Abstract
Magnetic seizure therapy (MST) is a novel neurotherapeutic intervention in development for the treatment of major affective disorders. Like other neurotherapeutic strategies such as electroconvulsive therapy (ECT) or transcranial magnetic stimulation (TMS), a primary interest will be to monitor the associated neurocognitive effects. Thus, the purpose of this systematic review was to synthesize the available data on the neurocognitive effects of MST. The authors performed two independent literature searches with the following terms terms: MST, magnetic, magnetic seizure therapy, depression, neurocognition, cognitive, preclinical. We included in this review a total of eleven articles that mentioned MST and neurocognition in the abstract. The articles were divided into three methodological domains that included virtual computer simulations, preclinical studies, and clinical investigations. Collectively, the available evidence suggests MST has little to no adverse cognitive effects. Specifically, virtual computer simulations found the magnetic field was localized to grey matter, and preclinical studies found no neurocortical or neurocognitive sequelae. Clinical investigations found MST to be associated with rapid reorientation and intact anterograde and retrograde memory. Future investigations using translational methods are warranted to confirm these findings and to further determine the effects of MST on neurocognitive functions.
Introduction
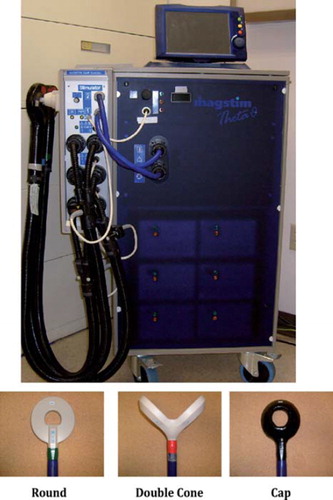
Magnetic seizure therapy (MST) is a novel neurotherapeutic intervention that is in development for the treatment of major affective disorders. The administration of MST has been described in detail elsewhere (CitationLisanby et al., 2003a; CitationWhite et al., 2006), but in brief it involves the use of a transcranial magnetic stimulation (TMS) device and coils (see ) to deliver a series of magnetic pulses at intensity levels sufficient for generating a seizure while a patient is under general anaesthesia. Different MST parameters that can be altered for treatment include the magnetic coil (configuration and placement), magnetic pulse (frequency, pulse-width, amplitude, duration), and dosage (threshold, suprathreshold). Similar to ECT, the MST parameters are integral to maximizing the ratio of efficacy to adverse effects. For example, ECT provided with right unilateral electrode placement, ultra-brief pulse width, and titrated dose results in high efficacy with low adverse cognitive effects, relative to other parameter configurations (CitationSackeim et al., 2008). A recent review (CitationHoy & Fitzgerald, 2010) highlighted that MST parameters do affect the provision of the treatment (e.g. more reliable seizure induction with 100 Hz than 50 Hz).
Figure 1. Magnetic seizure therapy device and coils. (A) Example of a device used to provide magnetic seizure therapy (Magstim Theta). (B) Examples of different coils used to provide magnetic seizure therapy including round, double cone, and cap.
The hypothesis of using magnetic pulses for the induction of therapeutic seizures appeared in the mid 1990s (CitationSackeim, 1994), but it was not until 2001 that the first case report was published that documented the feasibility and safety of MST (CitationLisanby et al., 2001b). In that study a patient diagnosed with major depressive disorder (MDD) was treated with four MST sessions (provided at 40 Hz either with a double cone coil or a figure-of-eight coil) that resulted in improved mood and no change in global cognitive function. Since that time, the development of MST has been platformed on translational research methods (CitationRowny et al., 2009) that integrate virtual computer simulated, preclinical, and clinical models in order to develop it as an efficacious convulsive therapy with little to no adverse effects, particularly in the domain of neurocognitive function.
Conceptually, MST is a hybrid of TMS and electroconvulsive therapy (ECT). The former neurotherapeutic modality produces an approximate 15% remission rate with minimal adverse effects (CitationGeorge et al., 2010; CitationJanicak et al., 2008), whereas the latter is one of the strongest antidepressants with an approximate 87% remission rate, but with moderate adverse effects (CitationHusain et al., 2004; CitationSemkovska & McLoughlin, 2010). Thus, the development of MST aims to combine the optimal antidepressant effects of ECT with the minimal adverse effect profile of TMS (CitationLisanby, 2002). Technical aspects of MST support that it may have benign cognitive effects. The magnetic field passes unimpeded through the scalp and skull, which allows for maximal efficiency and limiting of the magnetic field and resultant electrical field to select cortical areas (CitationLisanby et al., 2001a). The pulse has a dampened cosine shape and the pulsewidth is approximately 0.3ms (ultrabrief) (CitationLisanby et al., 2003c). Research with ECT has suggested that the pulsewidth, pulse shape, and spread of the electrical field indeed affect neurocognition (CitationPeterchev et al., 2010; CitationWeiner & Coffey, 1989; CitationWeiner et al., 1986).
The effects of neurotherapeutic interventions on neurocognitive outcomes has growingly received considerable attention (CitationMoreines et al., 2011). Research has found that ECT negatively affects neurocognitive function, particularly in the domains of orientation, anterograde memory, and retrograde memory (CitationPorter et al., 2008). For example, the negative effects on anterograde and retrograde memory can last up to 1 month and 6 months, respectively, after the last ECT session (CitationSackeim et al., 2007; CitationSemkovska & McLoughlin, 2010). Further, different ECT parameters have been associated with the degree of adverse effects (e.g. ultrabrief pulse width results in fewer negative cognitive effects relative to brief pulse width) (CitationLoo et al., 2008; CitationPrudic, 2008). Of recent note, the United States Food and Drug Administration (USFDA) is considering whether ECT should be classified as a Class 2 (general controls with special controls) or Class 3 (general controls with premarket approval) device due to its negative effects on neurocognition (CitationGoodman, 2011). Despite the antidepressant advantages conferred by ECT and its time-limited adverse cognitive effects, it is nonetheless limited by its side effects (CitationLisanby, 2007). Given that MST is currently in the developmental phase, it will be important to monitor its effects on neurocognition. Thus, the purpose of this systematic review was to synthesize the available data on the neurocognitive effects of MST.
Systematic literature review methods
To accomplish the systematic literature review, we (authors M.C. and O.T.) performed independent searches in the PsycInfo (1806–2011) and MEDLINE (1948–2011) databases with the following terms: MST, magnetic, magnetic seizure therapy, depression, neurocognition, cognitive, neuropsychology, preclinical. To control for duplicate information, the results of the two independent searches were imported into and managed with Endnote (version X4 for Windows, Thomson Reuters, Carlsbad, CA). We included only those articles in this systematic review that mentioned magnetic seizure therapy (or MST) and neurocognition (or one of the variants mentioned above) in the abstract, and provided original data. As such, we included a total of eleven studies (English language literature) in this review. These studies were published between the dates of 2003–2011, from the USA, UK and Germany, and the methodologies varied from computer virtual simulations to controlled clinical trials.
Neurocognitive effects of magnetic seizure therapy
The 11 studies (see ) found in the systematic literature review varied in terms of scientific methodology. Of the studies, two were based on computer virtual simulations, five provided data (neurohistological and neurocognitive) from preclinical models, and the remainder assessed the effects of MST on neurocognitive functions in controlled clinical investigations.
Table I. Virtual, preclinical, and clinical studies of the neurocognitive effects of magnetic seizure therapy.
Virtual computer simulation studies
Two studies have explored the focal propagation and field strength of MST compared to ECT in computer spherical head simulations. The layers of these virtual models simulate relative thicknesses and conductivities of scalp and skull, as well as variations of cranial volume. These studies elucidate the effect of unique anatomical variants on neurostimulation modalities, particularly ECT or MST (see ). This Virtual Head simulation model has been found to have spatial accuracy regarding electroencephalographic (EEG) and magnetoencephalographic (MEG) source localization (CitationDeng et al., 2009). Exploration of electric field strength and spatial distribution demonstrate how various subcortical structures are affected by neurotherapeutic interventions, which in turn may explain variations, either positive or negative, in cognitive effects for different neurotherapeutic modalities (CitationDeng et al., 2011).
Figure 2. Simulation models of electroconvulsive therapy electrode and magnetic seizure therapy coil configurations. (A) Bilateral ECT, (B) bifrontal ECT, (C) right unilateral ECT, (D) focal electrically administered seizure therapy, (E) circular coil MST, (F) cap coil MST, (G) double cone coil MST, and (H) interior of the five-layer spherical head model. Tissue layers from outer to inner shell: scalp, skull, cerebrospinal fluid, grey matter, and white matter. Reproduced with permission from Dr. Zhi De-Deng, 2011. ECT, electroconvulsive therapy; MST, magnetic seizure therapy.
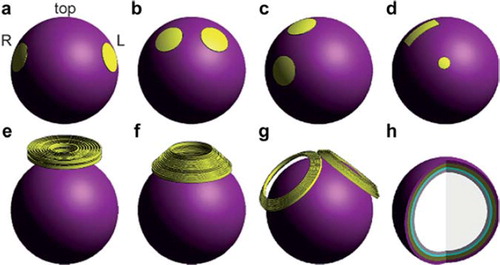
Deng et al. compared different ECT electrode configurations and MST coil types to see differing effects upon neural tissue for each stimulation modality. The ECT electrode configurations tested included: bitemporal (BT), bifrontal (BF), right unilateral (RUL), and focal electrically administered seizure therapy (FEAST). Regarding MST coil orientations, the following coil types were tested in the computer simulated mode: cap coil (inner diameter 68 mm; outer diameter 130 mm; with 15 turns), round circle (inner diameter 44 mm; outer diameter 120 mm; with 9 turns), and double cone (2 coils of inner diameter of 96 mm; outer diameter of 125 mm; with 7 turns positioned at 120 degrees relative to one another) (CitationDeng et al., 2011). The study by Deng et al. found stimulation strength in both grey and white matter were substantially higher in all forms of ECT relative to MST (CitationDeng et al., 2009). Brain volume stimulated by ECT was also much larger than MST, with 100% maximum volume stimulated with BL = bilateral ECT as compared to 21% of volume stimulated with MST (CitationDeng et al., 2009, Citation2011). Of importance, MST was found to only exert its stimulating effects primarily on cortical grey matter rather than deep white matter tracts. The opposite findings were noted for ECT (CitationDeng et al., 2009). This indicates that MST is generally more focal than ECT, regardless of the chosen electrode configuration, and may contribute to the differential effects between the two stimulation types on neurocognitive functions. In fact, MST can produce tonic-clonic seizures at stimulation intensities 3–6 times weaker, but with focality 10–60 times higher than ultra-brief pulse ECT administered at 800 mA (CitationDeng et al., 2011). Since the electrical field strength of MST stimulation is confined to and concentrated in the most superficial regions of the brain, and attenuates more rapidly in depth compared to ECT, it may spare the medial-temporal lobe structures, such as the hippocampus. Of the ECT electrode configurations studied, the slowest attenuation was observed in BL ECT, which also provided the strongest stimulation of subcortical structures (CitationDeng et al., 2011).
Regarding the anatomical variants studied in the virtual simulations, the scalp, skull thickness, and total brain volume resulted in the greatest field strength. The variant of head diameter had the least influence on field strength. Relative to ECT, MST stimulation strength was found to be less sensitive to variability of scalp, skull thickness and total brain volume (CitationDeng et al., 2009). This may be due to the differences in seizure induction between ECT and MST. For example, the electrical current used for seizure propagation is impeded by the scalp and skull; however, this is not the case for the magnetic current used for seizure propagation with MST. Of the coil types used in MST, the double cone coil induced the strongest and deepest electric field, and was the least affected by anatomical variation. The circle coil was observed to have the most superficial field strength with the smallest impact on overall brain volume (CitationDeng et al., 2011).
Although the virtual models provide information regarding the effects of ECT and MST treatment parameters on the brain, it is unclear at this time how these models will inform neurocognitive outcomes. Further, the authors noted that the virtual stimulation models were limited by subtle anatomical inaccuracies. In living human subjects tissue layers have uneven thicknesses, and conductivity is generally non-uniform within each tissue layer. Also, openings in the calvarium such as the orbital fissures and external auditory meatus can provide electrical shunting through pathways of low physical resistance. These virtual head models neglected cortical foldings and deeper, subcortical structures, which may attenuate focality and field strength for both MST and ECT. More anatomically accurate virtual models could inform the effects of stimulation on significant subcortical structures involved in neurocognitive functions (e.g. hippocampus), which may contribute to a better understanding of the side-effect profiles for both neurotherapeutic modalities (CitationDeng et al., 2009, Citation2011).
Pre-clinical study findings: Neurohistological
Preclinical studies have investigated the neurocortical and neurocognitive sequelae of ECT and MST. CitationDwork et al. (2004, Citation2009) performed two studies that explored histological changes in neurons and glia following ECT, MST or sham therapy. Subjects (Macaca mulatta) underwent procedures that were based on clinical treatment paradigms. As such, treatment sessions included general anaesthesia and oxygenation, and physiological monitoring. Formalin perfusion was performed prior to removal of the brain in order to avoid confounding from pharmacological effects, hypoxia, or damage artefact prior to histological evaluation (CitationDwork et al., 2004).
In the first study (CitationDwork et al., 2004), subjects (N = 12) were randomly assigned to receive either MST, ECT or sham (anaesthesia only) treatment. Of the pathological changes found, the majority occurred in those subjects randomized to the ECT condition. Specifically in the ECT condition, immunoreactivity to diffuse glial fibrillary acidic protein (GFAP) in the hippocampus, amygdala, and the frontal gyrus was most pronounced, indicating astrocytic activation throughout the neural parenchyma. In the MST condition, only one subject showed a region of microscopic cortical change in the parietal lobe, and in the sham condition, only one subject showed increased eosinophilia within the CA1 region of the hippocampus. The authors reported that the isolated changes were too acute, and thus were unrelated to the MST or the anaesthesia conditions. For instance, the changes observed in those subjects could have been related to artefacts from the histological procedures. This study showed how ECT and MST affect cortical regions, though it was unable to quantify the changes in neuronal number or finding lesions that occur infrequently due to the small cohort size (CitationDwork et al., 2004). To address these questions, the authors repeated the study in a larger cohort where they explored for specific changes in cortical regions important for neurocognitive functions.
The second study (CitationDwork et al., 2009) employed the same research conditions in 24 subjects who received MST, ECT or anaesthesia alone for 4 days a week over a 6-week period. The study found no effect of condition on neuronal or glial counts, densities, or volumes. Although all frontal and hippocampal subregions were evaluated for neural/glial volumes, densities and counts, only the evaluation of posterior-frontal glial density showed changes approaching statistical significance. In the region most sensitive to neuronal loss, the CA1 of the hippocampus, the differences in neuronal counts were still negligible.
Pre-clinical study findings: Neurocognitive
Seizure initiation site and pattern of activation can impact neurocognitive function. Magnetic seizure therapy has limited spreading of seizure activity, and fewer neuroanatomical changes in the dentate gyrus and hippocampus. Thus, MST is hypothesized to have little or no cognitive side effects. Two preclinical studies (CitationMoscrip et al., 2006; CitationSpellman et al., 2008) investigated the effects of MST compared to electroconvulsive shock (ECS), and anaesthesia-only conditions, on subjects’ (Macaca mulatta) performance on the Columbia University Primate Cognitive Profile (CUPCP) (CitationMoscrip et al., 2004), a touch-screen computerized neurocognitive test battery. The CUPCP was designed to measure those cognitive functions that are negatively affected by ECT including orientation, memory for newly (anterograde) and previously (retrograde) learned information, spatial working memory, and serial working memory (CitationSpellman et al., 2008).
CitationMoscrip et al. (2006) found overall that ECS resulted in greater impairments in both time to completion and accuracy of neurocognitive measures compared to MST or sham. For the measure of immediate learning and anterograde memory, the study found no differences in completion times or accuracy among MST, sham or baseline scores; but ECS resulted in longer completion times and decreased accuracy relative to those three conditions. Regarding performance on the serial learning test for recalling a newly learned list, mild deficits were noted for both MST and ECS relative to sham, although MST and ECS did not significantly differ from one another. For a serial learning test measuring recall memory for old lists, the ECS condition resulted in substantial impairment relative to sham and MST (CitationMoscrip et al., 2006).
CitationSpellman et al. (2008) followed the study of CitationMoscrip et al. (2006) to examine whether the neurocognitive effects of MST followed dose-dependent rules, and to examine the effects of ECS and MST on working memory for serial and spatial information. Spellman et al. followed the research method established in the Moscrip experiment, except the MST was delivered at 100 Hz for 10 s (total of 1000 pulses) with a round, pediatric-sized coil. Thus, the study compared moderate dose (MD-MST, 2.5 × seizure threshold) with high dose (HD-MST, 6 × seizure threshold) MST, as well as ECS and sham, in terms of neurocognitive outcome on the first three measures of the CUPCP.
The study (CitationSpellman et al., 2008) found no difference between the MD-MST and HD-MST conditions on time to complete neurocognitive measures. However, there was a difference in accuracy on two measures that required the subjects to recall previously learned information, with performance being better in the HD-MST condition relative to the MD-MST condition. The authors suggested that this could have been related to practice effects since two of the subjects had previously been exposed to the Columbia University Primate Cognitive Battery (CUPCB) stimuli. Relative to ECS, subjects showed quicker time to complete tasks in the HD-MST condition. Further, accuracy on measures of orientation, anterograde memory, and retrograde memory was significantly higher in the HD-MST condition.
In this study (CitationSpellman et al., 2008), the CUPCB was expanded and included two working memory measures, one for spatial information and the other for serial information. On the spatial working memory measure, subjects showed decreased accuracy in the ECS condition relative to sham, but there was no difference in performance between the ECS and HD-MST conditions. However, on the serial working memory measure, accuracy was significantly impaired in the ECS condition, relative to HD-MST and sham, with no difference between the latter two conditions.
CitationCycowicz et al. (2009) noted in an experiment that compared electroencephalographic (EEG) findings to the cognitive results of the CitationSpellman et al. (2008) study, that ictal power, as measured by EEG, was greater for ECS than MST at six times the seizure threshold. Accuracy on all CUPCP tasks were unaffected by EEG power, although time to completion of the orientation task was increased proportionally to EEG ictal power (CitationCycowicz et al., 2009). Magnetic seizure therapy administered at six times the seizure threshold produced seizure morphology that was distinct from that seen in ECS. This difference between treatment modalities indicates that some of the neurocognitive adverse effects observed with ECS may be related to ictal expression.
Clinical study findings
The clinical investigations of the neurocognitive effects of MST have primarily focused on the neurocognitive functions affected by ECT. These include reorientation after treatment, global cognitive function, and anterograde and retrograde memory. The research methods varied between one study that only examined the effects of MST in a case report (CitationKosel et al., 2003), and others contrasting the effects of MST and ECT in a case series (CitationKayser et al., 2011; CitationKirov et al., 2008; CitationLisanby et al., 2003a).
In a case report by CitationKosel et al. (2003), the authors reported the clinical and neurocognitive effects of a 66-year-old patient diagnosed with severe, recurrent, non-psychotic MDD with melancholic features treated with open-label MST. The investigators measured depression severity with the 21-item Hamilton Rating Scale of Depression (HRSD-21) (CitationHamilton, 1967) and the Beck Depression Inventory (BDI) (CitationBeck & Steer, 1987), while neurocognitive function was measured with a comprehensive battery of neuropsychological tests. The battery included the Münchner Verbaler Gedächtnistest (a measure similar to the California Verbal Learning Test (CVLT) (CitationDelis et al., 1987)) that assesses verbal learning and memory, and the Rey Visual Design and Learning Test (CitationRey, 1964) to assess non-verbal learning and memory. These measures were administered 35 days before the first MST treatment and 1 week after completion of the acute MST course. The study employed an adapted version of the Treatment Effects Battery (TEB) (CitationSackeim et al., 1986), which consists of various brief measures (i.e. verbal fluency, sentence recognition) in order to assess immediate, post-ictal neurocognitive effects and time to orientation. The time to orientation measure of the TEB, which assesses orientation to name, place, day of week, age, and date of birth was administered after each MST session. The complete TEB was administered immediately after MST treatment sessions 1, 4, 8, 10 and 12.
The MST stimulus was generated through a custom magnetic stimulator (Magstim Super Rapid, Whitland, Carmarthenshire) and delivered through a standard butterfly coil with an outer diameter of 13 cm. The coil was placed on the vertex of the patient's skull when providing treatment. Stimulation frequency and duration were set to 50 Hz and 8 s, respectively. The pulse had a dampened cosine waveform with a pulse width twice as wide as the unmodified rTMS device by the same manufacturer. Anaesthesia consisted of 0.5–1 mg alfentanil and 0.33–0.48 mg/kg etomidate, and muscle relaxation was accomplished with 0.93–1.4 mg/kg succinylcholine.
CitationKosel et al. (2003) reported several findings from this study, primarily that the patient's depression remitted following 12 MST sessions. Other significant findings included that the patient recovered on average within 5 min. This recovery time is significantly shorter relative to unilateral low, moderate, and high dosage ECT, as well as bilateral high dosage ECT. The patient's post treatment verbal learning and memory performance on the Münchner Verbaler Gedächtnistest significantly improved after MST treatment compared to baseline. However, the authors did not provide a rationale as to why there was improvement in neurocognitive test performance one week after the last MST session.
A case series by CitationLisanby et al. (2003a) compared and contrasted the neurocognitive effects of MST and ECT. The study enrolled 10 patients diagnosed with MDD who were treated with an acute course of ECT. The two initial treatments were provided to determine seizure threshold for ECT as well as MST, followed by two sessions of either suprathreshold ECT or MST. Electrode placement for ECT varied with nine patients undergoing right-unilateral (RUL) ECT and the tenth patient undergoing bifrontotemporal (BL) ECT. The ECT pulse width was 0.5 ms and treatment was provided at six times the seizure threshold for RUL ECT and 2.5 times the seizure threshold for BL ECT. After the fourth treatment session, ECT was provided for all subsequent treatments. Both ECT and MST were dose-titrated (using the empirical titration method) on the first session, followed by suprathreshold stimulation during subsequent treatment sessions. MST stimulus parameters were set at 60 Hz for 6.6 s, and treatment was provided using three different coil types including a figure-of-eight coil (7 cm diameter), double cone coil (9 and 12 cm), and a round coil (9 cm). The coils were also placed at three different sites including RUL-site F6, midline frontal site, and the vertex site.
The authors utilized a comprehensive neuropsychological battery to assess cognition in the patients undergoing neurostimulation treatments. To assess anterograde memory, the investigators utilized several measures including the TEB, Squire Memory Test (Sentences) (CitationShimamura & Squire, 1987), the Buschke Selective Reminding Test (CitationBuschke & Fuld, 1974), and a Complex Figure Test.
The TEB was administered prior to and following each ECT or MST session. Patients were instructed to memorize 12 words, eight shapes (four geometrical, four nonsensical), and eight neutral faces. The retention of these learned stimuli was immediately tested after each treatment session through recall or recognition of test stimuli. The study found that overall performance on neurocognitive tests was significantly better after MST compared to ECT. For both the threshold titration and supratheshold conditions, patients recovered and oriented more quickly after MST relative to ECT. Specifically, in the threshold condition, patients recognized a greater number of affective faces after MST. In the suprathreshold condition, patients recognized more neutral faces and more sentences from the Squire Memory Test, as well as exhibited superior category fluency following MST. However, recall of the Complex Figure Test after a 20-min delay was better following ECT (CitationLisanby et al., 2003a).
While the above studies administered MST at a moderate dose (50 Hz), a case series by CitationKirov et al. (2008) evaluated the neurocognitive effects of MST administered at 100 Hz (high-dose). As the neurocognitive effects of ECT are related in part to the stimulus dose with higher doses conferring increased adverse effects, the investigators sought to examine the safety of high dose MST. The study enrolled 11 patients diagnosed with MDD who were already undergoing ECT. Eight of these patients started their ECT course, during which each received one MST treatment in place of an ECT treatment. The treatment of the other three patients commenced with MST before they received ECT. The parameters for MST treatment were set at 100 Hz for a duration of up to 10 s with a brief pulse (340–400 μs). A round coil was utilized with an internal diameter of 47 mm and external diameter of 115 mm. The coil was positioned using standard 10–20 electroencephalogram (EEG) positions. The anaesthesia regimen administered to patients included intravenous etomidate dosed at 0.15 to 0.3 mg/kg and intravenous succinylcholine to induce muscle relaxation.
A significant finding of this study was that patients reorientated on average within 7 min after treatment with MST compared to the mean reorientation time of approximately 26 min following ECT. The study investigated the effects on several cognitive domains including verbal and visual memory, verbal fluency and psychomotor speed; however, the results were not reported.
Another study conducted by CitationKayser et al. (2011) investigated the antidepressant efficacy of combined MST and pharmacotherapy in patients with treatment resistant depression (TRD). Twenty patients with TRD were enrolled to receive 12 treatments of either MST or ECT. MST treatment parameters were set at 100 Hz with a train duration of up to 6 s, depending on the seizure threshold of each patient. A twin coil (a custom-made coil that combined two round coils (13 cm diameter)) was used to administer treatments. The ECT stimulus parameters were set at a brief pulse, with dose-titrated sessions and electrode placement following the RUL configuration. All patients underwent general anaesthesia with intravenous propofol ranging from 1.5 to 2.5 mg/kg. Muscle relaxation was induced via intravenous succinylcholine ranging from 1 to 1.5 mg/kg.
Several domains of cognition were assessed including orientation, language, processing speed, executive function, learning and memory at baseline, at treatments 1, 4, 8 and 12 as well as 4 weeks after the final treatment. Orientation was assessed by asking the patient for his or her name, date of birth, age, place, and day of the week. This was asked immediately after the patient began breathing independently. Further neuropsychological testing was then conducted 4 h after the patient had orientated. The measures employed included the TEB and the Logical Memory subtest from the Wechsler Memory Scale (WMS; specific edition of the WMS used was not stated). The only significant difference found in the study was that MST patients’ performance was superior to their ECT counterparts on the geometrical shapes portion of the Visual Neglect Test.
Summary of findings and discussion
Prior investigations of MST have found it to have little to no adverse neurocognitive effects. Converging evidence from virtual computer simulated models, preclinical, and clinical studies suggest that MST does not result in substantive impact on cortical or subcortical structures, which in turn allows for preservation of cognitive abilities. Data from virtual models showed that MST stimulation affected 21% of total brain volume and was limited only to grey matter. Preclinical data showed that MST had little impact on cortical areas and cognitive functions, even when administered at suprathreshold levels. The latter findings were also observed in the clinical investigations. Collectively, these data from different study methodologies provide support for the safety of MST.
The virtual and preclinical studies of MST help to explicate the underlying neurocircuitry that subserves cognitive functions, particularly memory. With the introduction of MST, experiments were able to compare the neurocognitive effects of different modalities of seizure induction, thereby discerning the between the effects of the seizure and its mode of induction (e.g. electric, magnetic). The cognitive profile associated with ECT is disorientation immediately after the treatment, and anterograde and retrograde amnesia following the treatment (CitationSemkovska & McLoughlin, 2010). The available data indicate that MST does not produce the same cognitive profile (see ) as ECT, which suggests that the mode of seizure induction rather than the seizure itself may be associated with adverse cognitive effects.
Table II. Comparing magnetic seizure therapy and electroconvulsive therapy effects on neurocognitive functions.
The clinical studies suggested that MST did not impact neurocognitive performance. Specifically, when administered at moderate dose (∼50 Hz), patients showed quick recovery of orientation and stable or improved performance on neurocognitive measures. Even when administered at a high dose (100 Hz), patients showed rapid recovery of orientation and preservation of cognitive abilities. However, methodological variability between the clinical investigations that included different MST parameters, treatment course length, and neuropsychological measures, prevent firm conclusions. For example, in the CitationLisanby et al. (2003a) study, only two MST sessions were administered at moderate dose with different coil configurations, whereas the patients in the CitationKayser et al. (2011) completed 12 treatments at high dose with a twin coil. Research on ECT has found an association between stimulus dose, treatment course length, electrode configuration, and neurocognitive effects (CitationMcCall et al., 2000; CitationSackeim et al., 2007). Thus, further examination of different MST parameters and treatment algorithms is needed to evaluate the effects, if any, on neurocognitive function.
Regarding neuropsychological measures, the clinical studies included at minimum a measure of orientation, one study (CitationLisanby et al., 2003a) only measured cognitive effects immediately after MST with the TEB, and two studies (CitationKayser et al., 2011; CitationKosel et al., 2003) utilized the TEB and additional cognitive measures to assess postictal and end of treatment effects. As such, there was limited information regarding the effects on other cognitive domains such as executive function, working memory, and processing speed which are interrelated with memory functions (CitationReid & Krasnegor, 1996). Future studies should employ neurocognitive measures that assess multiple domains to comprehensively examine neurocognitive effects, are psychometrically sound with available demographic-adjusted normative scores, and have equivalent alternate forms to prevent practice effects. Also, future studies are needed to replicate and extend these early findings (e.g. effects on visuospatial reproduction) to determine the neurocognitive profile of MST, and to establish whether the profile mimics or is different than ECT.
In conclusion, the available studies that examined the neurocognitive effects of MST found it to be a cognitively safe neurotherapeutic intervention. The evidence also suggests that MST and ECT are different in terms of their seizure propogation and focality, two variables that may account for their respective differential neurocognitive effects. Further research with translational methods that combines virtual computer simulations with preclinical and clinical investigations is warranted to comprehensively characterize the neurocognitive effects on other domains such as working memory and executive function, and then to link those effects to the underlying neurocircuitry. Such investigations would aid in the development, and help to confirm and establish MST as a cognitively benign therapeutic modality to treat severe mood disorders.
Take home points
(1) Magnetic seizure therapy (MST) does not appear to negatively impact cortical and subcortical areas that govern neurocognitive functions.
(2) Available preclinical and clinical evidence suggest that magnetic seizure therapy (MST) does not adversely impact neurocognitive functions.
Future directions
(1) The development of magnetic seizure therapy (MST) would benefit from continued research combining multimodal methods to inform any effects on cortical and subcortical areas, and neurocognitive functions.
(2) Large scale clinical investigations are warranted to determine the neurocognitive effects of magnetic seizure therapy treatment parameters including dosing algorithms and coil configurations.
Acknowledgements
We thank Zhi-De Deng for providing us with to use in this systematic review.
Declaration of interest: Shawn McClintock reports research grant support from the National Institutes of Health (NIH) grant number K23 MH087739, National Center for Research Resources (NCRR), and National Alliance for Research on Schizophrenia and Depression (NARSAD). Mustafa Husain reports research grant support from the NIH, the Stanley Foundation (grant number 05T-682), Cyberonics, Neuronetics, St Jude Medical and Magstim. The authors alone are responsible for the content and writing of the paper.
References
- Beck, A.T. & Steer, R.A. (1987). Beck Depression Inventory. San Antonio, TX: Psychological Corporation.
- Buschke, H. & Fuld, P.A. (1974). Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology, 24, 1019–1025.
- Cycowicz, Y.M., Luber, B., Spellman, T. & Lisanby, S.H. (2009). Neurophysiological characterization of high-dose magnetic seizure therapy: Comparisons with electroconvulsive shock and cognitive outcomes. Journal of ECT, 25, 157–164.
- Delis, D.C., Kramer, J.H., Kaplan, E. & Ober, B.A. (1987). The California Verbal Learning Test: Research Edition. San Antonio, TX: Psychological Corporation.
- Deng, Z.D., Lisanby, S.H. & Peterchev, A.V. (2009). Effect of anatomical variability on neural stimulation strength and focality in electroconvulsive therapy (ECT) and magnetic seizure therapy (MST). Conference Proceedings of the IEEE Engineering in Medicine and Biology Society, 2009, 682–688.
- Deng, Z.D., Lisanby, S.H. & Peterchev, A.V. (2011). Electric field strength and focality in electroconvulsive therapy and magnetic seizure therapy: A finite element study. Journal of Neural Engineering, 8, 1–13.
- Dwork, A.J., Arango, V., Underwood, M., Ilievski, B., Rosoklija, G., Sackeim, H.A. & Lisanby, S.H. (2004). Absence of histological lesions in primate models of ECT and magnetic seizure therapy. American Journal of Psychiatry, 161, 576–578.
- Dwork, A.J., Christensen, J.R., Larsen, K.B., Scalia, J., Underwood, M.D., Arango, V., … Lisanby, S.H. (2009). Unaltered neuronal and glial counts in animal models of magnetic seizure therapy and electroconvulsive therapy. Neuroscience, 164, 1557–1564.
- George, M.S., Lisanby, S.H., Avery, D., McDonald, W.M., Durkalski, V., Pavlicova, M., … Sackeim, H.A. (2010). Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: A sham-controlled randomized trial. Archives of General Psychiatry, 67, 507–516.
- Goodman, W.K. (2011). Electroconvulsive therapy in the spotlight. New England Journal of Medicine, 364, 1785–1787.
- Hamilton, M. (1967). Development of a rating scale for primary depressive illness. British Journal of Social Clinical Psychology, 6, 278–296.
- Hoy, K.E. & Fitzgerald, P.B. (2010). Introducing magnetic seizure therapy: A novel therapy for treatment resistant depression. Australian and New Zealand Journal of Psychiatry, 44, 591–598.
- Husain, M.M., Rush, A., Fink, M., Knapp, R., Petrides, G., Rummans, T., … Kellner, C.H. (2004). Speed of response and remission in major depressive disorder with acute Electroconvulsive therapy (ECT): A consortium for research in ECT (CORE) report. Journal of Clinical Psychiatry, 65, 485–491.
- Janicak, P.G., O'Reardon, J.P., Sampson, S.M., Husain, M.M., Lisanby, S.H., Rado, J.T., … Demitrack, M.A. (2008). Transcranial magnetic stimulation in the treatment of major depressive disorder: A comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. Journal of Clinical Psychiatry, 69, 222–232.
- Kayser, S., Bewernick, B.H., Grubert, C., Hadrysiewicz, B.L., Axmacher, N. & Schlaepfer, T.E. (2011). Antidepressant effects of magnetic seizure therapy and electroconvulsive therapy in treatment-resistant depression. Journal of Psychiatric Research, 45, 569–576.
- Kirov, G., Ebmeier, K.P., Scott, A.I.F., Atkins, M., Khalid, N., Carrick, L., … Lisanby, S.H. (2008). Quick recovery of orientation after magnetic seizure therapy for major depressive disorder. British Journal of Psychiatry, 193, 152–155.
- Kosel, M., Frick, C., Lisanby, S.H., Fisch, H.-U. & Schlaepfer, T.E. (2003). Magnetic seizure therapy improves mood in refractory major depression. Neuropsychopharmacology, 28, 2045–2048.
- Lisanby, S.H. (2002). Update on magnetic seizure therapy: A novel form of convulsive therapy. Journal of ECT, 18, 182–188.
- Lisanby, S.H. (2007). Electroconvulsive therapy for depression. New England Journal of Medicine, 357, 1939–1945.
- Lisanby, S.H., Gutman, D., Luber, B., Schroeder, C. & Sackeim, H.A. (2001a). Sham TMS: Intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biological Psychiatry, 49, 460–463.
- Lisanby, S.H., Luber, B., Schlaepfer, T.E. & Sackeim, H.A. (2003a). Safety and feasibility of magnetic seizure therapy (MST) in major depression: Randomized within-subject comparison with electroconvulsive therapy. Neuropsychopharmacology, 28, 1852–1865.
- Lisanby, S.H., Morales, O., Payne, N., Kwon, E., Fitzsimons, L., Luber, B., … Sackeim, H.A. (2003b). New developments in electroconvulsive therapy and magnetic seizure therapy. [Original]. CNS Spectrums, 8, 529–536.
- Lisanby, S.H., Moscrip, T., Morales, O., Luber, B., Schroeder, C. & Sackeim, H.A. (2003c). Neurophysiological characterization of magnetic seizure therapy (MST) in non-human primates. Supplements to Clinical Neurophysiology, 56, 81–99.
- Lisanby, S.H., Schlaepfer, T.E., Fisch, H.-U. & Sackeim, H.A. (2001b). Magnetic seizure therapy of major depression. Archives of General Psychiatry, 58, 303–305.
- Loo, C.K., Sainsbury, K., Sheehan, P. & Lyndon, B. (2008). A comparison of RUL ultrabrief pulse (0.3 ms) ECT and standard RUL ECT. International Journal of Neuropsychopharmaco logy, 11, 883–890.
- McCall, W.V., Reboussin, D.M., Weiner, R.D. & Sackeim, H.A. (2000). Titrated moderately suprathreshold vs fixed high-dose right unilateral electroconvulsive therapy: Acute antidepressant and cognitive effects. Archives of General Psychiatry, 57, 438–444.
- Moreines, J.L., McClintock, S.M. & Holtzheimer, P.E III. (2011). Neuropsychological effects of neuromodulation techniques for treatment-resistant depression: A review. Brain Stimulation, 4, 17–27.
- Moscrip, T.D., Terrace, H.S., Sackeim, H.A. & Lisanby, S.H. (2004). A primate model of anterograde and retrograde amnesia produced by convulsive treatment. Journal of ECT, 20, 26–36.
- Moscrip, T.D., Terrace, H.S., Sackeim, H.A. & Lisanby, S.H. (2006). Randomized controlled trial of the cognitive side-effects of magnetic seizure therapy (MST) and electroconvulsive shock (ECS). International Journal of Neuropsychopharmacology, 9, 1–11.
- Peterchev, A.V., Rosa, M.A., Deng, Z.-D., Prudic, J. & Lisanby, S. (2010). Electroconvulsive therapy stimulus parameters: Rethinking dosage. Journal of ECT, 26, 159–174.
- Porter, R., Heenan, H. & Reeves, J. (2008). Early effects of electroconvulsive therapy on cognitive function. Journal of ECT, 24, 35–39.
- Prudic, J. (2008). Strategies to minimize cognitive side effects with ECT: Aspects of ECT technique. Journal of ECT, 24, 46–51.
- Reid, L.G. & Krasnegor, N.A. (1996). Attention, Memory, and Executive Function. Baltimore, MD: Brookes.
- Rey, A. (1964). L'Examen Clinique en Psychologie. Paris: Presse Universitaire de France.
- Rowny, S.B., Benzl, K. & Lisanby, S.H. (2009). Translational development strategy for magnetic seizure therapy. Experimental Neurology, 219, 27–35.
- Sackeim, H.A. (1994). Magnetic stimulation and ECT. Convulsive Therapy, 10, 255–258.
- Sackeim, H.A., Portnoy, S., Neeley, P., Steif, B.L., Decina, P. & Malitz, S. (1986). Cognitive consequences of low-doseage electroconvulsive therapy. Annals New York Academy of Sciences, 462, 326–340.
- Sackeim, H.A., Prudic, J., Fuller, R., Keilp, J., Lavori, P.W. & Olfson, M. (2007). The Cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology, 32, 244–254.
- Sackeim, H.A., Prudic, J., Nobler, M.S., Fitzsimons, L., Lisanby, S.H., Payne, N., … Devanand, D.P. (2008). Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimulation, 1, 71–83.
- Semkovska, M. & McLoughlin, D.M. (2010). Objective cognitive performance associated with electroconvulsive therapy for depression: A systematic review and meta-analysis. Biological Psychiatry, 68, 568–577.
- Shimamura, A.P. & Squire, L.R. (1987). A neuropsychological study of fact memory and source amnesia. Journal of Experimental Psychology: Learning, Memory, and Cognition, 13, 464–473.
- Spellman, T., McClintock, S.M., Terrace, H., Luber, B., Husain, M.M. & Lisanby, S.H. (2008). Differential effects of high-dose magnetic seizure therapy and electroconvulsive shock on cognitive function. Biological Psychiatry, 63, 1163–1170.
- Weiner, R.D. & Coffey, C.E. (1989). Comparison of brief-pulse and sine wave ECT stimuli. Convulsive Therapy, 5, 184–185.
- Weiner, R.D., Rogers, H.J., Davidson, J.R.T. & Squire, L.R. (1986). Effects of stimulus parameters on cognitive side effects. Annals of the New York Academy of Sciences, 462, 315–325.
- White, P.F., Amos, Q., Zhang, Y., Stool, L., Husain, M.M., Thornton, L., … Lisanby, S.H. (2006). Anesthetic considerations for magnetic seizure therapy: A novel therapy for severe depression. Anesthesia and Analgesia, 103, 76–80.