Abstract
Diet in early infancy has an impact on early growth and the formation of flavour preferences, as well as on later life health outcomes. Although breast milk is the preferred source of nutrition during infancy, more than half of American infants receive infant formula by the age of 4 months. As a group, formula-fed infants weigh more by the age of one year and have a greater risk for later obesity than breastfed infants. However, a recent randomized study found that, when compared to breastfed infants, infants fed an extensively hydrolysed protein formula (ePHF) had more normative weight gain velocity than infants fed cow's milk formula (CMF). Therefore, grouping all formula-fed infants together with respect to certain health outcomes such as obesity may not be appropriate. Scientific evidence also suggests that there are sensitive periods for flavour learning. Infants become familiar with and learn to accept the flavours they experience through their mother's amniotic fluid and breast milk as well as formula. These early experiences influence flavour preferences of children that may affect food choices and therefore later life health. Further research on the influence of early diet on growth, flavour preferences, and food choices is imperative.
Introduction
Diet composition and subsequent growth during infancy affect later-life health outcome (CitationBarker, 2004; CitationLucas, 1998, Citation2005b). In particular, rapid rates of growth during the first year of life are associated with increased risk for later obesity (CitationBaird et al., 2008; CitationChomtho et al., 2009; CitationDennison et al., 2006; CitationEid, 1970; CitationMelbin & Vuille, 1976; CitationOng et al., 2009; CitationParsons et al., 2001; CitationStettler et al., 2002, Citation2003), metabolic syndrome (CitationEkelund et al., 2007), and mortality from cardiovascular disease (CitationBarker, 1997), leading some to argue that early life should be the focus for both preventive intervention and further scientific inquiry into body weight control (CitationEkelund et al., 2007; CitationGluckman & Hanson, 2008; CitationLucas, 2005a).
The notion that there are sensitive periods (sometimes called ‘critical periods’) when the organism is particularly susceptible to long-term effects of various sorts of environmental influences, or triggers, has been the focus of research for centuries. In particular, several animal model studies and some work in humans provide convincing evidence for a sensitive period during early life that influences a variety of metabolic, developmental, and pathological processes in later life (CitationLucas, 1990). For example, the ability of the young child to learn languages more rapidly and with greater facility than older (post-pubertal) individuals has been attributed to the existence of a sensitive period, probably related to neurological development (CitationHurford, 1991). That nutrition is a key environmental influence that acts on the genome during sensitive periods during ontogeny is suggested by the findings that brief periods of dietary manipulations, as well as diet in early infancy, can influence a variety of processes, including lipid and carbohydrate metabolism, blood pressure, and cognitive development (CitationLucas, 1998; CitationMcCance, 1962; CitationMott et al., 1991; CitationSinghal et al., 2003, Citation2004).
Since early diet can programme risks for later obesity and other chronic diseases (CitationGluckman & Hanson, 2008; CitationLucas, 1990), research has aimed at determining the mechanisms underlying growth differences as well as how early diet influences food and flavour likes and dislikes. In this chapter we review scientific evidence that suggests that there are age-related changes in functional plasticity, or sensitive periods, for growth and flavour learning, both of which provide the foundation for long-term health and food habits. We also focus on recent studies that examined how diets of different macronutrient composition affect growth and flavour learning and why some formula-fed infants differ substantially from infants who are breastfed, the gold standard of infant feeding. It is imperative that we understand the influence of infant formula composition on growth and subsequent diet, given that more than 50% of American infants receive infant formula while in hospital (either exclusively or as a supplement to breast milk), and this percentage increases steadily to more than 60% by 4 months of age (CitationGrummer-Strawn et al., 2008).
Sensitive periods in growth
An environmental trigger for early growth is diet. Infants who are fed infant formula, the vast majority of whom are fed a cow's milk formula (CMF) (CitationMartinez & Ballew, 2011; CitationOliveira et al., 2010), tend to weigh more and have a greater risk for later obesity than do infants who are breastfed (CitationArmstrong & Reilly, 2002; CitationBurke et al., 2005; CitationGrummer-Strawn & Mei, 2004; CitationOwen et al., 2005; CitationDewey et al., 1993). However, formula-fed infants are not a homogeneous group since infants randomized to feed one type of formula exhibited growth rates comparable to that of breastfed infants, whereas those randomized to feed another type of formula, although isocaloric, exhibited faster rates of growth (CitationMennella et al., 2011b).
Not all formulas are alike in macronutrient composition
Globally, several categories of infant formula are available. Among formulas for healthy term infants, one of the main distinctions is their protein source and/or degree of protein hydrolysis. CMF is the most common formula consumed by infants, accounting for 80% of all US infant formula sales (CitationOliveira et al., 2010). Its protein source is cow's milk, which usually includes combinations of intact casein and whey proteins (CitationCommittee on Nutrition, 2009b). Soy-based formulas account for 14% of infant formula sales (CitationOliveira et al., 2010); its protein source is intact soy protein isolate (CitationCommittee on Nutrition, 2009b)and these formulas are typically lactose free. Protein hydrolysate formulas (PHFs) account for 6 - 7% of all US infant formula sales (CitationOliveira et al., 2010). These formulas contain protein that has been enzymatically treated (cleaving the peptide bonds) to yield smaller peptides, as opposed to intact long-chain proteins (CitationCommittee on Nutrition, 2009b). Some hydrolysate formulas contain extensively hydrolysed protein (ePHF), meaning that most of the nitrogen is in the form of free amino acids (FAAs) and peptides < 1,500 kDa (CitationCommittee on Nutrition, 2000), whereas other hydrolysate formulas contain partially hydrolysed protein (pPHF), which provide protein mainly in the form of peptides; pPHF has higher concentrations of intact proteins (1,000–100,000 times higher) than does ePHF (CitationCommittee on Nutrition, 2000).
A primary nutritional role of protein in infant formula is to support infant growth by supplying appropriate amounts of essential and semi-essential amino acids (CitationCommittee on Nutrition, 2009b). Infants have a greater requirement for essential amino acids than do adults because of their rapid rates of growth, and certain amino acids are considered semi-essential during infancy because the metabolic pathways needed to create these amino acids are not fully developed (CitationChesney et al., 1998; CitationCommittee on Nutrition, 2009c). The US Federal Food, Drug, and Cosmetic Act (CitationUSFDA, 2004) provides guidance on the minimum and maximum protein concentration of US infant formula. Globally, the World Health Organization/Food and Agriculture Organization (WHO/FAO) and European Union also provide guidance on infant formula composition via the Codex Standard for Infant Formulas (CitationCodex Alimentarius Commission, 1981), and the European Commission Regulation (CitationEU Commission, 2006), respectively. These authoritative recommendations specify minimum and maximum protein and amino acid concentration for infant formula and follow-on infant formula.
Differences in protein source, degree of hydrolysis, and manufacturing processes lead to large differences in quantities and qualities of FAAs, with ePHF having the highest concentrations of FAAs and most diverse FAA profile (CitationVentura et al., in press). The total protein concentration of infant formula is the sum of true protein (intact proteins composed of amino acids) and non-protein nitrogen (low-molecular-weight compounds such as small peptides, FAAs, urea, uric acid, ammonia, creatinine, creatine) (CitationDonovan & Lonnerdal, 1989). FAAs in breast milk are thought to confer beneficial physiologic effects. For example, glutamic acid, the most predominant FAA in human milk (CitationDonovan & Lonnerdal, 1989), is proposed to play a role in zinc absorption (CitationMay et al., 1982); and taurine, the second most abundant FAA in human milk (CitationDonovan & Lonnerdal, 1989), is thought to support central nervous system function by aiding in bile acid conjugation (CitationVessey, 1978). We hypothesize that differences in the quantity of FAAs found in human milk and infant formulas play a role in differential infant growth.
Not all infant formulas are alike in terms of growth
Differences in total protein concentration of infant formula affect growth. Prospective randomized trials have shown that infants receiving a higher-protein cow's milk formula have greater weight gain or greater weight-for-age z-scores than do infants receiving a lower-protein cow's milk formula (CitationAxelsson et al., 1989; CitationKoletzko et al., 2009b; CitationRaiha et al., 1986). It has been hypothesized (CitationKoletzko et al., 2009a) that the higher total protein concentration of cow's milk infant formula relative to human milk leads to increased concentrations of circulating plasma amino acids; increased plasma concentrations of insulin-releasing amino acids are thought to stimulate insulin and insulin-like growth factor 1 (IGF-1) secretion, resulting in greater weight gain and greater adipogenic activity (CitationKoletzko et al., 2009a). Some randomized trials did not find a difference in weight gain when infants were fed formulas of differing protein composition (CitationJanas et al., 1987; CitationPicone et al., 1989; CitationTurck et al., 2006); however, these studies had smaller differences in protein concentration between the formulas, as well as a smaller sample size, and therefore may not have had the power to detect growth differences.
Emerging research suggests that the form of protein may be as important as the amount of protein in the diet when it comes to growth. Evidence for this hypothesis comes from three clinical trials, two that enrolled healthy infants with a family history of atopic disease (FHA) (CitationRoche et al., 1993; CitationRzehak et al., 2009) and one that enrolled healthy infants with no FHA (CitationMennella et al., 2011b) (see ). In these studies, infants were randomized at or shortly after birth to feed PHF (pPHF or ePHF) or CMF for the first 4 (CitationRzehak et al., 2009), 6 (CitationRoche et al., 1993b), or 7.5 (CitationMennella et al., 2011b) months of life. However, in the Rzehak et al. study (2009), many infants were not exposed to the study formula until 5 or 6 weeks after birth, and breastfeeding as well as postponing the introduction of solid foods until after the end of the fourth month were recommended for all infants. Despite these differences in experimental designs, findings were consistent: infants randomized to be fed either pPHF or ePHF gained less weight during the first year of life than did infants randomized to feed CMF. In the study by Mennella et al, length-for-age z-scores and linear growth velocity did not differ between groups (see ), indicating that growth differences were attributable to differences in gains in weight, not length, across the study period (CitationMennella et al., 2011b). The rate of weight gain of ePHF infants was comparable to national norms for breastfed infants (CitationMennella et al., 2011b). In contrast, infants fed CMF gained weight at a faster rate than breastfed infants, a finding consistent with epidemiological studies (CitationDewey et al., 1992; CitationKramer et al., 2004). Two other trials failed to find growth differences; both of these studies were of shorter duration (< 4 months) but reported that overall the CMF-fed infants consumed more formula than did the ePHF-fed infants (CitationHyams et al., 1995; CitationVandenplas et al., 1993), suggesting that formula composition (protein, FAAs) affects satiation. Thus, formula-fed infants are not a homogeneous group (CitationMennella et al., 2011b) – the form of the protein provided to the infant is also important.
Figure 1. Infant weight-for-length z-score trajectories from birth to 7.5 months by formula group. Z-scores were calculated using WHO growth standards. The arrow (↓) indicates the age at which infants were randomized to either CMF (Enfamil; circles) or ePHF (Nutramigen; triangles). A z-score of 0 is considered normative, and z-score tracking is a clinical indicator of normative growth. ↓Significant differences between groups; groups differed significantly at P < 0.05 in the post hoc comparison. Reproduced with permission from Pediatrics, 127, pp. 110–118, Copyright 2011 by the AAP.
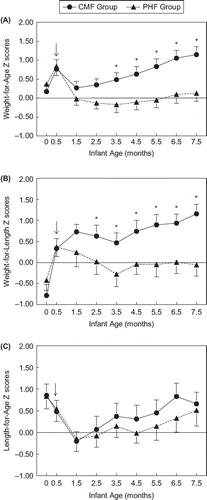
Table 1. Summary of growth studies in which infants were randomized to receive CMF or PHF.
The mechanism or mechanisms by which feeding ePHF results in growth normative to breastfed infants remain unknown. We suggest that there are several alternative, not mutually exclusive, hypotheses for the observed growth differences. First, infant feeding behaviours and consequent growth may differ because of the sensory characteristics of the formulas. To adults, protein hydrolysate formulas have a distinctive, unpleasant flavour (taste and odour) because the hydrolysis results in high levels of FAAs and small peptides, which taste sour, bitter, and savory, and elicits unpleasant sulphur-based odours (CitationCook & Sarett, 1982; CitationMennella & Beauchamp, 2005; CitationSchiffman & Dackis, 1975). It is possible that infants may dislike the taste of ePHF and consequently consume less, which in turn leads to slower weight gain. However, there is little evidence that negative sensory properties of food or beverages alone can result in decreased growth during infancy. For example, in animal models, total food intake and the growth efficiency of weanling rats were not affected by feeding a solid-food diet adulterated with aversive tastes (CitationMennella et al., 2004; CitationNaim et al., 1980). Moreover, infants who are introduced to ePHF during the first 3 months of life, a sensitive period for flavour learning, accept ePHF formula throughout infancy (see below) (CitationMennella et al., 2004). When infants were introduced to ePHF during this sensitive period, they fed ePHF to satiation, and their mothers perceived that they enjoyed the formula throughout the study. Together, these data suggest that the lower intakes and differences in growth were not attributable to rejection of the formula based on its negative sensory characteristics.
Second, the higher protein content of the ePHF may be more satiating to infants compared with CMF, a finding that has been observed in animal model studies and studies on older children and adults (CitationJohnson & Vickers, 1993; CitationPoppitt et al., 1998; CitationRolls et al., 1988; CitationWesterterp-Plantenga et al., 1999). While ePHF and CMF are isocaloric, the protein content of ePHF is 35% higher than that of CMF (CitationMead Johnson Nutrition, 2012). However, this explanation is contradictory to data obtained from a recent European clinical trial in which infants fed a high-protein cow's-milk infant formula consumed more formula and gained more weight than did infants who consumed lower-protein cow's milk infant formula, even when controlling for energy intake (CitationKoletzko et al., 2009b; CitationSchulze et al., 1987). Specifically, Koletzko and colleagues (CitationKoletzko et al., 2009b) randomized healthy newborns to either a lower-protein CMF infant and follow-on formula (1.8 and 2.2 g protein/100 kcal, respectively) or a high-protein CMF infant and follow-on formula (2.9 and 4.4 g protein/100 kcal, respectively; as a reference, the CMF and ePHF used in our study were 2.1 and 2.8 g protein equivalent/100 kcal, respectively). Infants consuming the higher-protein CMF and follow-on formula had higher weight-for-age z-scores than did infants consuming the lower-protein CMF formulas. Thus, absolute difference in the protein equivalencies of the two formulas, in and of themselves, was unlikely to be solely responsible for the relative differences in intake, body weight, and weight gain between the two groups.
The third and most plausible explanation for the findings is that the form in which the amino acids are delivered to the infants, rather than overall total protein concentration, was responsible for the differences in infant intake and growth. Specifically, the amino acids in ePHF are predominantly FAAs, meaning they are not contained within intact proteins, whereas very little of the amino acid content of CMF is in free form (CitationVentura et al., in press) a difference that may have important implications for nutrient absorption, metabolism, and signalling (CitationDiepvens et al., 2008; CitationFoltz et al., 2008; CitationKeohane et al., 1985; CitationKoopman et al., 2009). Differential intake and growth patterns of infants fed ePHF may result from the ability of FAAs to stimulate sensory receptors in the oral cavity and/or gastrointestinal tract (CitationAgostoni et al., 2000), which in turn may serve as key signals for satiation and satiety (CitationViarouge et al., 1992; CitationViarouge et al., 1991). Further support of the hypothesis comes from a recent within-subject experimental study (CitationVentura et al., 2012a). We found that infants self-regulate and satiate at lower intake volumes when feeding ePHF than when they feed either CMF (see also CitationHauser et al., 1993; CitationHyams et al., 1995; CitationMennella & Beauchamp, 1996a, Citation1998a; CitationMennella et al., 2011b) or CMF with free glutamate levels that approximate levels found in ePHF; thus, growth differences may be due to decreased energy intake.
It has been hypothesized that a key difference between breastfeeding and formula feeding is that breastfeeding is an infant-led process, thus fostering infants’ developing abilities to self-regulate intake, whereas formula feeding is a parent-led process that may lead to habitual overfeeding and infants losing their abilities to self-regulate intake (CitationBernal & Richards, 1970; CitationCrow et al., 1980). However, experimental research in infants illustrates that, regardless of prior feeding history, formula-fed infants can self-regulate intake in response to the formula they are fed when given the opportunity to do so through infant-led feeding practices (CitationVentura et al., 2012b); CitationMennella & Beauchamp, 1996a, Citation1998a; CitationMennella et al., 2004). Decreased energy intake may contribute to differential rates of weight gain in infants fed ePHF compared with those fed CMF.
In other words, what an infant is fed may be more important than how it is delivered. This point is also underscored by a recent pilot study evaluating growth of infants fed breast milk primarily via the breast versus by bottle. This study found no differences in weight or weight-for-age z-scores during the first 4 months of life, suggesting that the mode of feeding (breast versus bottle) may not influence early growth. This study should be interpreted with caution, however, because the sample size was small (36 infants total) and thus the study may be insufficiently powered to detect growth differences (CitationBartok, 2011).
Sensitive period in flavour learning
While much research has focused on the effects of the nutrient quality of the diet or on the long-term effects of early growth, relatively little attention has been paid to another important feature of nutrition: how humans learn to like the flavours of foods. During the past two decades, research has systematically studied the transfer of dietary volatiles to amniotic fluid and human milk to determine the effects this has on the behaviour of breastfed infants (see CitationMennella, 2007 for review). This has revealed that a wide variety of flavours either ingested (e.g. fruits, vegetables, spices) or inhaled (e.g. tobacco, perfumes) by the mother are transmitted to her amniotic fluid and/or breast milk (CitationMennella, 2007; CitationMennella & Beauchamp, 1991a, Citation1991b, Citation1998b, Citation1999; CitationMennella et al., 1995). In general, the intensity of the flavour in milk increased significantly within hours after consumption.
That amniotic fluid and breast milk share flavour profiles with foods eaten by the mother suggests that breast milk ‘bridges’ experiences with flavours in utero and while nursing and flavours experienced from solid food (CitationBarker, 1980). Variations in flavour from mother to mother and from feeding to feeding suggest that breastfeeding, unlike formula feeding, provides the infant with the potential for a rich source of sensory variety. The types and intensities of flavours experienced may be unique for each infant and characteristic of the culinary traditions of the family. These are the foods their mothers eat (CitationPark et al., 2003; CitationSkinner et al., 2002b) and will be the foods that their mothers will feed them as they grow.
Thus, learning about the diet of the mother through transmitted flavours may be a fundamental feature of dietary learning for humans, as well as for other mammals. Such experiences early in life cause a variety of neurological and physiological changes that influence later behaviours, and there is some evidence that dietary learning is more pronounced during early life. In a recent study, mice were exposed during nursing to odours that activate GFP-tagged olfactory receptors (ORs), and then the size of tagged glomeruli in the olfactory bulb where axons from olfactory sensory neurons coalesce by OR type was measured (CitationTodrank et al., 2011). Mice exposed to these activating odours in mother's milk had significantly larger glomeruli and significant preferences for the activating odour. Thus, early experiences with retronasally perceived odours (flavours) in milk result in enhanced detection of these learned odours that in nature would facilitate selection of foods that mothers find palatable.
This pattern makes evolutionary sense because the foods that a mother eats when she is pregnant and nursing are precisely the ones that her infant should prefer. All else being equal, these are the flavours associated with nutritious foods or, at the very least, with foods the mother has access to, and hence the foods to which the child will have the earliest exposure. Because food habits established during infancy track into later childhood and adolescence (CitationNicklaus et al., 2004; CitationSkinner et al., 2002b), early experiences with nutritious foods and flavour variety should increase the chance that, as infants grow, they will enjoy a more healthy diet because they like its tastes. In support of this hypothesis, longitudinal studies have shown that the strongest predictors of what foods young children eat are (1) whether they like how the foods taste, (2) how long they were breastfed and whether their mothers ate these foods, and (3) whether they have been eating these foods from an early age (CitationCooke et al., 2004; CitationNicklaus et al., 2005a; CitationResnicow et al., 1998; CitationSkinner et al., 2002a; CitationSkinner et al., 2002b). The varied sensory experiences with food flavours in mother's milk among children whose mothers eat a varied diet may explain why children who were breastfed are less picky (CitationGalloway et al., 2003) and more willing to try new foods during childhood (CitationCooke et al., 2004; CitationNicklaus et al., 2005b; CitationSkinner et al., 2002b).
Using the analogy reviewed above for growth patterns, we began investigating whether there were sensitive periods in flavour learning (CitationBeauchamp & Mennella, 1998; CitationMennella et al., 2004, Citation2011a). The absence of a robust experimental paradigm, like that employed for other sensory systems and other animals, inhibited progress until we began investigating a model system that exploits the naturally occurring flavour variation in infant formulas (CitationMennella et al., 2004). To adults, extensively hydrolysed protein hydrolysate formulas are extremely unpalatable compared with cow's milk formulas because of ePHF's distinctive, unpleasant flavours, including both volatile (odours) and non-volatile (bitter and sour tastes) components (CitationMennella & Beauchamp, 2005). In previous investigations we identified a ‘window’ of acceptance when young infants readily accept ePHF (CitationMennella & Beauchamp, 1998a). Then, beginning around 4 months of age and continuing through adulthood, its flavour is rejected unless the individual has been exposed to ePHF during early life (CitationMennella & Beauchamp, 1996a, Citation1998b). That is, ePHF acquires a completely different hedonic tone depending on whether the individual was exposed to this formula during the first few months of life (CitationMennella et al., 2004). Effects of early exposure on taste and food preferences were particularly persistent and lasted several years (CitationLiem & Mennella, 2002; CitationMennella & Beauchamp, 2002).
To characterize the sensitive period in early development when hedonic responses to flavours are established, we conducted randomized clinical trials and varied the age at which ePHF exposure began and the length of exposure. We tested the hypothesis that the infants’ acceptance of ePHF at 7.5 months is a function of exposure duration, by comparing infants exposed to ePHF for only 1 month with infants exposed for 3 months. To test the hypothesis that early exposure is more potent and persistent than later exposure, we held the duration of exposure constant at 1 month but altered its timing. For both hypotheses, treatment groups were compared to control groups of infants with either no ePHF exposure or 7 months of ePHF exposure.
Three months of ePHF exposure led to similar acceptance as 1 month of exposure. Although these infants were more accepting than infants with no exposure, they were less accepting than infants with seven months of exposure (CitationMennella et al., 2004). The most parsimonious explanation is that acceptance is a function of the absolute amount of exposure. We also found that when the flavour experiences began was also significant. Among infants exposed to PHF for 1 month, those who first fed ePHF at 3.5 months rejected PHF relative to CMF more than infants first fed ePHF at younger ages (1.5 and 2.5 months). This suggests that, in addition to quantity of exposure, timing is important. A relatively brief flavour experience of 1 month in duration before the baby is 3.5 months, was sufficient to cause a shift of hedonic tone from rejection to acceptance (CitationMennella et al., 2011a) (see ). Further, since those exposed to ePHF at 3.5 months had the most recent 1-month exposure to ePHF, we conclude that the recency of the exposure per se does not appear to be as important as when the exposure began.
Figure 2. Facial expressions suggest infants respond differently to ePHF at 7.5 months when they (A) have been consuming CMF for the previous 7 months and do not appear to have been programmed to like the flavour of the formula or (B) have been consuming the ePHF formula for the previous 7 months and are perceived to like the formula. Permission obtained from mothers of the infants for use of images. From the American Journal of Clinical Nutrition, 2011, 93, 1019–1024, American Society for Nutrition.
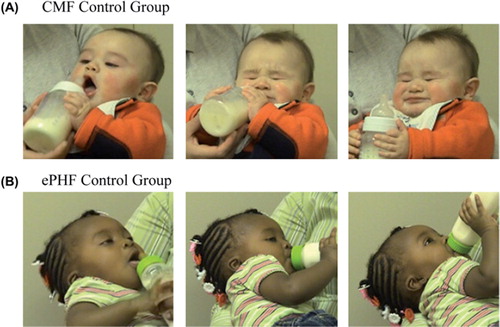
Children who were fed ePHF as infants are programmed to like not only the taste of ePHF () but also the taste of foods that are more savoury (e.g. chicken), sour (e.g. lemon), and bitter (e.g. broccoli) compared with children who were fed CMF (CitationLiem & Mennella, 2002; CitationMennella & Beauchamp, 2002; CitationMennella et al., 2009, Citation2006; CitationOwada et al., 2000; CitationSchuett et al. 1980, Citation1985). These findings provide further evidence the early diet can programme later dietary habits, which could in turn affect growth. We caution that one cannot assume there is only one sensitive period in flavour programming, any more than there is only one sensitive period for auditory (CitationRuben, 1997) or visual (CitationLewis & Maurer, 2005) learning. The model system that we identified allows us to explore the ability to change behavior based on experiences during only one period. As for other senses (CitationHuttenlocher, 2002; CitationSharma et al., 2002), windows of plasticity in flavour learning may not shut abruptly, and some level of plasticity is probably retained as the child ages.
The neural substrates underlying the observed developmental transition in human flavour learning remain unknown (but see CitationSullivan & Holman, 2010). We suggest that knowledge about the sensitive periods for other senses, particularly vision (CitationOlitsky et al., 2002), may shed light on aspects of flavour learning not yet explored. Before we gained scientific knowledge of the timing of the sensitive period of visual development, physicians rarely removed cataracts in children younger than 6 months, and this resulted in a lifetime of poor vision (CitationBeller et al., 1981; CitationOlitsky et al., 2002). Equipped with knowledge of the timing of this sensitive period, particularly the deleterious effects of monocular deprivation during the first 3 months of life, physicians now often remove cataracts during the first weeks of life, with significantly improved outcomes. Whether similar long-term effects occur if infants are deprived of food flavours during early life is an important area of future research.
Although our model system examined the flavours of hydrolysed formulas, the general principles observed are likely of much broader significance for food preferences, revealing a fundamental feature of human development. We suggest that the adaptive reason for this age-related plasticity reflects the importance of infants becoming familiar with and accepting the flavours that their mothers consume and transmit to breast milk (CitationMennella et al., 2001). By analogy, we hypothesize that it is important for the human infant to accept and be particularly (but not exclusively) attracted to the flavours that are consumed by the culture and, more specifically, by the mother. All else being equal, these are the flavours that (1) are associated with nutritious foods – or at least foods as nutritious as the mother has access to, and (2) the foods and flavours that the infant will be confronted with at weaning and probably thereafter. Under this hypothesis, much of the normal exposure to flavours would occur very early: in utero and during nursing, when flavours mothers consume are transferred to the infants’ chemosensory environment.
Conclusion
During the past decade, emerging research revealed that the composition of the diet fed during early infancy plays a role in both short- and long-term health outcomes. Breast milk is by far considered the preferred diet in infancy and is the gold standard for infant nutrition (CitationCommittee on Nutrition, 2009a; CitationWHO, 2003). While breastfeeding rates in the USA have continued to increase during the new millennium (CitationRyan et al., 2002), more than 50% of American infants receive infant formula while in hospital, and more than 60% of infants have received formula by 4 months of age (CitationGrummer-Strawn et al., 2008). Research has shown that infants who are fed infant formula (mostly CMF) (CitationMartinez & Ballew, 2011; CitationOliveira et al., 2010) tend to weigh more and have a greater risk for later obesity than do infants who are breastfed (CitationArmstrong & Reilly, 2002; CitationBurke et al., 2005; CitationDewey et al., 1993; CitationGrummer-Strawn & Mei, 2004; CitationOwen et al., 2005).
Today, there is a plethora of infant formulas on the market that differ in macronutrient composition. In this review we described how the protein composition differs between two types of formula, CMF and ePHF, and that infants fed ePHF show the same growth rates as breastfed infants. Therefore, when evaluating the effect of diet composition on growth and health outcomes, it may no longer be appropriate to consider all formula-fed infants as a homogeneous group with respect to certain health outcomes such as obesity. In addition, it is important to recognize that infant formulas may also differ in both fat and carbohydrate composition/structure as well as protein composition, and these differences may also affect growth. Because rapid rates of growth during the first year of life are associated with increased risk for later obesity (CitationBaird et al., 2008; CitationChomtho et al., 2009; CitationDennison et al., 2006; CitationEid, 1970; CitationMelbin & Vuille, 1976; CitationOng et al., 2009; CitationParsons et al., 2001; CitationStettler et al., 2002, Citation2003), metabolic syndrome (CitationEkelund et al., 2007), and mortality from cardiovascular disease (CitationBarker, 1997), it is imperative that we understand the influence of infant formula composition on growth and subsequent diet.
There is also increasing evidence for a sensitive window for flavour learning, encompassing in utero and nursing periods of growth. During this sensitive period, infants become familiar with and learn to accept the flavours they experience through their mothers’ amniotic fluid and breast milk. These early flavour experiences influence flavour and food preferences of children that last several years (CitationLiem & Mennella, 2002; CitationMennella & Beauchamp, 2002; CitationMennella et al., 2006, Citation2009; CitationOwada et al., 2000; CitationSchuett et al., 1980, Citation1985) and may affect food choices and health later in life. It is therefore also imperative that we understand how early diet influences later food choices.
Acknowledgements
We are thankful for the valuable comments and editing by Patricia Watson on an earlier version of the manuscript.
Declaration of interest: Preparation of this manuscript was supported in part by Award Number R01HD072307-01 and R01HD37119 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Schriver National Institute of Child Health and Human Development or the National Institutes of Health.
References
- Agostoni, C., Carratu, B., Boniglia, C., Riva, E. & Sanzini, E. (2000). Free amino acid content in standard infant formulas: Comparison with human milk. Journal of the American College of Nutrition, 19, 434–438.
- Armstrong, J. & Reilly, J.J. (2002). Breastfeeding and lowering the risk of childhood obesity. Lancet, 359, 2003–2004.
- Axelsson, I.E., Ivarsson, S.A. & Räihä, N.C. (1989). Protein intake in early infancy: Effects on plasma amino acid concentrations, insulin metabolism, and growth. Pediatric Research, 26, 214–217.
- Baird, J., Poole, J., Robinson, S., Marriott, L., Godfrey, K., Cooper, C., … Law, C. (2008). Milk feeding and dietary patterns predict weight and fat gains in infancy. Paediatric & Perinatal Epidemiology, 22, 575–586. doi: PPE963 [pii] 10.1111/j.1365 - 3016.2008.00963.x
- Barker, D.J. (2004). The developmental origins of chronic adult disease. Acta Paediatrica Supplement, 93, 26–33.
- Barker, D.J.P. (1997). Fetal nutrition and cardiovascular disease in later life. British Medical Bulletin, 53, 96–108.
- Barker, E. (1980). Sensory evaluation of human milk. MSc thesis, University of Manitoba, Manitoba, Canada.
- Bartok, C.J. (2011). Babies fed breastmilk by breast versus by bottle: A pilot study evaluating early growth patterns. Breastfeeding Medicine, 6, 117–124. doi: 10.1089/bfm.2010.0055
- Beauchamp, G.K. & Mennella, J.A. (1998). Sensitive periods in the development of human flavor perception and preference. Annales Nestlé;, 56, 19–31.
- Beller, R., Hoyt, C.S., Marg, E. & Odom, J.V. (1981). Good visual function after neonatal surgery for congenital monocular cataracts. American Journal of Ophthalmology, 91, 559–565.
- Bernal, J. & Richards, M.P. (1970). The effects of bottle and breast feeding on infant development. Journal of Psychosomatic Research, 14, 247–252.
- Burke, V., Beilin, L.J., Simmer, K., Oddy, W.H., Blake, K.V., Doherty, D., Stanley, F.J. (2005). Breastfeeding and overweight: Longitudinal analysis in an Australian birth cohort. Journal of Pediatrics, 147, 56–61.
- Chesney, R.W., Helms, R.A., Christensen, M., Budreau, A.M., Han, X. & Sturman, J.A. (1998). The role of taurine in infant nutrition. Advances in Experimental Medicine & Biology, 442, 463–476.
- Chomtho, S., Wells, J.C., Davies, P.S., Lucas, A. & Fewtrell, M.S. (2009). Early growth and body composition in infancy. Advances in Experimental Medicine & Biology, 646, 165–168. doi: 10.1007/978 - 1-4020 - 9173-5_19
- Codex Alimentarius Commission (1981). Standard for infant formula and formulas for special medical purposes intended for infants. CODEX STAN 72 - 1981. Rome: Codex Alimentarius.
- Committee on Nutrition, American Academy of Pediatrics (2000). Hypoallergenic infant formulas. Pediatrics, 106, 346–349.
- Committee on Nutrition (2009a). Breastfeeding. In R.E. Kleinman (Ed.), Pediatric Nutrition Handbook, 6th ed. (pp. 29–59). Elk Grove, IL: American Academy of Pediatrics.
- Committee on Nutrition (2009b). Formula feeding of term infants. In R.E. Kleinman (Ed.), Pediatric Nutrition Handbook, 6th ed. (pp. 61–78). Elk Grove, IL: American Academy of Pediatrics.
- Committee on Nutrition (2009c). Protein. In R.E. Kleinman (Ed.), Pediatric Nutrition Handbook, 6th ed. (pp. 325–342). Elk Grove, IL: American Academy of Pediatrics.
- Cook, D.A. & Sarett, H.P. (1982). Design of infant formulas for meeting normal and special need. Pediatric Nutrition: Infant Feeding, Deficiencies, Disease. New York: Dekker.
- Cooke, L.J., Wardle, J., Gibson, E.L., Sapochnik, M., Sheiham, A. & Lawson, M. (2004). Demographic, familial and trait predictors of fruit and vegetable consumption by pre-school children. Public Health Nutrition, 7, 295–302.
- Crow, R.A., Fawcett, J.N. & Wright, P. (1980). Maternal behavior during breast- and bottle-feeding. Journal of Behavioral Medicine, 3, 259–277.
- Dennison, B.A., Edmunds, L.S., Stratton, H.H. & Pruzek, R.M. (2006). Rapid infant weight gain predicts childhood overweight. Obesity (Silver Spring), 14, 491–499. doi: 14/3/491 [pii] 10.1038/oby.2006.64
- Dewey, K.G., Heinig, M.J., Nommsen, L.A., Peerson, J.M. & Lonnerdal, B. (1992). Growth of breast-fed and formula-fed infants from 0 to 18 months: the DARLING Study. Pediatrics, 89, 1035–1041.
- Dewey, K.G., Heinig, M.J., Nommsen, L.A., Peerson, J.M. & Lonnerdal, B. (1993). Breast-fed infants are leaner than formula-fed infants at 1 y of age: The DARLING study. American Journal of Clinical Nutrition, 57, 140–145.
- Diepvens, K., Haberer, D. & Westerterp-Plantenga, M. (2008). Different proteins and biopeptides differently affect satiety and anorexigenic/orexigenic hormones in healthy humans. International Journal of Obesity (London), 32, 510–518. doi: 10.1038/sj.ijo.0803758
- Donovan, S.M. & Lonnerdal, B. (1989). Isolation of the nonprotein nitrogen fraction from human milk by gel-filtration chromatography and its separation by fast protein liquid chromatography. American Journal of Clinical Nutrition, 50, 53–57.
- Eid, E.E. (1970). Follow-up study of physical growth of children who had excessive weight gain in first six months of life. British Medical Journal, 2, 74–76.
- Ekelund, U., Ong, K.K., Linne, Y., Neovius, M., Brage, S., Dunger, D.B., … Rossner, S. (2007). Association of weight gain in infancy and early childhood with metabolic risk in young adults. Journal of Clinical Endocrinology & Metabolism, 92, 98–103. doi 10.1210/jc.2006 - 1071
- EU Commission (2006). European Union Commission Directive 2006/141/EC of 22 December 2006 on infant formulae and follow-on formulae ammeding Directive 1999/21/EC with EEA relevance. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri = OJ:L:2006:401:0001:0001:EN:PDF
- Foltz, M., Ansems, P., Schwarz, J., Tasker, M.C., Lourbakos, A. & Gerhardt, C.C. (2008). Protein hydrolysates induce CCK release from enteroendocrine cells and act as partial agonists of the CCK1 receptor. Journal of Agriculture & Food Chemistry, 56, 837–843. doi: 10.1021/jf072611h
- Galloway, A.T., Lee, Y. & Birch, L.L. (2003). Predictors and consequences of food neophobia and pickiness in young girls. Journal of the American Dietetic Association, 103, 692–698.
- Giovannini, M., Agostoni, C., Fiocchi, A., Bellu, R., Trojan, S. & Riva, E. (1994). Antigen-reduced infant formulas versus human milk: Growth and metabolic parameters in the first 6 months of life. Journal of the American College of Nutrition, 13, 357–363.
- Gluckman, P.D. & Hanson, M.A. (2008). Developmental and epigenetic pathways to obesity: An evolutionary-developmental perspective. International Journal of Obesity (London), 32, S62–71. doi: ijo2008240 [pii] 10.1038/ijo.2008.240.
- Grummer-Strawn, L.M. & Mei, Z. (2004). Does breastfeeding protect against pediatric overweight? Analysis of longitudinal data from the Centers for Disease Control and Prevention Pediatric Nutrition Surveillance System. Pediatrics, 113, e81–86.
- Grummer-Strawn, L.M., Scanlon, K.S. & Fein, S.B. (2008). Infant feeding and feeding transitions during the first year of life. Pediatrics, 122, S36–42. doi: 10.1542/peds.2008 - 1315d
- Hauser, B., Keymolen, K., Blecker, U., Suys, B., Bougatef, A., Loeb, H. & Vandenplas, Y. (1993). A comparative evaluation of whey hydrolysate and whey-predominant formulas. How well do infants accept and tolerate them? Clinical Pediatrics (Philadelphia), 32, 433–437.
- Hernell, O. & Lonnerdal, B. (2003). Nutritional evaluation of protein hydrolysate formulas in healthy term infants: Plasma amino acids, hematology, and trace elements. American Journal of Clinical Nutrition, 78, 296–301.
- Hurford, J.R. (1991). The evolution of the critical period for language acquisition. Cognition, 40, 159–201.
- Huttenlocher, P.R. (2002). Neural Plasticity: The Effects of the Environment on the Development of the Cerebral Cortex. Cambridge, MA: Harvard University Press.
- Hyams, J.S., Treem, W.R., Etienne, N.L., Weinerman, H., MacGilpin, D., Hine, P., … Burke, G. (1995). Effect of infant formula on stool characteristics of young infants. Pediatrics, 95, 50–54.
- Janas L.M., Picciano M.F., Hatch T.F. (1987). Indices of proteinmetabolism in term infants fed either human milk or formulas with reduced protein concentration and various whey/case in ratios. J Pediatr 110, 838–848
- Johnson, J. & Vickers, Z. (1993). Effects of flavor and macronutrient composition of food servings on liking, hunger and subsequent intake. Appetite, 21, 25–39. doi: S0195666383710342 [pii]
- Keohane, P.P., Grimble, G.K., Brown, B., Spiller, R.C. & Silk, D.B. (1985). Influence of protein composition and hydrolysis method on intestinal absorption of protein in man. Gut, 26, 907–913.
- Koletzko, B., von Kries, R., Closa, R., Escribano, J., Scaglioni, S., Giovannini, M., … Grote, V. (2009a). Can infant feeding choices modulate later obesity risk? American Journal of Clinical Nutrition, 89, 1502S–1508S.
- Koletzko, B., von Kries, R., Closa, R., Escribano, J., Scaglioni, S., Giovannini, M., … Grote, V. (2009b). Lower protein in infant formula is associated with lower weight up to age 2 y: A randomized clinical trial. American Journal of Clinical Nutrition, 89, 1836–1845. doi: 10.3945/ajcn.2008.27091
- Koopman, R., Crombach, N., Gijsen, A.P., Walrand, S., Fauquant, J., Kies, A.K., … van Loon, L.J. (2009). Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. American Journal of Clinical Nutrition, 90, 106–115. doi: 10.3945/ajcn.2009.27474.
- Kramer, M.S., Guo, T., Platt, R.W., Vanilovich, I., Sevkovskaya, Z., Dzikovich, I., … Dewey, K. (2004). Feeding effects on growth during infancy. Journal of Pediatrics, 145, 600–605. doi: S0022347604006353 [pii]10.1016/j.jpeds.2004.06.069
- Lewis, T.L. & Maurer, D. (2005). Multiple sensitive periods in human visual development: Evidence from visually deprived children. Developmental Psychobiology, 46, 163–183. doi: 10.1002/dev.20055
- Liem, D.G. & Mennella, J.A. (2002). Sweet and sour preferences during childhood: Role of early experiences. Developmental Psychobiology, 41, 388–395.
- Lucas, A. (1990). Does early diet program future outcome? Acta Paediatrica Scandnavica Supplement, 365, 58–67.
- Lucas, A. (1998). Programming by early nutrition: An experimental approach. Journal of Nutrition, 128, 401S–406S.
- Lucas, A. (2005a). The developmental origins of adult health and well-being. Advances in Experimental Medicine & Biology, 569, 13–15.
- Lucas, A. (2005b). Long-term programming effects of early nutrition – implications for the preterm infant. Journal of Perinatology, 25, S2–6. doi: 10.1038/sj.jp.7211308
- Martinez, J.A. & Ballew, M.P. (2011). Infant formulas. Pediatrics Review, 32, 179–189, quiz 189. doi: 10.1542/pir.32 - 5–179
- May, P.M., Smith, G.L. & Williams, D.R. (1982). Computer calculation of zinc(II)-complex distribution in milk. Journal of Nutrition, 112, 1990–1993.
- McCance, R.A. (1962). Food, growth, and time. Lancet, 2, 671–676.
- Mead Johnson Nutrition (2012). Health Care Professional Resource Center. http://www.mjn.com/.
- Melbin, T. & Vuille, J. (1976). Weight gain in infancy and physical development development between 7 and 10 1/2 years of age. British Journal of Preventative and Social Medicine, 30, 233–238.
- Mennella, J.A. (2007). The chemical senses and the development of flavor preferences in humans. In T.W. Hale & P.E. Hartmann (Eds), Textbook on Human Lactation (pp. 403–414). Amarillo, TX: Hale.
- Mennella, J.A. & Beauchamp, G.K. (1991a). Maternal diet alters the sensory qualities of human milk and the nursling's behavior. Pediatrics, 88, 737–744.
- Mennella, J.A. & Beauchamp, G.K. (1991b). The transfer of alcohol to human milk. Effects on flavor and the infant's behavior. New England Journal of Medicine, 325, 981–985.
- Mennella, J.A. & Beauchamp, G.K. (1996a). Developmental changes in the acceptance of protein hydrolysate formula. Journal of Developmental & Behavioral Pediatrics, 17, 386–391.
- Mennella, J.A. & Beauchamp, G.K. (1996b). The human infants’ responses to vanilla flavors in human milk and formula. Infant Behavior and Development, 19, 13–19.
- Mennella, J.A. & Beauchamp, G.K. (1998a). Development and bad taste. Pediatric Asthma Allergy Immunology, 12, 161–163.
- Mennella, J.A. & Beauchamp, G.K. (1998b). Smoking and the flavor of breast milk. New England Journal of Medicine, 339, 1559–1560.
- Mennella, J.A. & Beauchamp, G.K. (1999). Experience with a flavor in mother's milk modifies the infant's acceptance of flavored cereal. Developmental Psychobiology, 35, 197–203.
- Mennella, J.A. & Beauchamp, G.K. (2002). Flavor experiences during formula feeding are related to preferences during childhood. Early Human Development, 68, 71–82.
- Mennella, J.A. & Beauchamp, G.K. (2005). Understanding the origin of flavor preferences. Chemical Senses, 30, Si242–i243.
- Mennella, J.A., Forestell, C.A., Morgan, L.K. & Beauchamp, G.K. (2009). Early milk feeding influences taste acceptance and liking during infancy. American Journal of Clinical Nutrition, 90, S780–788. doi: 10.3945/ajcn.2009.27462O
- Mennella, J.A., Griffin, C.E. & Beauchamp, G.K. (2004). Flavor programming during infancy. Pediatrics, 113, 840–845.
- Mennella, J.A., Jagnow, C.P. & Beauchamp, G.K. (2001). Prenatal and postnatal flavor learning by human infants. Pediatrics, 107, E88.
- Mennella, J.A., Johnson, A. & Beauchamp, G.K. (1995). Garlic ingestion by pregnant women alters the odor of amniotic fluid. Chemical Senses, 20, 207–209.
- Mennella, J.A., Kennedy, J.M. & Beauchamp, G.K. (2006). Vegetable acceptance by infants: Effects of formula flavors. Early Human Development, 82, 463–468.
- Mennella, J.A., Lukasewycz, L.D., Castor, S.M. & Beauchamp, G.K. (2011a). The timing and duration of a sensitive period in human flavor learning: A randomized trial. American Journal of Clinical Nutrition, 93, 1019–1024. doi: 10.3945/ajcn.110.003541
- Mennella, J.A., Ventura, A.K. & Beaucamp, G.K. (2011b). Differential growth patterns among healthy infants fed protein hydrolysate or cow-milk formulas. Pediatrics, 127, 110–118.
- Mott, G.E., Lewis, D.S. & McGill, H.C., Jr (1991). Programming of cholesterol metabolism by breast or formula feeding. Ciba Foundation Symposium, 156, 56–66; discussion 66–76.
- Naim, M., Brand, J.G., Kare, M.R., Kaufmann, N.A. & Kratz, C.M. (1980). Effects of unpalatable diets and food restriction on feed efficiency in growing rats. Physiology of Behavior, 25, 609–614.
- Nicklaus, S., Boggio, V., Chabanet, C. & Issanchou, S. (2004). A prospective study of food preferences in childhood. Food Quality and Preference, 15, 805–819.
- Nicklaus, S., Boggio, V., Chabanet, C. & Issanchou, S. (2005a). A prospective study of food variety seeking in childhood, adolescence and early adult life. Appetite, 44, 289–297.
- Nicklaus, S., Boggio, V. & Issanchou, S. (2005b). Food choices at lunch during the third year of life: High selection of animal and starchy foods but avoidance of vegetables. Acta Paediatrica, 94, 943–951.
- Olitsky, S.E., Nelson, B.A. & Brooks, S. (2002). The sensitive period of visual development in humans. Journal of Pediatric Ophthalmology Strabismus, 39, 69–72.
- Oliveira, V., Frazao, E. & Smallwood, D. (2010). Rising Infant Formula Costs to the WIC Program Recent Trends in Rebates and Wholesale Prices. Economic Research Report 93. Washington, DC: US Department of Agriculture.
- Ong, K.K., Emmett, P., Northstone, K., Golding, J., Rogers, I., Ness, A.R., … Dunger, D.B. (2009). Infancy weight gain predicts childhood body fat and age at menarche in girls. Journal of Clinical Endocrinology & Metabolism, 94, 1527–1532. doi: 10.1210/jc.2008 - 2489
- Owada, M., Aoki, K. & Kitagawa, T. (2000). Taste preferences and feeding behaviour in children with phenylketonuria on a semisynthetic diet. European Journal of Pediatrics, 159, 846–850.
- Owen, C.G., Martin, R.M., Whincup, P.H., Smith, G.D. & Cook, D.G. (2005). Effect of infant feeding on the risk of obesity across the life course: A quantitative review of published evidence. Pediatrics, 115, 1367–1377. doi: 10.1542/peds.2004 - 1176
- Park, S.Y., Paik, H.Y., Skinner, J.D., Ok, S.W. & Spindler, A.A. (2003). Mothers’ acculturation and eating behaviors of Korean American families in California. Journal of Nutrition Education & Behavior, 35, 142–147.
- Parsons, T.J., Power, C. & Manor, O. (2001). Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: Longitudinal study. British Medical Journal, 323, 1331–1335.
- Picone T.A., Benson J.D., Moro G., Minoli I., Fulconis F., Rassin D.K. . (1989). Growth, serum biochemistries, and amino acids of term infants fed formulas with amino acid and protein concentrations similar to human milk. J Pediatr Gastroenterol Nutr 9, 351–360.
- Poppitt, S.D., McCormack, D. & Buffenstein, R. (1998). Short-term effects of macronutrient preloads on appetite and energy intake in lean women. Physiology of Behavior, 64, 279–285. doi: S0031 - 9384(98)00061 - 4
- Raiha, N., Minoli, I. & Moro, G. (1986). Milk protein intake in the term infant. I. Metabolic responses and effects on growth. Acta Paediatrica, 75, 881–886.
- Resnicow, K., Smith, M., Baranowski, T., Baranowski, J., Vaughan, R. & Davis, M. (1998). 2-year tracking of children's fruit and vegetable intake. Journal of the American Dietetic Association, 98, 785–789.
- Roche, A.F., Guo, S., Siervogel, R.M., Khamis, H.J. & Chandra, R.K. (1993). Growth comparison of breast-fed and formula-fed infants. Canadian Journal of Public Health, 84, 132–135.
- Rolls, B.J., Hetherington, M. & Burley, V.J. (1988). The specificity of satiety: The influence of foods of different macronutrient content on the development of satiety. Physiology of Behavior, 43, 145–153. doi: 0031 - 9384(88)90230 - 2
- Ruben, R.J. (1997). A time frame of critical sensitive periods in language development. Acta Otolaryngology, 117, 202–205.
- Ryan, A.S., Wenjun, Z. & Acosta, A. (2002). Breastfeeding continues to increase into the new millennium. Pediatrics, 110, 1103–1109.
- Rzehak, P., Sausenthaler, S., Koletzko, S., Reinhardt, D., von Berg, A., Kramer, U., … Heinrich, J. (2009). Short- and long-term effects of feeding hydrolyzed protein infant formulas on growth at < or = 6 y of age: Results from the German Infant Nutritional Intervention Study. American Journal of Clinical Nutrition, 89, 1846–1856. doi: 10.3945/ajcn.2008.27373
- Schiffman, S.S. & Dackis, C. (1975). Taste of nutrients: Amino acids, vitamins, and fatty acids. Perception and Psychophysics, 17, 140–146.
- Schuett, V.E., Brown, E.S. & Michals, K. (1985). Reinstitution of diet therapy in PKU patients from twenty-two clinics. American Journal of Public Health, 75, 39–42.
- Schuett, V.E., Gurda, R.F. & Brown, E.S. (1980). Diet discontinuation policies and practices of PKU clinics in the United States. American Journal of Public Health, 70, 498–503.
- Schulze, K.F., Stefanski, M., Masterson, J., Spinnazola, R., Ramakrishnan, R., Dell, R.B. & Heird, W.C. (1987). Energy expenditure, energy balance, and composition of weight gain in low birth weight infants fed diets of different protein and energy content. Journal of Pediatrics, 110, 753–759.
- Sharma, A., Dorman, M.F. & Spahr, A.J. (2002). A sensitive period for the development of the central auditory system in children with cochlear implants: Implications for age of implantation. Ear Hear, 23, 532–539.
- Singhal, A., Cole, T.J., Fewtrell, M., Deanfield, J. & Lucas, A. (2004). Is slower early growth beneficial for long-term cardiovascular health? Circulation, 109, 1108–1113. doi: 10.1161/01.CIR.0000118500.23649.DF.
- Singhal, A., Fewtrell, M., Cole, T.J. & Lucas, A. (2003). Low nutrient intake and early growth for later insulin resistance in adolescents born preterm. Lancet, 361, 1089–1097. doi: 10.1016/S0140 - 6736(03)12895 - 4.
- Skinner, J.D., Carruth, B.R., Bounds, W., Ziegler, P. & Reidy, K. (2002a). Do food-related experiences in the first 2 years of life predict dietary variety in school-aged children? Journal of Nutrition Education & Behavior, 34, 310–315.
- Skinner, J.D., Carruth, B.R., Wendy, B. & Ziegler, P.J. (2002b). Children's food preferences: A longitudinal analysis. Journal of the American Dietetic Association, 102, 1638–1646.
- Stettler, N., Kumanyika, S.K., Katz, S.H., Zemel, B.S. & Stallings, V.A. (2003). Rapid weight gain during infancy and obesity in young adulthood in a cohort of African Americans. American Journal of Clinical Nutrition, 77, 1374–1378.
- Stettler, N., Zemel, B.S., Kumanyika, S. & Stallings, V.A. (2002). Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics, 109, 194–199.
- Sullivan, R.M. & Holman, P.J. (2010). Transitions in sensitive period attachment learning in infancy: The role of corticosterone. Neuroscience and Biobehavioral Reviews, 34, 835–844.
- Todrank, J., Heth, G. & Restrepo, D. (2011). Effects of in utero odorant exposure on neuroanatomical development of the olfactory bulb and odour preferences. Proceedings of the Royal Society B, 278, 1949–1955.
- Turck D., Grillon C., Lachambre E., Robiliard P., Beck L., Maurin J.L. . (2006). Adequacy and safety of an infant formula with a protein/energy ratio of 1.8 g/100 kcal and enhanced protein efficiency for term infants during the first 4 months of life. J Pediatr Gastroenterol Nutr 43, 364–371.
- USFDA (2004). Federal Food, Drug, and Cosmetic Act; Section 412 (21 USC §350a). Requirements for Infant Formulas. Silver Spring, MD: United States Food and Drug Administration. http://www.fda.gov/RegulatoryInformation/Legislation/Federal FoodDrugandCosmeticActFDCAct/FDCActChapterIVFood/ucm107864.htm.
- Vandenplas, Y., Hauser, B., Blecker, U., Suys, B., Peeters, S., Keymolen, K. & Loeb, H. (1993). The nutritional value of a whey hydrolysate formula compared with a whey-predominant formula in healthy infants. Journal of Pediatric Gastroenterology & Nutrition, 17, 92–96.
- Ventura, A.K., Beauchamp, G.K. & Mennella, J.A. (2012a). Infant regulation of intake: The effect of free glutamate content in infant formulas. American Journal of Clinical Nutrition, 95, 875–881.
- Ventura, A.K., Beauchamp, G.K., Mennella, J.A. (2012b) Infant regulation of intake: the effect of free glutamate content in infant formulas. 95, 875–891.
- Ventura, A.K., San Gabriel, A., Hirota, M. & Mennella, J.A. (in press). Determination of free amino acid content in infant formulas. Nutrition and Food Sciences.
- Vessey, D.A. (1978). The biochemical basis for the conjugation of bile acids with either glycine or taurine. Biochemical Journal, 174, 621–626.
- Viarouge, C., Cauliez, R. & Nicolaidis, S. (1992). Umami tast of monosodium glutamate enhances the thermic effect of food and affects the respiratory quotient in the rat. Physiology of Behavior, 52, 879–884.
- Viarouge, C., Even, P., Rougeot, C. & Nicolaïdis, S. (1991). Effects on metabolic and hormonal parameters of monosodium glutamate (umami taste) ingestion in the rat. Physiology of Behavior, 49, 1013–1018.
- Westerterp-Plantenga, M.S., Rolland, V., Wilson, S.A. & Westerterp, K.R. (1999). Satiety related to 24 h diet-induced thermogenesis during high protein/carbohydrate vs high fat diets measured in a respiration chamber. European Journal of Clinical Nutrition, 53, 495–502.
- WHO (2003). Global Strategy for Infant and Young Children Feeding. Geneva, Switzerland: World Health Organization. http://www.paho.org/english/ad/fch/ca/GSIYCF_infantfeeding_eng.pdf)