To the editor,
Abciximab is a platelet glycoprotein (GP) IIb/IIIa receptor blocker used, in association with other antiplatelet and anticoagulant drugs, during high-risk percutaneous coronary intervention (PCI) in the setting of acute coronary syndromes (ACS). Administration of abciximab is associated with development of thrombocytopenia [Citation1]. In approximately one-third of patients, thrombocytopenia is a laboratory artefact (pseudothrombocytopenia) [Citation2]. Immune-mediated thrombocytopenia, either acute (4–96 hours from drug exposure) or delayed (5–8 days) is also well recognised [Citation3–8]. While the acute form is secondary to naturally occurring cross-reacting antibodies, the delayed form reflects an immune response to a neo-antigen resulting from abciximab being bound to the platelet membrane; platelets sensitized by the antibody are cleared from the circulation mainly by splenic macrophages [Citation9]. The immune response is drug-dependent and therefore thrombocytopenia is self-limiting, resolving with clearance of abciximab from the blood stream and bone marrow production of new platelets [Citation7].
Delayed thrombocytopenia has been described following a drug regimen of abciximab bolus plus 12-hour infusion after PCI [Citation10, Citation11]. It usually follows an uneventful course and its detection is occasional [Citation9].
We present two patients who developed severe delayed abciximab-induced thrombocytopenia while on prasugrel, a P2Y12 blocker that has been associated with increased risk of bleeding events [Citation12], whose clinical picture was that of an emergency situation due to the coexisting risk of stent thrombosis and of severe bleeding.
Case 1: A 46-year-old man presented with large anterior ST-segment elevation myocardial infarction (STEMI) secondary to occlusion of the proximal left anterior descending artery which was treated by PCI using three bare metal stents. A single periprocedural bolus of abciximab (0.25 mg/kg) was administrated along with 5000 UI of unfractionated heparin. Dual antiplatelet therapy (DAPT) – aspirin and prasugrel – was initiated in the cathlab. Platelet count at admission and discharge were 276 and 220 × 109/l, respectively. Eight days after PCI, the patient was readmitted to the emergency room for petechiae and significant gingival bleeding; platelet count was 1 × 109/l. DAPT was discontinued, a platelet pool was transfused and dexamethasone was administered. Because of high risk of stent thrombosis, the patient was admitted to the Intensive Cardiac Care Unit (ICCU) for monitoring. Prompt response to platelet transfusion was observed ruling out ITP and steroids were discontinued. Platelet kinetics are depicted in , along with instituted therapy. Aspirin was resumed on day 9, and prasugrel on day 10. At discharge (day 14 from PCI), platelet counts had returned to normal levels (168 × 109/l); subsequent follow-up was uneventful.
Figure 1. Platelet count trend (expressed as 109/l) during hospitalization for both patient 1 (A) and patient 2 (B). Day 1 is the day of initial abciximab administration (day of PCI).
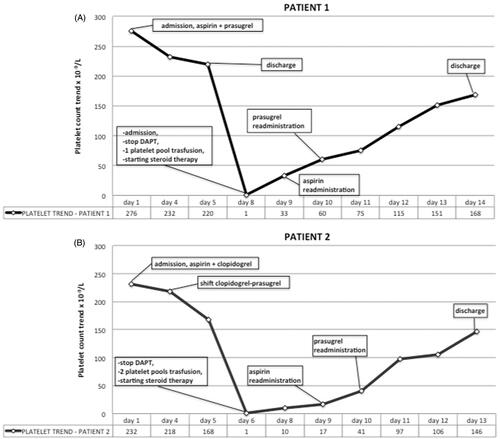
Case 2: A 50-year-old man presented with infero-postero-lateral STEMI secondary to occlusion of the mid circumflex artery which was treated by PCI using a drug-eluting stent. Abciximab (bolus followed by 12-hour infusion 0.125 mcg/kg/min) was administrated along with 5000 UI of unfractionated heparin. DAPT – aspirin and clopidogrel – was initiated in the cathlab. Platelet count at admission was 232 × 109/l, and remained stable over the next 5 days. On day 4, prasugrel was substituted for clopidogrel. On day 6, acute severe thrombocytopenia (platelet count of 1 × 109/l) developed not associated with bleeding signs. The patient was transferred to the ICCU for monitoring. DAPT was discontinued, platelet transfusion every 12 hours and dexamethasone were started. Platelet kinetics are depicted in , along with instituted therapy. Aspirin was resumed on day 9, and prasugrel on day 10. At discharge (day 13 from PCI), platelet counts had returned to normal levels (146 × 109/l); subsequent follow-up was uneventful.
Although seldom characterized by severe (i.e. platelet count <20 × 109/l) thrombocytopenia, delayed abciximab-induced thrombocytopenia developing after PCI may represent a medical emergency due to the combined risk of stent thrombosis and of major bleeding. Although abciximab is usually administered only during PCI or shortly thereafter, it remains in the blood stream for up to 2 weeks [Citation1, Citation9], adding to the anti-platelet effect of aspirin and P2Y12 blockers, and potentially worsening the risk of bleeding complications. This might be more significant with the new and more powerful agents, such as prasugrel and ticagrelor.
Management of severely thrombocytopenic patients with a recently positioned coronary stent may be challenging. Since abciximab is mostly bound to platelets with negligible amounts of free circulating drug [Citation1, Citation7], platelet transfusions are effective as emergency treatment [Citation8]. However, platelet transfusions do carry the risk of inducing thrombus formation in newly implanted coronary stents due to transient damage to the endothelial lining in the stent area. This is why ICCU monitoring for signs of myocardial ischemia was felt appropriate. High-dose steroids were also administered, although there is no clear indication of their effectiveness in drug-induced thrombocytopenia [Citation13], since it is impossible in the emergency setting to definitely rule out acute-onset primary immune-mediated thrombocytopenia. This choice is debatable; however, in the face of the severity of the clinical setting and considering the minor side effects of short-term steroid treatment, it was felt nevertheless appropriate.
Aspirin is seldom associated with development of thrombocytopenia, while so far this adverse reaction has not been reported with prasugrel. Re-challenge with aspirin and prasugrel did not result in recurrence of thrombocytopenia at follow-up, confirming the diagnosis of delayed abcximab-induced thrombocytopenia.
The patients presented underlie the need of strict post-discharge monitoring of platelet levels after PCI since, albeit often not recognized and asymptomatic, delayed abiciximab-induced thrombocytopenia may increase the bleeding risk of patients on DAPT.
Declaration of interest
The authors declare that they have no conflict of interest.
References
- Tamhane UU, Gurm HS. The chimeric monoclonal antibody abciximab: A systematic review of its safety in contemporary practice. Expert Opin Drug Saf 2008;7:809–819
- Sane DC, Damaraju LV, Topol EJ, Cabot CF, Mascelli MA, Harrington RA, Simoons ML, Califf RM. Occurrence and clinical significance of pseudothrombocytopenia during abciximab therapy. J Am Coll Cardiol 2000;36:75–83
- Berkowitz SD, Harrington RA, Rund MM, Tcheng JE. Acute profound thrombocytopenia after c7E3 Fab (abciximab) therapy. Circulation 1997;95:809–813
- Berkowitz SD, Sane DC, Sigmon KN, Shavender JH, Harrington RA, Tcheng JE, Topol EJ, Califf RM. Occurrence and clinical significance of trombocytopenia in a population undergoing high-risk percutaneous coronary revascularizatiCon. Evaluation of c7E3 for the Prevention of Ischemic Complications (EPIC) Study Group. JACC 1998;32:311–319
- Coller BS. Monitoring platelet GP IIb/IIIa (corrected) antagonist therapy. Circulation 1997;96:3828–3832
- Kereiakes DJ, Berkowitz SD, Lincoff AM, Tcheng JE, Wolski K, Achenbach R, Melsheimer R, Anderson K, Califf RM, Topol EJ. Clinical correlates and course of thrombocytopenia during percutaneous coronary intervention in the era of abciximab platelet glycoprotein IIb/IIIa blockade. Am Heart J 2000;140:74–80
- De Caterina R, Zimarino M. Understanding the complexity of abciximab-related thrombocytopenia. Thromb Haemost 2010;103:484–486
- Arnold DM, Nazi I, Warkentin TE, Smith JW, Toltl LJ, George JN, Kelton JG. Approach to the diagnosis and management of drug-induced immune thrombocytopenia. Transfus Med Rev 2013;27:137–145
- Curtis BR, Divgi A, Garritty M, Aster RH. Delayed thrombocytopenia after treatment with abciximab: A distinct clinical entity associated with the immune response to the drug. J Thromb Haemost 2004;2:985–992
- Webb GJ, Swinburn JM, Grech H. Profound delayed thrombocytopenia presenting 16 days after Abciximab (Reopro®) administration. Platelets 2011;22:302–304
- Trapolin G, Savonitto S, Merlini PA, Caimi MT, Klugmann S. Delayed profound thrombocytopenia after abciximab administration for coronary stenting in acute coronary syndrome. Case reports and review of the literature. Ital Heart J 2005;6:647–651
- Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2011;32:2999–3054
- Pedersen-Bjergaard U, Andersen M, Hansen PB. Drug-induced thrombocytopenia: Clinical data on 309 cases and the effect of corticosteroid therapy. Eur J Clin Pharmacol 1997;52:183–189

