Abstract
We live in a world replete with opportunities to overeat highly calorific, palatable foods – yet not everyone becomes obese. Why? We propose that individuals show differences in appetitive traits (e.g. food cue responsiveness, satiety sensitivity) that manifest early in life and predict their eating behaviours and weight trajectories. What determines these traits? Parental feeding restriction is associated with higher child adiposity, pressure to eat with lower adiposity, and both strategies with less healthy eating behaviours, while authoritative feeding styles coincide with more positive outcomes. But, on the whole, twin and family studies argue that nature has a greater influence than nurture on adiposity and eating behaviour, and behavioural investigations of genetic variants that are robustly associated with obesity (e.g. FTO) confirm that genes influence appetite. Meanwhile, a growing body of neuroimaging studies in adults, children and high risk populations suggests that structural and functional variation in brain networks associated with reward, emotion and control might also predict appetite and obesity, and show genetic influence. Together these different strands of evidence support a biobehavioural risk model of obesity development. Parental feeding recommendations should therefore acknowledge the powerful – but modifiable – contribution of genetic and neurological influences to children's eating behaviour.
Introduction
The developed world bulges with opportunities to consume palatable high-calorie foods in vast quantities – yet not all of us become obese. One explanation for this could be biologically and environmentally influenced individual differences in appetitive characteristics that emerge early in life (CitationCarnell & Wardle, 2007; CitationLlewellyn et al., 2011), and persist through development (CitationAshcroft et al., 2008; CitationFarrow & Blissett, 2012; CitationParkinson et al., 2010). Since the beginning of the child obesity epidemic, a wealth of cross-sectional evidence has accumulated to suggest that adiposity is related in a stepwise fashion to higher food cue responsiveness and/or lower sensitivity to internal satiety cues in children and infants, as assessed by either behavioural tests (CitationHill et al., 2008; CitationLlewellyn et al., 2008), or parent-report questionnaire (CitationCarnell & Wardle, 2008; CitationSpence et al., 2011; CitationWebber et al., 2009). Now, a number of prospective studies are beginning to extend this research to provide persuasive support for the theory that these traits predict weight trajectories in childhood (CitationBerkowitz et al., 2010; CitationWebber et al., 2010b) and infancy (CitationFaith & Hittner, 2010; Citationvan Jaarsveld et al., 2011).
A host of important questions remain, though. Where do these traits originate? Are they shaped by environmental factors? Or do they have a biological basis and if so, what is it? Most importantly, what are the practical implications of this information? Can the forces that shaped obesogenic traits be harnessed to bend an individual's dispositions back towards a healthier course? Our goal in this paper is to present evidence for an environmentally, genetically and neurologically informed biobehavioural susceptibility model that provides a useful framework for understanding the sources of individual variability in body weight in childhood and later in life. To do this we give a brief overview of relevant key findings from the diverse fields of parental feeding behaviour, obesity genetics, and neuroimaging of appetite and obesity, highlighting what they teach us about the origins of appetite. We finish by discussing some potential future research directions and the implications of our model for obesity prevention and treatment.
Methods
For this narrative review, we conducted three initial PubMed searches: (1) parent feeding AND child obesity OR weight OR appetite OR eating; (2) obesity genes; (3) obesity AND fMRI OR PET OR MRI. The parent feeding literature was then whittled down to contain only studies whose abstracts indicated content pertaining to relationships between the most commonly assessed feeding behaviours/styles (restriction, pressure to eat), and appetitive traits/behaviours known to predict weight (e.g. food cue responsiveness, satiety sensitivity, disinhibited eating, external eating, dietary intake, eating in the absence of hunger in the laboratory). Other feeding strategies (e.g. emotional feeding, instrumental feeding) and more general measures of the home environment (e.g. fruit and vegetable availability, family meals, TV watching) may also have long-term effects on appetite but are beyond our scope here. For the genetic literature we focused on twin and family studies generating heritability estimates for appetitive behaviours, and studies that directly related the genetic variants emerging from recent genome-wide association studies to such behaviours (e.g. associations between FTO, MC4R or combined variants and reported eating behaviours, dietary intake, eating in the absence of hunger). For the neuroimaging literature we focused on studies of appetitive traits (e.g. food cue responsiveness, emotional eating), child and adolescent obesity, and populations at high familial obesity risk. In order to represent the current state of each field, publications since 2007 are given the most attention; however, key findings prior to this date are described where context is needed.
Parent power
Ask someone why they weigh what they do and you can be sure that most of them will begin telling you about the way their parents (usually their mother) fed them during childhood. But is there really evidence that the explicit strategies parents commonly employ to control their children's intake (e.g. restriction, pressure to eat) have long-term impacts on children's enduring appetitive traits, and therefore their risk of developing obesity later in life?
Restriction
Parental restriction may be defined as parents’ deliberate limiting of the amount and type of food accessible to the child, typically their access to high-calorie ‘junk’ foods. The strategy is generally adopted to promote a healthy diet. However, some experimental studies have suggested that restricting access to a certain food may have the paradoxical effect of increasing preferences for and intake of that food when restrictions are lifted (CitationFisher & Birch, 1999b; CitationJansen et al., 2007), leading investigators to ask whether restriction could have long-lasting negative effects on children's eating behaviour and ultimately their body weight.
Cross-sectional studies of samples of children ranging from 3 to 12 years have shown that higher restriction, usually measured via the Child Feeding Questionnaire (CFQ) (CitationBirch et al. (2001); e.g. ‘I have to be sure my child does not eat too many sweets’), is associated with: greater daily energy intake (CitationBirch & Fisher, 2000), higher intake of snack foods following a satiating lunch (eating in the absence of hunger test (EAH)) (CitationFisher & Birch, 1999a), higher self-reported disinhibited eating (eating in response to external cues) (CitationCarper et al., 2000), increased parent-reported food responsiveness (CitationWebber et al., 2010a), and higher weight (CitationLewis & Worobey, 2011; CitationVentura & Birch, 2008). But associations with greater dietary restraint and lower levels of emotional and external eating (Citationvan Strien & Bazelier, 2007), better parent-reported self-regulation (CitationTan & Holub, 2011), and self-selection of lower energy-dense foods at a lab meal (among overweight children only) (CitationSud et al., 2010), have also been observed. ‘Softer’ forms of restriction also seem to show more positive associations – CitationBrann & Skinner (2005) found that fathers with high scores on the ‘Monitoring’ scale of the CFQ, which measures how much parents ‘keep track’ of children's intake of unhealthy foods (CitationBirch et al., 2001), had 8–10-year-old sons with lower body mass indexes (BMIs).
Longitudinal studies, which have the potential to reveal more about cause and effect, have also produced mixed results. CitationFisher & Birch (2002) found that restriction at 5 years predicted greater EAH at 7 years, CitationBirch et al. (2003) showed that girls who were overweight and most restricted at 5 years had the highest EAH at 9 years and the greatest EAH increases from 5 years to 9 years, and CitationFaith et al. (2004) found that greater restriction scores in 5 year olds with heavier mothers were associated with greater increases in BMI z-scores 2 years later, even controlling for child weight at 3 years. The latter studies are both consistent with an obesogenic gene–environment interaction. However, CitationCampbell et al. (2010) found associations between restriction and less 3 year weight gain in 5–6 year olds, and the CitationFaith et al. (2004) study also found that among low-risk children, parental ‘monitoring’ of children's intake of less healthy foods predicted reduced child BMI z-scores at 7 years, suggesting that for some kinds of parenting strategies and some families, restriction may be protective.
Other studies have also shed light on the cause–effect issue. For example, CitationWebber et al. (2010c) found that maternal concern about weight significantly mediated the association between the use of restrictive feeding practices and adiposity in 7–9 year olds. Further, using a discordant sibling design, which partially controls for genetic influences, CitationRoemmich et al. (2010) found that parents exerted higher levels of restriction for their overweight 8–17-year-old boys compared with their lean siblings, and CitationPayne et al. (2011) found that although parents of 6–12 year olds generally used similar feeding practices with both siblings, differences in parental concern for a sibling's weight (rather than perceived weight or actual weight) predicted differences in the degree of restriction used. These results support a model in which some forms of restriction may promote healthy weight maintenance, but restriction is at least sometimes a response to maternal concern about overweight, rather than a cause of child weight gain per se.
Pressure to eat
Another common parental feeding strategy is ‘pressure to eat’, which normally describes parents’ attempts to encourage their child to eat food perceived as healthy, typically at meal times. One might think that this would promote eating in the short term, and this is supported by observational results (CitationDrucker et al., 1999). However, some evidence suggests that persistent pressure can lead to less intake and negative food-related attitudes (CitationGalloway et al., 2006), and others have suggested that systematic pressure to eat may ultimately override children's internal satiety signals, leaving them less able to regulate their own intake.
In support of these rather pessimistic hypotheses, higher pressure shows associations with parent reports of lower child enjoyment of food, and higher satiety sensitivity, slowness in eating and food fussiness (CitationGregory et al., 2010b; CitationMcPhie et al., 2011; CitationWebber et al., 2010a), greater child dietary restraint, disinhibited eating, and emotional eating (CitationCarper et al., 2000), greater food avoidance behaviours (CitationPowell et al., 2011), greater neophobia (fear of new foods) and intake of unhealthy snack foods (CitationBrown et al., 2008), and lower fruit and vegetable intake (CitationFisher et al., 2002; CitationWardle et al., 2005; CitationWyse et al., 2011). CitationJohnson & Birch (1994) additionally found that high scores on a general scale of parental control over feeding (e.g. ‘My child should eat everything on his/her plate’) were associated with poorer compensation for preload calories at a subsequent lunch.
In contrast, CitationVan Strien et al. (2009) found that higher pressure as perceived by children was associated with less overweight and greater breakfast- eating and physical activity, suggesting that perceived pressure could be a marker for other health- supporting behaviours. Additionally, CitationSleddens et al. (2010) found that a scale measuring a ‘softer’ form of encouragement (e.g. I encourage my child to enjoy his/her food) showed negative associations with unhealthy snacking. It should also be noted that the measure of pressure used in most of these studies was the pressure to eat scale from the CFQ, which might be thought of as representing an ‘authoritarian’ form of control. Both authoritarian and indulgent feeding styles as defined by CitationHughes et al. (2005) (largely based on items describing encouragement to eat) have been associated with higher child BMI (CitationHughes et al., 2008), whereas authoritative and authoritarian feeding have been associated with higher fruit and vegetable intake (CitationBlissett, 2011; CitationPatrick et al., 2005). These results suggest that, on balance, authoritative forms of encouragement may have the best outcomes.
The long-term effects of pressure to eat are unclear. However, it is striking that parent-report measures of pressure to eat are associated with lower (rather than higher) weight, in both cross-sectional (CitationVentura & Birch, 2008) and longitudinal (CitationFarrow & Blissett, 2008) studies. Longitudinal studies by Gregory et al. have additionally found that pressure to eat predicts lower interest in food and greater food fussiness in children (CitationGregory et al., 2010a), and less fruit intake, even when controlling for initial intake (CitationGregory et al., 2011). These studies could reflect pressure causing low weight and unhealthy eating behaviour, but even the longitudinal results do not rule out the possibility that pressure simply tracks children's eating behaviour and weight. Gene–environment interactions should also be considered: CitationFarrow & Blissett (2006) found that if maternal control (observed during a home meal) was low/moderate, then higher weight gain in the first 6 months was related to lower weight gain in the next 6 months, but if maternal control was high, the child maintained an accelerated weight trajectory, suggesting a lack of self-regulation. Together, the results suggest long-term effects may depend crucially on the type of pressure exerted, and the growth trajectory displayed by the child. In many cases relationships may reflect parents responding to unhealthy eating preferences and low weight in children.
Greedy genes
If parents are in some cases merely responding to their children's eating dispositions and body weight, then what other influences are at work? Many sources of evidence suggest substantial genetic influences.
Twin and family studies
The heritability of body weight in adults is well-established, with two reviews of twin studies estimating that 50–90% of variance in BMI is attributable to genes (CitationMaes et al., 1997; CitationSchousboe et al., 2003). Heritability is also high in children (CitationSilventoinen et al., 2010; CitationWardle et al., 2008), and longitudinal genetic analyses have shown that although, new genetic factors become influential from early childhood to adulthood, there is also significant genetic continuity, suggesting that the genes that influence weight early in life are the same ones that influence weight in early adulthood (CitationSilventoinen & Kaprio, 2009).
Although people often assume that quantitative genetic effects on weight act via metabolism, there is a good deal of evidence that appetitive traits are heritable, suggesting some of this genetic effect on weight could also be mediated behaviourally. For example, although estimates vary widely, twin and family studies of adults have indicated significant heritability for scores on cognitive restraint, disinhibition and hunger (Citationde Castro & Lilenfeld, 2005; CitationNeale et al., 2003; CitationProvencher et al., 2005; CitationSteinle et al., 2002; CitationTholin et al., 2005). Parent-reported satiety responsiveness and food cue responsiveness have demonstrated 63% and 75% heritability respectively, in a large sample of 8–11 year olds (CitationCarnell et al., 2008), and similar data from a large study of twin infants have suggested heritability of 84% for slowness in eating, 72% for satiety responsiveness, 59% for food responsiveness, and 53% for enjoyment of food (CitationLlewellyn et al., 2010). Data from this cohort have also demonstrated significant shared genetic influence (22–37%) for infant appetite and infant weight, which is able to explain 41–45% of phenotypic associations, and supports the genetic mediation model outlined above (CitationLlewellyn et al., 2012).
There is also evidence for heritability of eating behaviours as assessed in the lab or in the field. Eating rate measured during a meal eaten at home was found to be 62% heritable in 10–12-year-old twins (CitationLlewellyn et al., 2008); a family study by CitationFisher et al. (2007) reported 52% heritability for energy intake during an ad libitum dinner (52%) and 51% for post-dinner EAH; and CitationFaith et al. (2004) observed significant sibling aggregation for total energy intake and percentages of fat, carbohydrate and protein intakes at a lab meal. Interestingly, twin designs may also help to reveal dietary differences between children which are independent of genetic similarity: CitationRissanen et al. (2002) studied weight-discordant monozygotic twin pairs and found a three-fold preference among the obese twin for fatty foods. These studies tell us nothing about the actual genes involved, but they argue for strong genetic influence on variation in weight and appetite in children and adults, and are consistent with the action of multiple genetic variants of small effect combining to influence the umbrella phenotype of body weight.
The search for ‘obesity genes’
Several mutations in single genes, including LEP, LEPR, MC4R and POMC, are known to give rise to extreme, early-onset obesity (CitationFarooqi & O'Rahilly, 2008). But these are estimated to account for a maximum of 5–10% of cases of obesity (CitationChoquet & Meyre, 2011), leaving much variation unexplained. Some of this remaining variance might be explained by common variants in the genome, and the sequencing of the human genome and creation of databases of single nucleotide polymorphisms (SNPs), together with technological advances making genotyping cheaper and faster, have recently paved the way for genome-wide association studies (GWAS) to be conducted in large populations, giving power to detect very small effects (CitationDay & Loos, 2011).
The first gene to be associated with BMI via GWAS was FTO (CitationFrayling et al., 2007; CitationScuteri et al., 2007). The high-risk allele is present in 42% of white Europeans, and each additional risk allele is accompanied by a 0.39 increase in BMI (CitationSpeliotes et al., 2010). Frequency of the high-risk A allele varies with ethnicity, and there is some variation in effect size, but the association with BMI appears relatively consistent across backgrounds (CitationDay & Loos, 2011). The latest and largest GWAS (n ≈ 250,000) confirmed 32 BMI-associated loci, including FTO and several near MC4R (CitationSpeliotes et al., 2010). However, even when combined these SNPs explained only 1.45% of population variation in BMI, significantly limiting their ability to predict obesity (CitationSpeliotes et al., 2010). Notably, many of the BMI-associated loci identified in adults are similarly associated with BMI in children. For example, in a meta-analysis of n ≈ 13,000 children and adolescents Citationden Hoed et al. (2010) found that 13 of the variants observed in adults showed similar BMI associations in children (although only nine reached significance), and whereas the effect size of certain variants differed by age, the cumulative effect size was similar across ages.
Consistent with diet-influenced expression of FTO in the hypothalamus, a structure known for its role in energy intake (CitationGerken et al., 2007; CitationStratigopoulos et al., 2008; CitationTung et al., 2010), studies have revealed associations between the high-risk allele and higher fat and energy intake in both adults (CitationSpeakman et al., 2008) and children (CitationTimpson et al., 2008). CitationWardle et al. (2009) found that 4–5 year olds homozygous for the low-risk T allele ate significantly less than children heterozygous or homozygous for the high-risk A allele and demonstrated that the high-risk allele was associated with lower levels of parent-reported satiety sensitivity (CitationWardle et al., 2008), while CitationTanofsky-Kraff et al. (2009) reported that children with 1 or 2 (versus 0) high-risk alleles were more likely to experience loss of control and have greater intake of fat during an eating episode. Variants near MC4R (CitationStutzmann et al., 2009) as well as other common variants (SH2B1, KCTD15, MTCH2, NEGR1, BDNF) (CitationBauer et al., 2009), may also be associated with increased intake of high-energy foods.
Of course, genes do not cause obesity in isolation and increasing evidence suggests FTO and other common variants may interact with environmental factors to determine body weight. For example, physical activity has been shown to attenuate the association between FTO and obesity in adults (CitationKilpelainen et al., 2011) and adolescents (CitationRuiz et al., 2010). Low-fat diets (CitationSonestedt et al., 2009) and embarking on a Mediterranean diet (CitationRazquin et al., 2010) may also ‘protect against’ FTO-related obesity, and some have suggested that education (CitationCorella et al., 2010) and breast-feeding may also be protective (CitationDedoussis et al., 2011). There is also evidence that high genetic risk in terms of common variants could mitigate against weight loss via bariatric surgery (CitationStill et al., 2011), caloric restriction (CitationMatsuo et al., 2011), or within a diabetes prevention programme (CitationDelahanty et al., 2012).
In addition to the common variants identified via GWAS, growing evidence suggests that genes influencing dopamine and serotonin function may play a role in obesity and appetite. For example, CitationSpitz et al. (2000) and others have reported greater obesity rates in those with the Taq1A A1 allele, which is linked with decreased dopamine receptor density, and CitationEpstein et al. (2007) have observed that food reinforcement, (i.e. the willingness to work for food) was particularly high in individuals with that allele. Polymorphisms in the serotonin gene SLC6A4, and the monoamine-regulating gene MAOA, which produces an enzyme that metabolizes dopamine, serotonin and noradenaline, may be associated with BMI, and SLC6A4 may be additionally associated with changes in BMI from adolescence to young adulthood (CitationFuemmeler et al., 2008). The next wave of genetic studies is also likely to unearth lower frequency obesity-associated variants with larger effects (e.g. copy number variants), as well as evidence for specific epigenetic changes which might influence weight (CitationDay & Loos, 2011).
Fat brains
As we outlined above, there is substantial evidence for genetic influence on appetitive characteristics, and many of the common obesity-associated genetic variants are highly expressed in the human brain. But what about the missing link between the brain and appetitive traits? Is there evidence that individual differences in appetite and body weight are associated with differential neurobiology? Might certain functional or structural characteristics of the brain predispose someone to eat more and gain weight? And, if so, is there evidence that the obesity and appetite genes identified thus far are related to these characteristics?
Neuroimaging studies of obesity and appetitive traits
There are now a considerable number of neuroimaging studies comparing obese and lean adults. Many of them have examined responses to food cues, which might be considered an implicit operationalization of the appetitive trait ‘food cue responsiveness’, and have revealed abnormal patterns of activation and connectivity among the obese in areas associated with reward, somatosensory processing, emotion, memory and cognitive control. Others have tested responses to food ingestion, revealing evidence for blunted responses in the striatum, hypothalamus and dorsolateral prefrontal cortex (dlPFC), which are suggestive of impaired ingestion reward and satiety/inhibition mechanisms (see CitationCarnell et al. (2012) for a review).
In addition, a small but growing number of studies relate established appetitive phenotypes assessed by questionnaire measures to neuroimaging data. For example, in a study of lean adults, ‘external food cue sensitivity’ scores were found to be associated with greater functional connectivity between the ventral striatum and emotion/motor preparation structures (amygdala, premotor cortex), and lesser connectivity between the ventral striatum and amygdala and attention-related regions (anterior cingulate cortex (ACC)), in response to appetizing versus bland pictures (CitationPassamonti et al., 2009). Across obese and lean subjects, scores on disinhibition (i.e. the tendency for restraint to break down when confronted by emotional or external cues to eat) has been associated with lesser pre-meal ACC responses to visual food versus non-food cues (CitationMartin et al., 2010), and scores on food addiction (i.e. a pattern of compulsive eating typified by tolerance, withdrawal and loss of control) have been associated with greater medial orbitofrontal cortex (OFC), amygdala and ACC responses to anticipated receipt of a milkshake, and lesser activation in the lateral OFC in response to actual receipt (CitationGearhardt et al., 2011).
Other studies have examined phenotypes that may involve eating in response to negative emotion. For example, CitationVolkow et al. (2003) found that higher emotional eating scores were associated with greater dopaminergic striatal responses to the taste and smell of food among lean adults, and in a study of lean and overweight adolescent girls CitationBohon et al. (2009) found that those high in emotional eating showed greater parahippocampal gyrus and ACC activation in response to a cue signalling receipt of a milkshake – as well as greater activation in the ventral pallidum, ACC and thalamus when actually tasting the milkshake. Meanwhile, studies of binge eating have suggested that binge eaters show heightened responses to high-palatability food cues in the frontal premotor area (CitationGeliebter et al., 2006), and medial OFC (CitationSchienle et al., 2009), increased responses to the sight and smell of real cooked food in the frontal and prefrontal cortex (CitationKarhunen et al., 2000), and caudate/putamen (increased dopamine activity) (CitationWang et al., 2011), and greater grey matter volume in the ACC and medial OFC (CitationSchafer et al., 2010).
Together these studies suggest that appetitive endophenotypes may be associated with subtly distinct neural traces, although since each study examined associations with a single trait it is not possible to tell whether these traces are independent. Studying them separately, however, may help refine understanding of the neural basis of appetite and possibly increase our power to detect gene–brain associations by allowing us to map specific genes to specific endophenotypes relating to their functional pathways.
Neuroimaging studies of children and high familial or genetic obesity risk
The studies above suggest that the obese state and appetitive traits have measurable neural correlates. However, they do not tell us whether these neural traits are capable of predicting weight gain. Indeed, since the majority of studies described were conducted in adults, any obesogenic neural dispositions may already have been translated into weight differences – or may have been prevented from doing so via the exertion of cognitive control – making predictive relationships harder to see. Studying children may shed light on causal mechanisms since observed abnormalities are less likely to be the result of long-term obesity or exposure to high-energy foods.
With the exception of the study by CitationBohon et al. (2009), no neuroimaging studies have assessed appetitive traits in children per se. However, a number of studies have compared fMRI responses to food cues in obese versus lean children, and these seem to largely replicate findings in adults. For example, CitationBruce et al. (2010) found greater pre-meal (after 4 h fasting) responses to food pictures in the PFC, greater post-meal (after 500 kcal standardized mixed meal) activation in the OFC, and relatively smaller post-meal (versus pre-meal) decreases in nucleus accumbens, limbic, and prefrontal activation among obese versus lean 10–17 year olds. In adolescent girls, CitationStice et al. (2010) reported positive associations between BMI and post-fast responses to appetizing food pictures in the putamen, OFC and frontal operculum. Most recently, CitationDavids et al. (2010) described increased dLPFC responses, but lesser caudate and hippocampus responses to food pictures in overweight/obese versus lean 9–16 year olds. CitationStice et al. (2008b) have also examined responses to conditioned cues signifying imminent milkshake delivery, and have shown greater cue-associated activation in obese versus lean adolescent girls in the anterior and middle insula and somatosensory cortex. Obese versus lean adolescent girls additionally show relatively decreased caudate responses to actual delivery of 0.5 mL tastes of milkshake (CitationStice et al., 2008a). There may also be structural differences between obese and lean adolescents: in a large study of obese and lean 14–21 year olds, CitationMaayan et al. (2011) reported lower OFC volume in the obese, and found that lower OFC volume was associated with higher dietary disinhibition scores – although only among the lean group.
While studies of children are informative, studying populations at high risk for obesity based on either family weight history (i.e. familial (genetic and environmental) risk) or possession of obesity-associated genes (i.e. genetic risk) presents an even better opportunity to assess ‘raw’ neural patterns before they have been behaviourally translated into an obese phenotype. Taking this approach, CitationStice et al. (2011) found that adolescents with two obese (versus two lean) parents showed greater caudate, frontal operculum and parietal operculum responses to milkshake tastes. No studies have yet linked commonly occurring obesity- and appetite-associated genetic variants to neural food cue responses. However, high-risk variants on FTO have been associated with volume reductions in the frontal and occipital lobes (CitationHo et al., 2010). There is also some evidence to suggest that genetic markers of dopamine function (e.g. Taq1A A1 allele) may exaggerate relationships between blunted neural responses to tastes of a milkshake and obesity (CitationStice et al., 2008a), and interact with patterns of brain responsivity to food cues to predict 1-year weight increases in adolescence (CitationStice et al., 2010). Together these studies suggest that certain functional and structural characteristics of the brain (e.g. abnormal reward area responses to food cues, blunted striatal responses to food ingestion, reduced volume in areas associated with reward and inhibition) may be influenced by familial and genetic factors, and may be associated with the risk of developing obesity in the future.
Discussion
We have described an array of interesting studies from the worlds of psychology, epidemiology, behavioural genetics, molecular genetics and neuroscience. But how can we make sense of these multi-disciplinary findings and what do they mean for an individual during his or her lifespan? We believe that individuals come into the world genetically and epigenetically loaded with powerful biologically influenced predispositions towards food (e.g. reduced satiety and heightened food cue responsiveness as a result of possessing high risk alleles for FTO, or Taq1A). These are captured in our biology and particularly in our brains. Environmental factors can certainly affect these dispositions (e.g. authoritative forms of parental restriction and encouragement may promote moderate appetites and eating behaviour in children), but often these influences are overwhelmed by the expression of innate tendencies, and environmental factors correlate with rather than cause appetitive behaviour (e.g. evidence for restriction and pressure to eat as responses to children's eating behaviour and weight). This model is roughly illustrated in .
Figure 1. Biobehavioural susceptibility model of child and adult obesity. In this putative model, individuals develop enduring, measurable appetitive traits early in life. These traits are influenced by genetic factors as well as factors in the early environment (e.g. parental feeding behaviour), and may be associated with differential patterns of neural activation in response to food and food cues. Together, these traits impact how each person responds to obesogenic influences in the modern-day food environment, which in turn influences his or her energy intake and adiposity level.
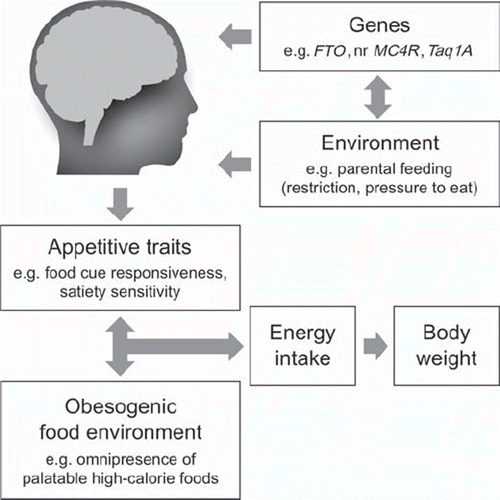
The model has empirical gaps. For example, very little is understood about how even the known obesity-associated genetic variants affect the brain to influence appetitive traits. Although many studies have looked for parental and environmental influences on appetitive traits, we do not thoroughly understand how these are represented at the neural level. We also lack large-scale prospective studies with the right measures and sufficient power to understand how genetic and environmental factors interact at a behavioural and neurological level to influence eating and predict weight trajectories. That said, new genetic and imaging technologies promise to rapidly accelerate progress over the coming decades, and continual improvements in our understanding of and ability to capture the relevant behavioural factors (e.g. interactions between parent feeding behaviours and children's eating behaviours) will allow us to make the most of these advances when they arrive. For example, it is becoming increasingly feasible to genotype large numbers of individuals and collect validated psychometric information about their eating traits. In parallel, neuroimaging methods are increasing in their spatial and temporal resolution and may one day be able to provide more information regarding pathways of information transfer, and therefore the precise causal mechanisms behind the influence of genes and environment on neural appetite networks. It should be noted that, as well as revealing potential neural precursors of overeating and obesity, brain imaging may help to establish biomarkers for appetitive responses which are independent of the social desirability effects that plague other assessments of appetite such as self-report questionnaires, food records, and public eating behaviour.
Future insights and those already gained may aid the development of targeted behavioural and biological interventions to increase healthy appetitive behaviours. For example, we may be able to behaviourally retrain children's appetitive traits (CitationJohnson, 2000; CitationFord et al., 2009), and neuroimaging could provide a nuanced and unbiased way of evaluating resulting alterations in the responsiveness of reward networks. The personal desire to change behaviour might be awakened by individualized genetic feedback (CitationMeisel et al., 2011), and personalized neurological feedback could have the same effect. Indeed, evidence that individuals can be trained to engage the dlPFC (CitationDelgado et al., 2008) – a key control area associated with successful dieting in a number of studies in adults (see CitationCarnell et al., 2012) – provides hope that fMRI-aided interventions, perhaps even incorporating real-time fMRI feedback (CitationHinds et al., 2011), could help individuals develop self-control over their reward responses to food. The identification of neural networks involved in specific obesogenic feeding phenotypes could also aid the development of novel pharmacological interventions to weight loss – although the enmeshment of appetite networks with motivation circuits involved in many essential human behaviours makes this goal a challenging one. The key message to convey to adults and children is that we all have different biologically determined predispositions towards food, and – even if these are largely immutable – we still have the power to exercise conscious control over the eating environments which elicit these predispositions. Some of us, though, will need to wield that control more than others.
Declaration of interest: Support was provided in part by US National Institutes of Health grant K99 DK 088360 (to S.C.). All authors participated sufficiently in this work to take public responsibility for the content. The authors alone are responsible for the content and writing of the paper.
References
- Ashcroft, J., Semmler, C., Carnell, S., van Jaarsveld, C.H. & Wardle, J. (2008). Continuity and stability of eating behaviour traits in children. European Journal of Clinical Nutrition, 62, 985–990.
- Bauer, F., Elbers, C.C., Adan, R.A., Loos, R.J., Onland-Moret, N.C., Grobbee, D.E., … van der Schouw, Y.T. (2009). Obesity genes identified in genome-wide association studies are associated with adiposity measures and potentially with nutrient-specific food preference. American Journal of Clinical Nutrition, 90, 951–959.
- Berkowitz, R.I., Moore, R.H., Faith, M.S., Stallings, V.A., Kral, T.V. & Stunkard, A.J. (2010). Identification of an obese eating style in 4-year-old children born at high and low risk for obesity. Obesity, 18, 505–512.
- Birch, L.L. & Fisher, J.O. (2000). Mothers’ child-feeding practices influence daughters’ eating and weight. American Journal of Clinical Nutrition, 71, 1054–1061.
- Birch, L.L., Fisher, J.O. & Davison, K.K. (2003). Learning to overeat: Maternal use of restrictive feeding practices promotes girls’ eating in the absence of hunger. American Journal of Clinical Nutrition, 78, 215–220.
- Birch, L.L., Fisher, J.O., Grimm-Thomas, K., Markey, C.N., Sawyer, R. & Johnson, S.L. (2001). Confirmatory factor analysis of the Child Feeding Questionnaire: A measure of parental attitudes, beliefs and practices about child feeding and obesity proneness. Appetite, 36, 201–210.
- Blissett, J. (2011). Relationships between parenting style, feeding style and feeding practices and fruit and vegetable consumption in early childhood. Appetite, 57, 826–831.
- Bohon, C., Stice, E. & Spoor, S. (2009). Female emotional eaters show abnormalities in consummatory and anticipatory food reward: A functional magnetic resonance imaging study. International Journal of Eating Disorders, 42, 210–221.
- Brann, L.S. & Skinner, J.D. (2005). More controlling child-feeding practices are found among parents of boys with an average body mass index compared with parents of boys with a high body mass index. Journal of the American Dietetcs Association, 105, 1411–1416.
- Brown, K.A., Ogden, J., Vogele, C. & Gibson, E.L. (2008). The role of parental control practices in explaining children's diet and BMI. Appetite, 50, 252–259.
- Bruce, A.S., Holsen, L.M., Chambers, R.J., Martin, L.E., Brooks, W.M., Zarcone, J.R., … Savage, C.R. (2010). Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. International Journal of Obesity, 34, 1494–1500.
- Campbell, K., Andrianopoulos, N., Hesketh, K., Ball, K., Crawford, D., Brennan, L., … Timperio, A. (2010). Parental use of restrictive feeding practices and child BMI z-score. A 3-year prospective cohort study. Appetite, 55, 84–88.
- Carnell, S., Cooke, L., Cheng, R., Robbins, A. & Wardle, J. (2011). Parental feeding behaviours and motivations. A qualitative study in mothers of UK pre-schoolers. Appetite, 57, 665–673.
- Carnell, S., Haworth, C.M., Plomin, R. & Wardle, J. (2008). Genetic influence on appetite in children. International Journal of Obesity, 32, 1468–1473.
- Carnell, S. & Wardle, J. (2007). Measuring behavioural susceptibility to obesity: Validation of the child eating behaviour questionnaire. Appetite, 48, 104–113.
- Carnell, S. & Wardle, J. (2008). Appetite and adiposity: A behavioral susceptibility model of obesity. American Journal of Clinical Nutrition, 88, 22–29.
- Carnell, S., Gibson, C., Benson, L., Ochner, C.N. & Geliebter, A. (2012). Neuroimaging and obesity: current knowledge and future directions. Obesity Reviews, 13, 43–56.
- Carper, J.L., Orlet Fisher, J. & Birch, L.L. (2000). Young girls’ emerging dietary restraint and disinhibition are related to parental control in child feeding. Appetite, 35, 121–129.
- Choquet, H. & Meyre, D. (2011). Genetics of obesity: What have we learned? Current Genomics, 12, 169–179.
- Corella, D., Carrasco, P., Sorli, J.V., Coltell, O., Ortega-Azorin, C., Guillen, M., … Ordovas, J.M. (2010). Education modulates the association of the FTO rs9939609 polymorphism with body mass index and obesity risk in the Mediterranean population. Nutrition, Metabolism & Cardiovascular Diseases. doi:10.1016/j.numecd.2010.10.006
- Davids, S., Lauffer, H., Thoms, K., Jagdhuhn, M., Hirschfeld, H., Domin, M., … Lotze, M. (2010). Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. International Journal of Obesity, 34, 94–104
- Day, F.R. & Loos, R.J. (2011). Developments in obesity genetics in the era of genome-wide association studies. Journal of Nutrigenetics and Nutrigenomics, 4, 222–238.
- de Castro, J.M. & Lilenfeld, L.R. (2005). Influence of heredity on dietary restraint, disinhibition, and perceived hunger in humans. Nutrition, 21, 446–455.
- Dedoussis, G.V., Yannakoulia, M., Timpson, N.J., Manios, Y., Kanoni, S., Scott, R.A., … Lyon, H.N. (2011). Does a short breastfeeding period protect from FTO-induced adiposity in children? International Journal of Pediatric Obesity, 6, e326–335.
- Delahanty, L.M., Pan, Q., Jablonski, K.A., Watson, K.E., McCaffery, J.M., Shuldiner, A., … Diabetes Prevention Program Research Group (2012). Genetic predictors of weight loss and weight regain after intensive lifestyle modification, metformin treatment, or standard care in the Diabetes Prevention Program. Diabetes Care, 35, 363–366.
- Delgado, M.R., Gillis, M.M. & Phelps, E.A. (2008). Regulating the expectation of rewards via cognitive strategies. Nature Neuroscience, 11, 880–881.
- den Hoed, M., Ekelund, U., Brage, S., Grontved, A., Zhao, J.H., Sharp, S.J., … Loos, R.J. (2010). Genetic susceptibility to obesity and related traits in childhood and adolescence: influence of loci identified by genome-wide association studies. Diabetes, 59, 2980–2988.
- Drucker, R.R., Hammer, L.D., Agras, W.S. & Bryson, S. (1999). Can mothers influence their child's eating behavior? Journal of Developmental & Behavioral Pediatrics, 20, 88–92.
- Epstein, L.H., Temple, J.L., Neaderhiser, B.J., Salis, R.J., Erbe, R.W. & Leddy, J.J. (2007). Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behavioral Neuroscience, 121, 877–886.
- Faith, M.S., Berkowitz, R.I., Stallings, V.A., Kerns, J., Storey, M. & Stunkard, A.J. (2004). Parental feeding attitudes and styles and child body mass index: Prospective analysis of a gene-environment interaction. Pediatrics, 114, e429–436.
- Faith, M.S. & Hittner, J.B. (2010). Infant temperament and eating style predict change in standardized weight status and obesity risk at 6 years of age. International Journal of Obesity, 34, 1515–1523.
- Farooqi, I.S. & O'Rahilly, S. (2008). Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nature Clinical Practice Endocrinology & Metabolism, 4, 569–577.
- Farrow, C. & Blissett, J. (2006). Does maternal control during feeding moderate early infant weight gain? Pediatrics, 118, e293–298.
- Farrow, C. & Blissett, J. (2012). Stability and continuity of parentally reported child eating behaviours and feeding practices from 2 to 5 years of age. Appetite, 58, 151–156.
- Farrow, C.V. & Blissett, J. (2008). Controlling feeding practices: Cause or consequence of early child weight? Pediatrics, 121, e164–169.
- Fisher, J.O. & Birch, L.L. (1999a). Restricting access to foods and children's eating. Appetite, 32, 405–419.
- Fisher, J.O. & Birch, L.L. (1999b). Restricting access to palatable foods affects children's behavioral response, food selection, and intake. American Journal of Clinical Nutrition, 69, 1264–1272.
- Fisher, J.O. & Birch, L.L. (2002). Eating in the absence of hunger and overweight in girls from 5 to 7 y of age. American Journal of Clinical Nutrition, 76, 226–231.
- Fisher, J.O., Cai, G., Jaramillo, S.J., Cole, S.A., Comuzzie, A.G. & Butte, N.F. (2007). Heritability of hyperphagic eating behavior and appetite-related hormones among Hispanic children. Obesity, 15, 1484–1495.
- Fisher, J.O., Mitchell, D.C., Smiciklas-Wright, H. & Birch, L.L. (2002). Parental influences on young girls’ fruit and vegetable, micronutrient, and fat intakes. Journal of the American Dietetics Association, 102, 58–64.
- Ford, A.L., Bergh, C., Sodersten, P., Sabin, M.A., Hollinghurst, S., Hunt, L.P., … Shield, J.P.H. (2009). Treatment of childhood obesity by retraining eating behaviour: Randomised controlled trial. British Medical Journal, 340, b5388.
- Frayling, T.M., Timpson, N.J., Weedon, M.N., Zeggini, E., Freathy, R.M., Lindgren, C.M., … McCarthy, M.I. (2007). A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science, 316, 889–894.
- Fuemmeler, B.F., Agurs-Collins, T.D., McClernon, F.J., Kollins, S.H., Kail, M.E., Bergen, A.W., & Ashley-Koch, A.E. (2008). Genes implicated in serotonergic and dopaminergic functioning predict BMI categories. Obesity, 16, 348–355.
- Galloway, A.T., Fiorito, L.M., Francis, L.A. & Birch, L.L. (2006). ‘Finish your soup’: Counterproductive effects of pressuring children to eat on intake and affect. Appetite, 46, 318–323.
- Gearhardt, A.N., Yokum, S., Orr, P.T., Stice, E., Corbin, W.R. & Brownell, K.D. (2011). Neural correlates of food addiction. Archives of General Psychiatry, 68, 808–816.
- Geliebter, A., Ladell, T., Logan, M., Schneider, T., Sharafi, M. & Hirsch, J. (2006). Responsivity to food stimuli in obese and lean binge eaters using functional MRI. Appetite, 46, 31–35.
- Gerken, T., Girard, C.A., Tung, Y.C., Webby, C.J., Saudek, V., Hewitson, K.S., … Schofield, C.J. (2007). The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science, 318, 1469–1472.
- Gregory, J.E., Paxton, S.J. & Brozovic, A.M. (2010a). Maternal feeding practices, child eating behaviour and body mass index in preschool-aged children: A prospective analysis. International Journal of Behavioral Nutrition and Physical Activity, 7, 55.
- Gregory, J.E., Paxton, S.J. & Brozovic, A.M. (2010b). Pressure to eat and restriction are associated with child eating behaviours and maternal concern about child weight, but not child body mass index, in 2- to 4-year-old children. Appetite, 54, 550–556.
- Gregory, J.E., Paxton, S.J. & Brozovic, A.M. (2011). Maternal feeding practices predict fruit and vegetable consumption in young children. Results of a 12-month longitudinal study. Appetite, 57, 167–172.
- Hill, C., Llewellyn, C.H., Saxton, J., Webber, L., Semmler, C., Carnell, S., … Wardle, J. (2008). Adiposity and ‘eating in the absence of hunger’ in children. International Journal of Obesity, 32, 1499–1505.
- Hinds, O., Ghosh, S., Thompson, T.W., Yoo, J.J., Whitfield-Gabrieli, S., Triantafyllou, C. & Gabrieli, J.D. (2011). Computing moment-to-moment BOLD activation for real-time neurofeedback. Neuroimage, 54, 361–368.
- Ho, A.J., Stein, J.L., Hua, X., Lee, S., Hibar, D.P., Leow, A.D., … Alzheimer's Disease Neuroimaging Initiative (2010). A commonly carried allele of the obesity-related FTO gene is associated with reduced brain volume in the healthy elderly. Proceedings of the National Academy of Sciences of the United States of America, 107, 8404–8409.
- Hughes, S.O., Power, T.G., Orlet Fisher, J., Mueller, S. & Nicklas, T.A. (2005). Revisiting a neglected construct: Parenting styles in a child-feeding context. Appetite, 44, 83–92.
- Hughes, S.O., Shewchuk, R.M., Baskin, M.L., Nicklas, T.A. & Qu, H. (2008). Indulgent feeding style and children's weight status in preschool. Journal of Developmental & Behavioral Pediatrics, 29, 403–410.
- Jansen, E., Mulkens, S. & Jansen, A. (2007). Do not eat the red food!: Prohibition of snacks leads to their relatively higher consumption in children. Appetite, 49, 572–577.
- Johnson, S.L. (2000). Improving preschoolers’ self-regulation of energy intake. Pediatrics, 106, 1429–1435.
- Johnson, S.L. & Birch, L.L. (1994). Parents’ and children's adiposity and eating style. Pediatrics, 94, 653–661.
- Karhunen, L.J., Vanninen, E.J., Kuikka, J.T., Lappalainen, R.I., Tiihonen, J. & Uusitupa, M.I. (2000). Regional cerebral blood flow during exposure to food in obese binge eating women. Psychiatry Research, 99, 29–42.
- Kilpelainen, T.O., den Hoed, M., Ong, K.K., Grontved, A., Brage, S., Jameson, K., … Loos, R.J. (2011). Obesity-susceptibility loci have a limited influence on birth weight: A meta-analysis of up to 28,219 individuals. American Journal of Clinical Nutrition, 93, 851–860.
- Lewis, M. & Worobey, J. (2011). Mothers and toddlers lunch together. The relation between observed and reported behavior. Appetite, 56, 732–736.
- Llewellyn, C.H., van Jaarsveld, C.H., Boniface, D., Carnell, S. & Wardle, J. (2008). Eating rate is a heritable phenotype related to weight in children. American Journal of Clinical Nutrition, 88, 1560–1566.
- Llewellyn, C.H., van Jaarsveld, C.H., Johnson, L., Carnell, S. & Wardle, J. (2010). Nature and nurture in infant appetite: Analysis of the Gemini twin birth cohort. American Journal of Clinical Nutrition, 91, 1172–1179.
- Llewellyn, C.H., van Jaarsveld, C.H., Johnson, L., Carnell, S. & Wardle, J. (2011). Development and factor structure of the Baby Eating Behaviour Questionnaire in the Gemini birth cohort. Appetite, 57, 388–396.
- Llewellyn, C.H., van Jaarsveld, C.H., Plomin, R., Fisher, A. & Wardle, J. (2012). Inherited behavioral susceptibility to adiposity in infancy: A multivariate genetic analysis of appetite and weight in the Gemini birth cohort. American Journal of Clinical Nutrition. doi:10.3945/ajcn.111.023671
- Maayan, L., Hoogendoorn, C., Sweat, V. & Convit, A. (2011). Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity, 19, 1382–1387.
- Maes, H.H., Neale, M.C. & Eaves, L.J. (1997). Genetic and environmental factors in relative body weight and human adiposity. Behavioral Genetics, 27, 325–351.
- Martin, L.E., Holsen, L.M., Chambers, R.J., Bruce, A.S., Brooks, W.M., Zarcone, J.R., … Savage, C.R. (2010). Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity, 18, 254–260.
- Matsuo, T., Nakata, Y., Murotake, Y., Hotta, K. & Tanaka, K. (2011). Effects of FTO genotype on weight loss and metabolic risk factors in response to calorie restriction among Japanese women. Obesity, doi: 10.1038/oby.2011.322
- McPhie, S., Skouteris, H., McCabe, M., Ricciardelli, L.A., Milgrom, J., Baur, L.A., … Dell'Aquila, D. (2011). Maternal correlates of preschool child eating behaviours and body mass index: A cross-sectional study. International Journal of Pediatric Obesity, 6, 476–480.
- Meisel, S.F., Walker, C. & Wardle, J. (2011). Psychological responses to genetic testing for weight gain: A vignette study. Obesity, 20, 540–546.
- Neale, B.M., Mazzeo, S.E. & Bulik, C.M. (2003). A twin study of dietary restraint, disinhibition and hunger: an examination of the eating inventory (three factor eating questionnaire). Twin Research, 6, 471–478.
- Parkinson, K.N., Drewett, R.F., Le Couteur, A.S. & Adamson, A.J. (2010). Do maternal ratings of appetite in infants predict later Child Eating Behaviour Questionnaire scores and body mass index? Appetite, 54, 186–190.
- Passamonti, L., Rowe, J.B., Schwarzbauer, C., Ewbank, M.P., von dem Hagen, E. & Calder, A.J. (2009). Personality predicts the brain's response to viewing appetizing foods: The neural basis of a risk factor for overeating. Journal of Neuroscience, 29, 43–51.
- Patrick, H., Nicklas, T.A., Hughes, S.O. & Morales, M. (2005). The benefits of authoritative feeding style: Caregiver feeding styles and children's food consumption patterns. Appetite, 44, 243–249.
- Payne, L.O., Galloway, A.T. & Webb, R.M. (2011). Parental use of differential restrictive feeding practices with siblings. International Journal of Pediatric Obesity, 6, e540–546.
- Powell, F.C., Farrow, C.V. & Meyer, C. (2011). Food avoidance in children. The influence of maternal feeding practices and behaviours. Appetite, 57, 683–692.
- Provencher, V., Perusse, L., Bouchard, L., Drapeau, V., Bouchard, C., Rice, T., … Lemieux, S. (2005). Familial resemblance in eating behaviors in men and women from the Quebec Family Study. Obesity Research, 13, 1624–1629.
- Razquin, C., Martinez, J.A., Martinez-Gonzalez, M.A., Bes-Rastrollo, M., Fernandez-Crehuet, J. & Marti, A. (2010). A 3-year intervention with a Mediterranean diet modified the association between the rs9939609 gene variant in FTO and body weight changes. International Journal of Obesity, 34, 266–272.
- Rissanen, A., Hakala, P., Lissner, L., Mattlar, C.E., Koskenvuo, M. & Ronnemaa, T. (2002). Acquired preference especially for dietary fat and obesity: A study of weight-discordant monozygotic twin pairs. International Journal of Obesity Related Metabolic Disorders, 26, 973–977.
- Roemmich, J.N., White, T.M., Paluch, R. & Epstein, L.H. (2010). Energy intake, parental control of children's eating, and physical activity in siblings discordant for adiposity. Appetite, 55, 325–331.
- Ruiz, J.R., Labayen, I., Ortega, F.B., Legry, V., Moreno, L.A., Dallongeville, J., … HELENA Study Group (2010). Attenuation of the effect of the FTO rs9939609 polymorphism on total and central body fat by physical activity in adolescents: The HELENA study. Archives of Pediatrics & Adolescent Medicine, 164, 328–333.
- Schafer, A., Vaitl, D. & Schienle, A. (2010). Regional grey matter volume abnormalities in bulimia nervosa and binge-eating disorder. Neuroimage, 50, 639–643.
- Schienle, A., Schafer, A., Hermann, A. & Vaitl, D. (2009). Binge-eating disorder: Reward sensitivity and brain activation to images of food. Biological Psychiatry, 65, 654–661.
- Schousboe, K., Willemsen, G., Kyvik, K.O., Mortensen, J., Boomsma, D.I., Cornes, B.K., … Harris, J.R. (2003). Sex differences in heritability of BMI: A comparative study of results from twin studies in eight countries. Twin Research, 6, 409–421.
- Scuteri, A., Sanna, S., Chen, W.M., Uda, M., Albai, G., Strait, J., … Abecasis, G.R. (2007). Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genetics, 3, e115.
- Silventoinen, K. & Kaprio, J. (2009). Genetics of tracking of body mass index from birth to late middle age: Evidence from twin and family studies. Obesity Facts, 2, 196–202.
- Silventoinen, K., Rokholm, B., Kaprio, J. & Sorensen, T.I. (2010). The genetic and environmental influences on childhood obesity: A systematic review of twin and adoption studies. International Journal of Obesity, 34, 29–40.
- Sleddens, E.F., Kremers, S.P., De Vries, N.K. & Thijs, C. (2010). Relationship between parental feeding styles and eating behaviours of Dutch children aged 6–7. Appetite, 54, 30–36.
- Sonestedt, E., Roos, C., Gullberg, B., Ericson, U., Wirfalt, E. & Orho-Melander, M. (2009). Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity. American Journal of Clinical Nutrition, 90, 1418–1425.
- Speakman, J.R., Rance, K.A. & Johnstone, A.M. (2008). Polymorphisms of the FTO gene are associated with variation in energy intake, but not energy expenditure. Obesity, 16, 1961–1965.
- Speliotes, E.K., Willer, C.J., Berndt, S.I., Monda, K.L., Thorleifsson, G., Jackson, A.U., … Loos, R.J. (2010). Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nature Genetics, 42, 937–948.
- Spence, J.C., Carson, V., Casey, L. & Boule, N. (2011). Examining behavioural susceptibility to obesity among Canadian pre-school children: The role of eating behaviours. International Journal of Pediatric Obesity, 6, e501–507.
- Spitz, M.R., Michelle, A., Detry, P.P., Yaohua, H., Christopher, I., Amos, W.K.H., … Wu, X. (2000). Variant alleles of the D2 dopamine receptor gene and obesity. Nutrition Research, 20, 371–380.
- Steinle, N.I., Hsueh, W.C., Snitker, S., Pollin, T.I., Sakul, H., St Jean, P.L., … Shuldiner, A.R. (2002). Eating behavior in the Old Order Amish: Heritability analysis and a genome-wide linkage analysis. American Journal of Clinical Nutrition, 75, 1098–1106.
- Stice, E., Spoor, S., Bohon, C. & Small, D.M. (2008a). Relation between obesity and blunted striatal response to food is moderated by Taq1A A1 allele. Science, 322, 449–452.
- Stice, E., Spoor, S., Bohon, C., Veldhuizen, M.G. & Small, D.M. (2008b). Relation of reward from food intake and anticipated food intake to obesity: A functional magnetic resonance imaging study. Journal of Abnormal Psychology, 117, 924–935.
- Stice, E., Yokum, S., Bohon, C., Marti, N. & Smolen, A. (2010). Reward circuitry responsivity to food predicts future increases in body mass: Moderating effects of DRD2 and DRD4. Neuroimage, 50, 1618–1625.
- Stice, E., Yokum, S., Burger, K.S., Epstein, L.H. & Small, D.M. (2011). Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. Journal of Neuroscience, 31, 4360–4366.
- Still, C.D., Wood, G.C., Chu, X., Erdman, R., Manney, C.H., Benotti, P.N., … Gerhard, G.S. (2011). High allelic burden of four obesity SNPs is associated with poorer weight loss outcomes following gastric bypass surgery. Obesity, 19, 1676–1683.
- Stratigopoulos, G., Padilla, S.L., LeDuc, C.A., Watson, E., Hattersley, A.T., McCarthy, M.I., … Leibel, R.L. (2008). Regulation of FTO/FTM gene expression in mice and humans. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology, 294, R1185–1196.
- Stutzmann, F., Cauchi, S., Durand, E., Calvacanti-Proenca, C., Pigeyre, M., Hartikainen, A.L., … Froguel, P. (2009). Common genetic variation near MC4R is associated with eating behaviour patterns in European populations. International Journal of Obesity, 33, 373–378.
- Sud, S., Tamayo, N.C., Faith, M.S. & Keller, K.L. (2010). Increased restrictive feeding practices are associated with reduced energy density in 4–6-year-old, multi-ethnic children at ad libitum laboratory test-meals. Appetite, 55, 201–207.
- Tan, C.C. & Holub, S.C. (2011). Children's self-regulation in eating: Associations with inhibitory control and parents’ feeding behavior. Journal of Pediatric Psychology, 36, 340–345.
- Tanofsky-Kraff, M., Han, J.C., Anandalingam, K., Shomaker, L.B., Columbo, K.M., Wolkoff, L.E., … Yanovski, J.A. (2009). The FTO gene rs9939609 obesity-risk allele and loss of control over eating. American Journal of Clinical Nutrition, 90, 1483–1488.
- Tholin, S., Rasmussen, F., Tynelius, P. & Karlsson, J. (2005). Genetic and environmental influences on eating behavior: The Swedish Young Male Twins Study. American Journal of Clinical Nutrition, 81, 564–569.
- Timpson, N.J., Emmett, P.M., Frayling, T.M., Rogers, I., Hattersley, A.T., McCarthy, M.I. & Davey Smith, G. (2008). The fat mass- and obesity-associated locus and dietary intake in children. American Journal of Clinical Nutrition, 88, 971–978.
- Tung, Y.C., Ayuso, E., Shan, X., Bosch, F., O'Rahilly, S., Coll, A.P. & Yeo, G.S.H. (2010). Hypothalamic-specific manipulation of FTO, the ortholog of the human obesity gene FTO, affects food intake in rats. PLoS ONE, 5, e8771.
- van Jaarsveld, C.H., Llewellyn, C.H., Johnson, L. & Wardle, J. (2011). Prospective associations between appetitive traits and weight gain in infancy. American Journal of Clinical Nutrtion, 94, 1562–1567.
- van Strien, T. & Bazelier, F.G. (2007). Perceived parental control of food intake is related to external, restrained and emotional eating in 7–12-year-old boys and girls. Appetite, 49, 618–625.
- Van Strien, T., van Niekerk, R. & Ouwens, M.A. (2009). Perceived parental food controlling practices are related to obesogenic or leptogenic child life style behaviors. Appetite, 53, 151–154.
- Ventura, A.K. & Birch, L.L. (2008). Does parenting affect children's eating and weight status? International Journal of Behavioral Nutrition and Physical Activity, 5, 15.
- Volkow, N.D., Wang, G.J., Maynard, L., Jayne, M., Fowler, J.S., Zhu, W., … Pappas, N. (2003). Brain dopamine is associated with eating behaviors in humans. International Journal of Eating Disorders, 33, 136–142.
- Wang, G.J., Geliebter, A., Volkow, N.D., Telang, F.W., Logan, J., Jayne, M.C., … Fowler, J.S. (2011). Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity, 19, 1601–1608.
- Wardle, J., Carnell, S. & Cooke, L. (2005). Parental control over feeding and children's fruit and vegetable intake: How are they related? Journal of the American Dietetics Association, 105, 227–232.
- Wardle, J., Carnell, S., Haworth, C.M. & Plomin, R. (2008). Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. American Journal of Clinical Nutrition, 87, 398–404.
- Wardle, J., Llewellyn, C., Sanderson, S. & Plomin, R. (2009). The FTO gene and measured food intake in children. International Journal of Obesity, 33, 42–45.
- Webber, L., Cooke, L., Hill, C. & Wardle, J. (2010a). Associations between children's appetitive traits and maternal feeding practices. Journal of the American Dietetics Association, 110, 1718–1722.
- Webber, L., Cooke, L., Hill, C. & Wardle, J. (2010b). Child adiposity and maternal feeding practices: A longitudinal analysis. American Journal of Clinical Nutrition, 92, 1423–1428.
- Webber, L., Hill, C., Cooke, L., Carnell, S. & Wardle, J. (2010c). Associations between child weight and maternal feeding styles are mediated by maternal perceptions and concerns. European Journal of Clinical Nutrition, 64, 259–265.
- Webber, L., Hill, C., Saxton, J., van Jaarsveld, C.H. & Wardle, J. (2009). Eating behaviour and weight in children. International Journal of Obesity, 33, 21–28.
- Wyse, R., Campbell, E., Nathan, N. & Wolfenden, L. (2011). Associations between characteristics of the home food environment and fruit and vegetable intake in preschool children: A cross-sectional study. BMC Public Health, 11, 938.