Abstract
In the PLATelet inhibition and patient Outcomes (PLATO) study of patients with acute coronary syndromes, ticagrelor reduced mortality compared to clopidogrel but the mechanisms for this mortality reduction remain uncertain. We analysed adverse events (AEs) consistent with either pulmonary infection or sepsis, and subsequent mortality, in 18,421 PLATO patients treated with ticagrelor or clopidogrel. AEs occurring within 7 days of last dose of study medication were defined as “on-treatment”. Serial measurements of blood leukocyte counts, C-reactive protein and interleukin-6 were performed. Fewer on-treatment pulmonary AEs occurred in the ticagrelor compared to the clopidogrel group (275 vs. 331 respectively; p = 0.019), with fewer deaths following these AEs (33 vs. 71; p < 0.001), particularly in those who remained on study medication three days after AE onset (10 vs. 43; p < 0.001). There were fewer deaths attributed to sepsis in the ticagrelor group (7 vs. 23; p = 0.003). Leukocyte counts were lower in the clopidogrel group during treatment (p < 0.0001 at 1, 3 and 6 months) but not at 1 month post-discontinuation. C-reactive protein increased more at discharge in the ticagrelor group (28.0 ± 38.0 vs. 26.1 ± 36.6 mg/l; p < 0.001) and interleukin-6 remained higher during the first month of treatment with ticagrelor. We conclude that the mortality risk following pulmonary AEs and sepsis in acute coronary syndrome patients appears to be lower during ticagrelor compared to clopidogrel therapy. Further work should assess whether ticagrelor and clopidogrel have differential effects on immune signalling.
Introduction
The PLATelet inhibition and patient Outcomes (PLATO) trial compared ticagrelor with contemporary regimens of clopidogrel in patients with acute coronary syndromes (ACS) and demonstrated superiority of ticagrelor in reduction of recurrent ischaemic events [Citation1]. There was also an observed reduction in total mortality in the ticagrelor group compared to the clopidogrel group (4.5% vs. 5.9%; hazard ratio (HR) 0.78, 95% confidence intervals (CI) 0.69–0.89), including both a significant reduction in cardiovascular death (4.0% vs. 5.1%; HR 0.79, 95% CI 0.69–0.91), which included deaths of unknown cause, and a trend towards reduction in non-cardiovascular mortality (0.4% vs. 0.8%; HR 0.71, 95% CI 0.49–1.04) [Citation1].
Ticagrelor is an oral, reversibly-binding platelet P2Y12 receptor inhibitor belonging to a novel chemical class that also inhibits adenosine reuptake [Citation2]. This is in distinction to clopidogrel and other thienopyridines that are prodrugs acting through hepatic metabolites that bind irreversibly to the P2Y12 receptor and are not known to have any effect on adenosine metabolism. Ticagrelor yielded greater inhibition of platelet aggregation than clopidogrel in the PLATO study [Citation3], which might at least partly explain its superiority in reducing the incidence of myocardial infarction and associated cardiovascular death. However, the extent of overall mortality reduction seen with ticagrelor in the PLATO study warrants scrutiny of other potential mechanisms. Platelets play a key role in the inflammatory response to vascular injury and also contribute to innate immune responses [Citation4]. Since the platelet P2Y12 receptor markedly amplifies the release of pro-inflammatory chemokines from platelet granules, it has a dominant role in supporting platelet-mediated inflammation [Citation5, Citation6]. Adenosine not only has an inhibitory effect on platelet responses but also has wide-ranging effects on other pathways involved in innate immunity and may have a protective effect against pulmonary injury [Citation7]. In order to explore whether the mortality benefit of ticagrelor compared to clopidogrel in the PLATO study may be related to mechanisms in addition to more effective prevention of arterial thrombotic events, we therefore performed a post hoc analysis of adverse events (AEs) attributable to pulmonary infection and sepsis.
Higher levels of inflammatory markers such as neutrophil count, C-reactive protein (CRP) and interleukin-6 (IL-6) have been associated with adverse outcomes and mortality in previous studies of ACS patients [Citation8–11]. We performed an analysis of serial blood leukocyte counts and inflammatory markers in patients participating in the PLATO study to assess their impact on clinical outcomes [Citation12] and were able, in the current study, to assess any differences of ticagrelor compared to clopidogrel on leukocyte counts and acute and chronic inflammatory responses following acute coronary syndrome.
Methods
Study design
The study design and primary results of the PLATO study have been previously reported [Citation1, Citation13]. Of 18 624 patients randomised to receive either ticagrelor or clopidogrel, 18 421 received at least one dose of study medication and were considered here in exploratory post hoc analyses looking for associations with mortality, including an analysis of the incidence of the following AEs reported by investigators prior to unblinding of the study: (1) any pulmonary AE, consisting of the Medical Dictionary for Regulatory Activities (MedDRA) preferred terms of “lower respiratory tract infection” (LRTI), “pneumonia,” “lung infection,” “respiratory failure” or the higher level group term of “pleural disorders”; and (2) any sepsis AE consisting of the MedDRA terms containing “sepsis,” “septic” or “bacteraemia.” Event rates were described both for events occurring at any time during the study and for events occurring not later than 7 days after last dose of study medication (defined as “on-treatment” in view of the timing of offset of the study drugs as well as incubation time for infections). The incidence of death following a pulmonary or sepsis AE was determined. In order to analyse the effects on mortality of continuing study medication after AE onset, sub-analysis was performed of deaths occurring after a dose of study medication had been taken on the third day after the onset of the pulmonary or sepsis AE and these deaths were defined as occurring with “continuing medication” (this analysis did not include deaths within 3 days of the AE onset since these could not meet this definition). Event rates were also investigated in the subgroups who did or did not have coronary-artery bypass graft (CABG) surgery during the study.
Haematology and biomarker substudy
Full blood counts including differential leukocyte counts were performed on EDTA-anti-coagulated blood samples for all patients at randomisation and, in the subset of patients recruited in the early phase of the study (prior to review of the data by the Data Safety Monitoring Board and its decision that safety monitoring was no longer required), at 1, 3 and 6 months, at end of treatment and at 1 month after discontinuation of study medication by a central laboratory (Quintiles Laboratories). The lower limits of the normal laboratory ranges were 3.0 × 109/l for total white cell counts and 1.5 × 109/l for neutrophil counts. Investigators reported whether or not patients were planned for open-label clopidogrel at the end-of-treatment visit, allowing assessment of the impact of transition, or not, to open-label clopidogrel on inflammatory markers at 1 month after discontinuation of study medication.
All study sites were invited to participate in a biomarker substudy using a separate informed consent process, according to a protocol approved by the relevant ethics committees. At those sites accepting to participate, representing the majority of patients recruited to the PLATO study, blood samples were collected at randomisation prior to administration of study medication. A subset of patients participating in this blood core substudy had additional samples taken at hospital discharge with intended recruitment of 4000 patients at 1 month and 2000 patients at 6 month follow-up visits. Plasma was obtained from EDTA-anti-coagulated venous blood samples and stored initially at −20 °C prior to transfer to the Uppsala Clinical Research Center Biobank for storage at −80 °C prior to measurement of levels of (1) CRP using immunoturbidimetry with the CRP Vario kit (Abbott) and spectrophotometric analysis (Architect, Abbott) and (2) IL-6 using a high-sensitivity enzyme-linked immunosorbent assay (Quantikine human IL-6 kit, R&D Systems) and spectrophotometric analysis (Freedom EVOlyzer, Tecan Group Ltd). All laboratory measurements were performed blinded to treatment allocation and clinical events.
Statistical analysis
The analysis set comprised all subjects who were randomly assigned to a treatment group and received at least one dose of study medication (safety population). Fisher’s exact test was used to compare proportions of pulmonary and sepsis AEs between treatment groups. The time from randomization to pulmonary AE and death following pulmonary AE, respectively, were described with cumulative Kaplan–Meier curves and analysed with Cox proportional hazards model with treatment group (ticagrelor or clopidogrel) as independent variable.
Baseline characteristics and procedures in hospital or at discharge were presented by treatment group for patients with and without pulmonary AEs. Continuous variables were measured as median and interquartile interval and categorical variables as frequency and rate (%). To assess the association between baseline factors and the rate of pulmonary AEs as well as death following these AEs, multivariable logistic regression with the baseline factors and randomized treatment group were performed. p Values and odds ratios (not present vs. present for binary factors; per unit increase for continuous factors) for the influence of baseline factor on the risk of pulmonary AE and subsequent mortality were calculated. Subgroup analyses of on-treatment pulmonary AEs were performed with logistic regression with treatment group, subgroup factor and their interaction as independent variables. p Values for the subgroup-by-treatment group interaction test and odds ratios (ticagrelor vs. clopidogrel) for the risk of on-treatment pulmonary AE were calculated (assessing whether the treatment group difference depended on patient’s subgroup). We used logistic regression for the subgroup analyses as a robust alternative to the Cox model considering the low event rates and the proportional hazard assumption in the Cox model. However, in general the two models gave similar results and conclusions were not altered. Baseline characteristics of those with and without subsequent pulmonary AE are presented descriptively only since the AE was a post-randomisation variable.
The leukocyte counts (total leukocyte and neutrophil counts), C-reactive protein and interleukin-6 were compared between treatments using analysis of covariance (ANCOVA) with the baseline value and treatment group as independent variables. The values were transformed with the natural logarithmic function before analysis due to skew distributions. Hence, model-adjusted (baseline value-adjusted) estimates of treatment group differences were provided as ratios of geometric means. The mean individual change in leukocyte and differential neutrophil counts following discontinuation of study drug were analysed with Wilcoxon’s signed rank test. Subsets of patients were analysed according to whether or not investigators planned transition to open-label clopidogrel after discontinuation of study medication. Cox proportional hazards model was used to assess the relationship between quartile of WBC count at 1 month and subsequent clinical outcomes, with treatment group as an independent variable.
No correction was performed for multiple analyses and p values of less than 5% were considered to be nominally significant and exploratory in nature. 95% CI were calculated where appropriate. All hypotheses tests were two-sided. All analyses were done with SAS software (version 9.3, SAS Institute, Cary, NC).
Results
Pulmonary and sepsis AEs and associated mortality
Pulmonary and sepsis AEs were determined in 9235 patients receiving at least one dose of ticagrelor and 9186 patients receiving at least one dose of clopidogrel ( and Supplement Table IA). Fewer pulmonary AEs were seen within 7 days of study medication (“on-treatment”) in the ticagrelor group compared to the clopidogrel group and there were fewer deaths following pulmonary AEs in the ticagrelor group, with all of the difference accounted for by deaths following on-treatment pulmonary AEs ( and ). There were also fewer deaths attributed to sepsis in the ticagrelor group, including fewer deaths attributed to sepsis following a pulmonary AE (). Approximately one-third of pulmonary and sepsis AEs occurred in patients who underwent CABG surgery at any time in the study and similar trends in AE rates and associated mortality were seen in those who either did not or did have CABG surgery (Supplement Table IB). Deaths following pulmonary or sepsis AEs that were adjudicated as both “cardiovascular” and “non-cardiovascular” contributed to the differences between the treatment groups (Supplement Table IC). There were fewer subsequent pulmonary or sepsis AEs following a pulmonary AE with “continuing medication,” and fewer deaths following these recurrent AE, in the ticagrelor group (). There were no significant differences between the treatment groups in the numbers of sepsis AEs that did not follow a pulmonary AE (Supplement Table ID).
Figure 1. Cumulative Kaplan–Meier estimates of (A) time to pulmonary AE on-treatment and (B) the time to death following pulmonary AE on-treatment, out of all randomized patients who received at least one dose of study medication, comparing treatment with ticagrelor (“T”, N = 9235) and clopidogrel (“C”, N = 9186). p Values and odds ratio (OR) are from multivariable logistic regression for modelling the probability of the event using treatment group and baseline factors (gender, age, weight, asthma, chronic obstructive pulmonary disease, diabetes, smoker, congestive heart failure, chronic renal disease, peripheral arterial disease and clopidogrel pre-randomization) as independent variables. CI = confidence intervals.
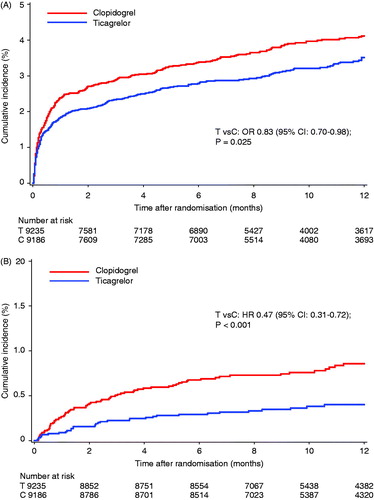
Table I. Incidence of pulmonary and sepsis AEs and associated mortality according to treatment group.
Patients with pulmonary AEs in the ticagrelor group tended to be slightly younger (67 vs. 69 years) and heavier (80 vs. 76 kg) compared to those with pulmonary AEs in the clopidogrel group (). For comparison of the rates of on-treatment pulmonary AE between the treatment groups, there were significant interactions noted for lower body weight (less than 80 kg) and, to a lesser extent, gender and age, and the most marked difference occurred in those with weight less than 60 kg (). Multivariable analysis showed that these interactions were attributable to higher age, female gender and co-morbid conditions (Supplement Table II). Patients with a history of asthma and chronic obstructive pulmonary disease had greater risk of pulmonary AE but similarly reduced incidences of these AE in the ticagrelor group as those without such a history. The reduced mortality observed in the ticagrelor group following on-treatment pulmonary AE remained significant after adjustment for baseline factors (, Supplement Table III).
Table II. Baseline and other characteristics of patients with pulmonary AEs according to treatment group.
Table III. Rates and odds ratios for on-treatment pulmonary AEs in subgroups of patients according to baseline characteristics.
Leukocyte counts
Central-laboratory leukocyte counts were available for 4121 and 4093 patients in the ticagrelor and clopidogrel groups, respectively, at baseline and a subset of these at later time points. There were no relevant differences at randomisation or at 1, 3 and 6 months in the rare incidences of leukopaenia or neutropaenia defined as total leukocyte or differential neutrophil counts below the lower reference range limits, respectively (Supplement Table IV). However, despite no difference in baseline counts, the mean leukocyte and neutrophil counts in the ticagrelor group were significantly higher than those in the clopidogrel group during treatment at 1, 3 and 6 months, with resolution of these differences at 1 month after discontinuation of study medication (). Data from patients with paired 6-month and follow-up data showed that this resolution was associated with significant fall in the mean neutrophil count in the ticagrelor group and significant increases in the mean leukocyte and neutrophil counts in the clopidogrel group after discontinuation of study medication (). For patients who had received study medication within 7 days of blood sampling, analysis of the subset of patients who transitioned to open-label clopidogrel after study medication discontinuation showed significant falls in mean total leukocyte and neutrophil counts in the ticagrelor group but no changes in the clopidogrel group, whereas in the subset of patients not transitioning to open-label clopidogrel, there was only a significant fall in neutrophil count in the ticagrelor group in contrast to significant rises in leukocyte and neutrophil counts in the clopidogrel group (). To assess whether subtle reductions in neutrophil counts contributed to subsequent infection risk, landmark analysis was performed for pulmonary or sepsis AEs after 1 month of treatment in patients with leukocyte counts in the lowest quartile at 1 month and showed no evidence of increased risk in these patients (data not shown).
Table IV. Total leukocyte and differential neutrophil counts during the study according to treatment group.
Table V. Mean individual change in leukocyte and differential neutrophil counts following discontinuation of study medication according to treatment group.
C-reactive protein and interleukin-6
The increase in the plasma levels of the inflammation biomarkers C-reactive protein and interleukin-6 from randomisation levels in the acute stage was larger in the ticagrelor group compared to the clopidogrel group. There were however no differences in C-reactive protein levels between the treatment groups at 1 and 6 months follow-up or in interleukin-6 levels at 6 months ().
Table VI. C-reactive protein and interleukin-6 levels at randomisation, at discharge and after 1 and 6 months according to treatment group.
Discussion
In this post hoc analysis of factors associated with the differences in mortality rates between the ticagrelor and clopidogrel groups in the PLATO study, more deaths were observed in the clopidogrel group following pulmonary AE as well as sepsis events. The lower rate of pulmonary AE during treatment with ticagrelor compared to clopidogrel is in contrast to the higher rates of dyspnoea that occur during ticagrelor therapy as a consequence of ticagrelor-related dyspnoea [Citation14, Citation15] and it is possible that some of the reported pulmonary AE in the ticagrelor group were partly or wholly related to this rather than infection, which could explain the small differences in age and weight between the pulmonary AE cohorts as well as some of the mortality differences. However, if this hypothesis is correct then it implies an even more significant reduction in the incidence of true pulmonary AE during treatment with ticagrelor than the reduction observed in this analysis. Furthermore, there was a nominally significant reduction in deaths attributed to sepsis with ticagrelor compared to clopidogrel therapy in the entire trial cohort regardless of the observations on pulmonary AE.
Platelets are recognised to play a role in innate immunity [Citation16]. Platelet activation in sepsis may contribute to organ dysfunction [Citation17–19], so it is possible that the greater platelet inhibition with ticagrelor compared to clopidogrel contributed to reduced deaths associated with pulmonary AE and sepsis. Preclinical and observational studies have suggested that clopidogrel may have a protective effect during sepsis [Citation20, Citation21] and additionally a preclinical study has shown ticagrelor to reduce lung injury during sepsis [Citation22]. On the other hand, large clinical studies of clopidogrel compared to placebo and prasugrel compared to clopidogrel have not reported any differences in deaths following pulmonary AEs or sepsis despite differences in platelet inhibition between the treatment groups and have also not shown significant differences in total mortality except related to early reductions in cardiovascular mortality in patients with ST-elevation myocardial infarction [Citation23–26], so other mechanisms for the mortality benefit of ticagrelor compared to clopidogrel should be strongly considered.
In conjunction with the observations on pulmonary and sepsis AE and associated mortality, we observed differences in inflammatory marker responses in the ticagrelor and clopidogrel groups that were apparent at hospital discharge, according to plasma C-reactive protein and interleukin-6 levels, and sustained throughout the course of treatment, according to leukocyte counts and interleukin-6 levels. This represents a paradoxical finding in view of the previous evidence that higher levels of these inflammatory markers are associated with higher mortality in ACS patients rather than the lower mortality that was observed with ticagrelor compared to clopidogrel in PLATO. There were, however, no differences in the incidence of clinical neutropaenia between the treatment groups to explain a potential difference in susceptibility to bacterial infection. Although these collected observations do not prove any causal link between the subtle differences in inflammatory responses and the different rates of mortality following pulmonary events and associated lung injury, they contribute to a hypothesis that the lower mortality associated with ticagrelor compared to clopidogrel in ACS patients may be partly due to effects of one or both drugs on host defence pathways in the lung and/or mechanisms of lung injury and support further work in this area.
The finding of higher inflammatory markers associated with ticagrelor compared to clopidogrel therapy is unlikely to be explained by the more potent P2Y12 inhibition achieved by ticagrelor in ACS patients [Citation3] since the P2Y12 receptor contributes to the vascular inflammation associated with platelet activation [Citation6] and so greater P2Y12 inhibition may be anticipated to have a greater anti-inflammatory effect. Potent platelet inhibition with abciximab at the time of percutaneous coronary intervention has previously been shown to reduce subsequent inflammatory response [Citation27]. This raises the hypothesis that mechanisms unrelated to P2Y12 inhibition explain the differences in inflammatory markers. Ticagrelor belongs to a different chemical class to the thienopyridines clopidogrel and prasugrel; unlike these thienopyridines, ticagrelor is an inhibitor of adenosine reuptake by erythrocytes and other cells and has been shown to augment the vascular response to infused adenosine [Citation2, Citation28]. It has been speculated, but not yet proven, that this might explain the increased incidence of dyspnoea as well as sinoatrial pauses related to ticagrelor therapy [Citation14, Citation15, Citation29]. Adenosine is involved in some inflammatory responses and, at low concentrations, may promote neutrophil chemotaxis and phagocytosis [Citation30]. Some studies have shown potential for adenosine to reduce pulmonary injury [Citation7]. Thus, it is possible that ticagrelor could modulate susceptibility to serious bacterial infection and lung injury through its impact on adenosine metabolism. Further studies are warranted to explore this hypothesis.
An alternative or additional explanation for the differences in inflammatory markers and infection-related deaths in PLATO is an immunosuppressant effect of clopidogrel. It is well recognised that clopidogrel is rarely associated with haematological disorders [Citation31]. However, we did not see any differences in absolute leukopaenia or neutropaenia that would support this hypothesis. On the other hand, there was a slight but significant rise in the neutrophil count at 1 month after cessation of study medication in the clopidogrel group (in contrast to a slight fall in this count in the ticagrelor group), which seemed to be accounted for by the subgroup of patients who did not transition to open-label clopidogrel suggesting that clopidogrel at least has a weak quantitative effect on neutrophils in vivo. The clinical evidence regarding any link between clopidogrel and bacterial infection is conflicting. In contrast to studies cited above suggesting potential beneficial effects of clopidogrel in sepsis, one study has reported an increased risk of infection and numerically higher mortality rates in patients treated with aspirin plus clopidogrel undergoing CABG surgery compared to those treated with aspirin alone [Citation32]. However, there have been no such reports in randomised, controlled trials of clopidogrel compared to placebo. On the other hand, in a separate analysis of the causes of death following CABG surgery in PLATO patients who received study medication within seven days of surgery, we have found an excess of deaths in the clopidogrel group where infection appeared to be the cause or a contributory factor [Citation33]. More recently, an observational study has suggested that clopidogrel may increase the incidence of community-acquired pneumonia, but not the severity or associated mortality, compared to patients not exposed to clopidogrel [Citation34].
Limitations
Our analysis of pulmonary and sepsis AEs was post hoc and therefore susceptible to bias so the findings should be considered hypothesis-generating and the p values, as stated in the Methods section, are exploratory in nature. Furthermore, the presentation of the AE data alongside data on the inflammatory markers is intended to stimulate avenues of future research and not suggest a definite mechanistic link between the observations. Pulmonary and sepsis AEs were reported by investigators and not independently adjudicated.
Conclusions
Overall, our findings suggest that the all-cause mortality reduction seen with ticagrelor compared to clopidogrel may be partly explained by differential effects on the responses to pulmonary events and sepsis, particularly in patients who continue ticagrelor or clopidogrel after onset of a pulmonary AE. These results point to the need for further research into the effects of different P2Y12 inhibitors on host defence mechanisms and lung injury.
Declaration of interest
This work was supported by AstraZeneca. R. F. Storey reports receiving research grants from AstraZeneca, Eli Lilly/Daiichi Sankyo, and Merck; research support from Accumetrics; honoraria from AstraZeneca, Eli Lilly/Daiichi Sankyo, Merck, Novartis, Iroko, Sanofi-Aventis/BMS, Accumetrics, and Medscape; consultancy fees from AstraZeneca, Merck, Novartis, Accumetrics, Roche, Sanofi Aventis, and Regeneron.
S. K. James reports institutional research grant and honoraria from AstraZeneca, Eli Lilly, Merck and Bristol-Myers Squibb and being an advisory board member for AstraZeneca, Eli Lilly and Merck. Honoraria from The Medicines Company.
A. Siegbahn reports Institutional grants from Boehringer-Ingelheim and Astrazeneca; and honoraria from Boehringer-Ingelheim.
C. Varenhorst has received a research grant from AstraZeneca to perform this research; and is a member of the Speakers’ Bureaus for AstraZeneca, Eli Lilly & Company, and The Medicines Company.
C. Held reports institutional research grants from AstraZeneca, Merck, GlaxoSmithKline, Roche and Bristol-Myers Squibb and being an advisory board member for AstraZeneca, Honoraria from AstraZeneca.
J. Ycas reports being an employee of AstraZeneca.
S. Husted reports being an advisory board member for AstraZeneca, Bristol-Myers Squibb, Pfizer, and Bayer; research support from GlaxoSmithKline, Pfizer, and Sanofi-Aventis.
C. P. Cannon reports receiving research grants/support from Accumetrics, AstraZeneca, Essentialis, GlaxoSmithKline, Merck, Regeneron, Sanofi, and Takeda; being on advisory boards for Alnylam, Bristol-Myers Squibb, CSL Behring, and Pfizer (funds donated to charity); and a Clinical Advisor with equity in Automedics Medical Systems.
R. C. Becker reports honoraria from AstraZeneca and scientific advisory board for Merck, Portola, Boehringher-Ingelheim, Bayer.
P. G. Steg has received research grants from NYU School of Medicine, Sanofi, Servier; consultancy fees/honoraria from Ablynx, Amarin, Amgen, Astellas, AstraZeneca, Bayer, Boehringer-Ingelheim, BMS, Daiichi/sankyo, Eisai, GSK, Lilly, Medtronic, MSD, Novartis, Otsuka, Pfizer, Roche, Sanofi, Servier, The Medicines Company, and Vivus; and has equity ownership in Aterovax.
N. Åsenblad reports no disclosures.
L. Wallentin reports receiving research grants from AstraZeneca, Merck/Schering-Plough, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline; being a consultant for Merck/Schering-Plough, Regado Biosciences, Evolva, Portola, C. S. L. Behring, Athera Biotechnologies, Boehringer Ingelheim, AstraZeneca, GlaxoSmithKline, and Bristol-Myers Squibb/Pfizer; lecture fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline, Schering-Plough; and honoraria from Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline, and Schering-Plough/Merck.
Supplementary Material
Download PDF (73.7 KB)Acknowledgements
The authors are indebted to Johanna Tilly at Uppsala Clinical Research Center for statistical support and to Ulla Nässander Schikan PhD at Uppsala Clinical Research Center for assistance in preparation of the article.
References
- Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. New Engl J Med 2009;361:1045–1057
- Van Giezen JJ, Sidaway J, Glaves P, Kirk I, Bjorkman JA. Ticagrelor inhibits adenosine uptake in vitro and enhances adenosine-mediated hyperemia responses in a canine model. J Cardiovasc Pharmacol Therapeut 2011;17:164–172
- Storey RF, Angiolillo D, Patil S, Desai B, Ecob R, Husted S, Emanuelsson H, Cannon C, Becker R, Wallentin L. Inhibitory effects of ticagrelor compared to clopidogrel on platelet function in patients with acute coronary syndromes: The PLATO PLATELET Substudy. J Am Coll Cardiol 2010;56:1456–1462
- Nurden AT. Platelets, inflammation and tissue regeneration. Thrombosis Haemostasis 2011;105(Suppl 1):S13–S33
- Storey RF. Biology and pharmacology of the platelet P2Y12 receptor. Curr Pharmaceut Design 2006;12:1255–1259
- Evans DJW, Jackman LE, Chamberlain J, Crosdale DJ, Judge HM, Jetha K, Norman KE, Francis SE, Storey RF. The platelet P2Y12 receptor influences the vessel wall response to arterial injury and thrombosis. Circulation 2009;119:116–122
- Jacobson KA, Gao Z-G. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov 2006;5:247–264
- Guasti L, Dentali F, Castiglioni L, Maroni L, Marino F, Squizzato A, Ageno W, Gianni M, Gaudio G, Grandi AM, et al. Neutrophils and clinical outcomes in patients with acute coronary syndromes and/or cardiac revascularisation. A systematic review on more than 34,000 subjects. Thrombosis Haemostasis 2011;106:591–599
- Verma S, Szmitko PE, Ridker PM. C-reactive protein comes of age. Nat Clin Pract Cardiovasc Med 2005;2:29–36
- James SK, Lindahl B, Timmer JR, Ottervanger JP, Siegbahn A, Stridsberg M, Armstrong P, Califf R, Wallentin L, Simoons ML. Usefulness of biomarkers for predicting long-term mortality in patients with diabetes mellitus and non–ST-elevation acute coronary syndromes (A GUSTO IV Substudy). Am J Cardiol 2006;97:167–172
- Zamani P, Schwartz GG, Olsson AG, Rifai N, Bao W, Libby P, Ganz P, Kinlay S, for the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering Study Investigators. Inflammatory biomarkers, death, and recurrent nonfatal coronary events after an acute coronary syndrome in the MIRACL study. J Am Heart Assoc 2013;2:E-pub
- Storey RF, James S, Åsenblad N, Siegbahn A, Emanuelsson H, Katus H, Cannon C, Husted S, Steg P, Wallentin L. Consistent benefit of ticagrelor compared to clopidogrel in acute coronary syndrome patients regardless of baseline inflammatory markers. J Am Coll Cardiol 2012;59:E505 (abstract)
- James S, Åkerblom A, Cannon CP, Emanuelsson H, Husted S, Katus H, Skene S, Steg PG, Storey RF, Harrington R, et al. Comparison of Ticagrelor, the first reversible oral P2Y12 receptor antagonist, with clopidogrel in patients with acute coronary syndromes: Rationale, design, and baseline characteristics of the PLATelet inhibition and patient Outcomes (PLATO) trial. Am Heart J 2009;157:599–605
- Storey RF, Bliden K, Patil SB, Karunakaran A, Ecob R, Butler K, Teng R, Wei C, Tantry US, Gurbel P. Incidence of dyspnea and assessment of cardiac and pulmonary function in patients with stable coronary artery disease receiving ticagrelor, clopidogrel or placebo in the ONSET/OFFSET study. J Am Coll Cardiol 2010;56:185–193
- Storey RF, Becker RC, Harrington RA, Husted S, James SK, Cools F, Steg PG, Khurmi NS, Emanuelsson H, Cooper A, et al. Characterisation of dyspnoea in PLATO study patients treated with ticagrelor or clopidogrel and its association with clinical outcomes. Eur Heart J 2011;32:2945–2953
- Cox D, Kerrigan SW, Watson SP. Platelets and the innate immune system: Mechanisms of bacterial-induced platelet activation. J Thrombosis Haemostasis 2011;9:1097–1107
- Russwurm S, Vickers J, Meier-Hellmann A, Spangenberg P, Bredle D, Reinhart K, Lösche W. Platelet and leukocyte activation correlate with the severity of septic organ dysfunction. Shock 2002;17:263–268
- Winning J, Reichel J, Eisenhut Y, Hamacher J, Kohl M, Deigner HP, Claus RA, Bauer M, Lösche W. Anti-platelet drugs and outcome in severe infection: Clinical impact and underlying mechanisms. Platelets 2009;20:50–57
- Otto GP, Sossdorf M, Boettel J, Kabisch B, Breuel H, Winning J, Lösche W. Effects of low-dose acetylsalicylic acid and atherosclerotic vascular diseases on the outcome in patients with severe sepsis or septic shock. Platelets 2013;24:480–485
- Seidel M, Winning J, Claus RA, Bauer M, LÖSche W. Beneficial effect of clopidogrel in a mouse model of polymicrobial sepsis. J Thrombosis Haemostasis 2009;7:1030–1032
- Winning J, Neumann J, Kohl M, Claus RA, Reinhart K, Bauer M, Lösche W. Antiplatelet drugs and outcome in mixed admissions to an intensive care unit*. Critical Care Med 2010;38:32–37
- Rahman M, Gustafsson D, Wang Y, Thorlacius H, Braun OO. Ticagrelor reduces neutrophil recruitment and lung damage in abdominal sepsis. Platelets 2013;E-pub July 15
- The Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. New Engl J Med 2001;345:494–502
- Wiviott S, Braunwald E, McCabe C, Montalescot G, Ruzyllo W, Gottlieb S, Neumann F-J, Ardissino D, De Servi S, Murphy S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. New Engl J Med 2007;357:2001–2015
- Roe MT, Armstrong PW, Fox KAA, White HD, Prabhakaran D, Goodman SG, Cornel JH, Bhatt DL, Clemmensen P, Martinez F, et al. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. New Engl J Med 2012;367:1297–1309
- Chen ZM, Jiang LX, Chen YP, Xie JX, Pan HC, Peto R, Collins R, Liu LS, COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) collaborative group. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: Randomised placebo-controlled trial. The Lancet 2005;366:1607–1621
- Lincoff AM, Kereiakes DJ, Mascelli MA, Deckelbaum LI, Barnathan ES, Patel KK, Frederick B, Nakada MT, Topol EJ. Abciximab suppresses the rise in levels of circulating inflammatory markers after percutaneous coronary revascularization. Circulation 2001;104:163–167
- Wittfeldt A, Emanuelsson H, Brandrup-Wognsen G, van Giezen JJJ, Jonasson J, Nylander S, Gan L-M. Ticagrelor enhances adenosine-induced coronary vasodilatory responses in humans. J Am Coll Cardiol 2013;61:723–727
- Scirica B, Cannon C, Emanuelsson H, Michelson E, Harrington R, Husted S, James S, Katus H, Pais P, Raev D, et al. The incidence of arrhythmias and clinical arrhythmic events in patients with acute coronary syndromes treated with ticagrelor or clopidogrel in the PLATO trial. J Am Coll Cardiol 2011;57:1908–1916
- Barletta KE, Ley K, Mehrad B. Regulation of neutrophil function by adenosine. Arteriosclerosis, Thrombosis, Vasc Biol 2012;32:856–864
- Balamuthusamy S, Arora R. Hematologic adverse effects of clopidogrel. Am J Therapeut 2007;14:106–112
- Blasco-Colmenares E, Perl TM, Guallar E, Baumgartner W, Conte J, Alejo D, Pastor-Barriuso R, Sharrett A, Faraday N. Aspirin plus clopidogrel and risk of infection after coronary artery bypass surgery. Arch Intern Med 2009;169:788–795
- Varenhorst C, Alstrom U, Scirica BM, Hogue CW, Asenblad N, Storey RF, Steg PG, Horrow J, Becker RC, James S, et al. Factors contributing to the lower mortality with ticagrelor compared with clopidogrel in patients undergoing coronary artery bypass surgery. J Am Coll Cardiol 2012;60:1623–1630
- Gross AK, Dunn SP, Feola DJ, Martin CA, Charnigo R, Li Z, Abdel-Latif A, Smyth SS. Clopidogrel treatment and the incidence and severity of community acquired pneumonia in a cohort study and meta-analysis of antiplatelet therapy in pneumonia and critical illness. J Thromb Thrombolysis 2013;35:147–154
