in mediating agonist-evoked Ca2+ signalling in these cells Citation[1]. Our Western blot studies have been independently reproduced by Savini et al. using a different antibody Citation[2]. Furthermore, TRPV1 has also been identified in a human platelet proteomic study Citation[3], Citation[4]. These earlier studies are, however, yet to address the role that this non-selective cation channel might play in regulating platelet activity in vivo. A common strategy to assess the functions of signalling molecules in platelets has been to knockout the gene for the protein of interest in mice and then compare the responses of platelets from the knockout and wild-type animals. As a TRPV1-knockout mouse has been generated Citation[3], we conducted pilot experiments to assess whether this channel is expressed in the platelets of wild-type mice and thus whether the knockout mouse would provide a useful model for studying the function of TRPV1 in platelets in vivo.
Blood was taken by venepuncture from healthy human volunteers or by venepuncture of the abdominal vein in wild-type C57/Bl6 or Swiss mice terminally anaesthetised with ketamine (75 mg/kg) and medetomidine (1 mg/kg). After blood collection, mice were euthanized by cervical dislocation. Washed platelet suspensions from both species were prepared and the platelets were then loaded with Fura-2 by incubation with 5 µM Fura-2/AM for 30 minutes at 37°C. Platelets were then collected and resuspended in Hepes-buffered saline supplemented with glucose (10 mM), bovine serum albumin (1 mg/ml) and apyrase (0.1 U/ml) Citation[1].
In Fura-2-loaded platelets from wild-type C57/Bl6 or Swiss mice, capsaicin (100 µM), in the presence of 1 mM external Ca2+, evoked no rise in [Ca2+]cyt over that observed in vehicle-treated controls (; n = 6). This lack of response was not due to a failure of Fura-2 loading or an ability to detect changes in [Ca2+]cyt as samples from all mouse platelet suspensions demonstrated changes in [Ca2+]cyt upon treatment with thapsigargin in the absence of extracellular Ca2+ followed by the addition of Ca2+ to the medium to elicit store-operated calcium entry (data not shown). In parallel experiments, human platelets, at the same cell density, responded to capsaicin from the same stock solution with a rise in [Ca2+]cyt as previously reported (; n = 6) Citation[1]. We also examined whole cell lysates of murine (Swiss) and human platelets, prepared from the samples used for fluorescence experiments, for the presence of TRPV1 by Western blotting. We detected a band at around 100 kDa, consistent with the molecular weight of hTRPV1, in the human whole platelet lysates as previously reported Citation[1], Citation[2], but could not detect a band at around 95 kDa, as would be expected for mTRPV1 Citation[5], in the mouse platelet lysates (; n = 3). The antibody was, however, able to detect a protein of around 95 kDa in mouse brain lysate (; n = 3). We also observed a secondary band in the mouse brain lysate, which we believe is likely to represent a truncated variant of mTRPV1 expressed in hypothalamic osmoreceptors Citation[6]. Another N-terminal variant of TRPV1, TRPV1b, has also been reported to be present in mice Citation[7]. The anti-TRPV1 antibody used in this study was directed to the C-terminal of TRPV1, thus our data suggest the absence of all known murine TRPV1 isoforms from mouse platelets. Recent transcriptome profiles of murine and human platelets have demonstrated low levels of TRPV1 mRNA in human platelets, whilst it appeared to be absent from murine platelets Citation[8], supporting our observations.
Figure 1. TRPV1 is expressed in human but not mouse platelets. (A,B) Fura-2-loaded platelets from mice (A) or humans (B) were stimulated in the presence of 1 mM Ca2+, with either 100 µM capsaicin (Caps) or an equal volume of its vehicle DMSO. (C,D) Western blot analysis of whole cell lysates of mouse or human platelets (C) or whole cell lysates of mouse brain and platelets (D). Whole cell lysates were analyzed following sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) by Western blotting using an anti-TRPV1 polyclonal antibody. In panels marked “No Primary”, the primary antibody was omitted to rule out non-specific binding of the secondary antibody. Positions of molecular mass markers (kDa) are shown on the left. These results are representative of 6 (C) or 3 (D) independent experiments.
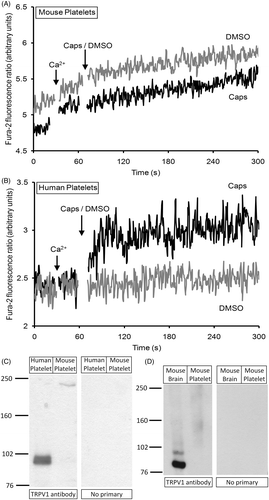
These results demonstrate that murine platelets lack TRPV1, and thus cannot provide a model system in which to study the physiological function of TRPV1 in platelets in vivo. However, the demonstration that these cells lack both the TRPV1 protein and responsiveness to capsaicin, even at high concentrations, does provide a cell that can be used as a negative control for screening for non-specific effects of TRPV1 agonists and antagonists in platelets. The negative results from the murine platelets also support our previous conclusion that capsaicin activates calcium signalling and serotonin secretion in human platelets through a specific effect on TRPV1 Citation[1].
There is growing recognition of platelet involvement in a range of inflammatory conditions such as atherosclerosis, inflammatory bowel disease and rheumatoid arthritis Citation[9]. We have previously suggested that the presence of TRPV1 in human platelets may be involved in a signalling pathway that could elicit platelet activation under these conditions Citation[1]. The results presented here, however, suggest that the molecular identity of signalling pathways eliciting platelet activity under inflammatory conditions is likely to be different in mice.
The absence of TRPV1 from murine platelets adds to a growing body of evidence of differences in both the proteomes and transcriptomes of mouse and human platelets Citation[8]. Thus, while platelets from genetically-modified mice provide a useful tool for an initial evaluation of the role of a variety of proteins in platelet function, caution must be exercised when extrapolating from mouse studies to consider human platelet function.
Acknowledgements
We thank Dr Pedro Redondo and Professor Gines Salido for assistance with some experiments.
Declaration of interest: The authors report no declaration of interest. This work was supported by The Spanish Ministry of Innovation (BFU2010-21043-C02-01).
References
- Harper AGS, Brownlow SL, Sage SO. A role for TRPV1 in agonist-evoked activation of human platelets. J Thromb Haemost 2009; 7: 330–338
- Savini I, Arnone R, Rossi A, Catani MV, Del Principe D, Avigliano L. Redox modulation of Ecto-NOX1 in human platelets. Mol Membr Biol 2010; 27: 160–169
- Coppinger JA, Cagney G, Toomey S, Kislinger T, Belton O, McRedmond JP, Cahill DJ, Emili A, Fitzgerald DJ, Maguire PB. Characterisation of the proteins released from activated platelets leads to localisation of novel platelet proteins in atherosclerotic lesions. Blood 2004; 103: 2096–2104
- Boyanova D, Nilla S, Birschmann I, Dandekar T, Dittrich M. PlateletWeb: A systems biologic analysis of signalling networks in human platelets. Blood 2012; 119: e22–e34
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000; 288: 306–313
- Sharif Naeini R, Witty MF, Séguéla P, Bourque CW. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat Neurosci 2006; 9: 93–98
- Wang C, Hu H-Z, Colton CK, Wood JD, Zhu MX. An alternative splicing product of the murine trpv1 gene dominant negatively modulates the activity of TRPV1 channels. J Biol Chem 2004; 279: 37423–37430
- Rowley JW, Oler AJ, Tolley ND, Hunter BN, Low EN, Nix DA, Yost CC, Zimmerman GA, Weyrish AS. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood 2011; 118: e101–e111
- McNicol A, Israels SJ. Beyond Haemostasis: The role of platelets in inflammation, malignancy and infection. Cardiovasc Hematol Disord Drug Targ 2008; 8: 99–117
