Abstract
Objective: Tofacitinib is a novel, oral Janus kinase inhibitor being investigated for psoriasis. This study assessed the relationship between pruritus and clinical signs of psoriasis (assessed by Physician’s Global Assessment [PGA]) in patients with moderate-to-severe chronic plaque psoriasis receiving tofacitinib.
Methods: In this 16-week (12-week treatment period, 4-week observation period), double-blind, placebo-controlled, phase IIb study (NCT00678210), 197 patients were randomized to tofacitinib 2, 5 or 15 mg BID, or placebo. Pruritus was patient assessed using the Itch Severity Score (ISS), a 0–10 (10 = worst itching) rating scale recorded daily from baseline to week 2 and at study visits. Mediation modeling was used to determine relationships between ISS (average score weeks 2–12), PGA (average score weeks 2–12) and treatment groups.
Results: Mediation analysis showed that 70.2–80.5% (p < 0.001 versus placebo) of tofacitinib’s effect on pruritus was direct, and mostly independent of improvements in erythema, induration and scaling. ISS measurements had acceptable test–retest reliability. Correlation analyses with clinical outcomes supported the validity of the ISS as a pruritus measure.
Conclusions: Tofacitinib has a direct, beneficial effect on patient-reported pruritus independent from improvements in clinician-reported psoriasis severity signs. The ISS demonstrated favorable psychometric characteristics, supporting its use as a pruritus assessment tool.
Introduction
Psoriasis is a chronic and debilitating immune-mediated disease that causes systemic inflammation with prominent skin manifestations and is characterized by well-demarcated erythematous and scaling plaques, that can be both painful and pruritic (Citation1).
Pruritus is a frequent and bothersome symptom of psoriasis that is observed in approximately 70–90% of patients during disease exacerbation (Citation2–6). In a study by Globe and colleagues, most patients rated pruritus as the most important (79%), most severe (79%) and most troublesome (61%) symptom that also had a negative impact on daily activities. In the same study, dermatologists most frequently mentioned pruritus as a key symptom of psoriasis and also acknowledged its importance to patients (Citation7).
Several therapies are currently used to treat pruritus for patients with psoriasis, including moisturizers and emollients, antihistamines, topical corticosteroids, topical salicylates, phototherapy and biologics (Citation8). However, current therapies have been shown to be largely ineffective, with a substantial proportion of patients reporting dissatisfaction with available treatment options (Citation9).
Tofacitinib is a novel, oral Janus kinase inhibitor that is being investigated for psoriasis. A phase IIb study was conducted to assess the efficacy and safety of three twice-daily (BID) regimens of tofacitinib versus placebo in patients with moderate-to-severe chronic plaque psoriasis who were candidates for systemic therapy or phototherapy. Full details of the trial and results are reported elsewhere (Citation10). Briefly, treatment with tofacitinib resulted in early and sustained clinical improvement with a manageable safety profile (Citation10), and was shown to favorably impact on a wide range of health-related quality of life (HRQoL) outcomes and self-assessment of disease severity and symptoms (Citation11). Tofacitinib had rapid and sustained clinically meaningful improvements in pruritus, as measured by the Itch Severity Score (ISS) (Citation12).
Although pruritus is an important symptom of psoriasis, it is not assessed by the Psoriasis Area and Severity Index (PASI) or Physician’s Global Assessment (PGA) scales, which are typically used as primary and/or secondary endpoints in psoriasis clinical trials. The PASI and PGA examine three clinical signs of psoriasis: erythema, induration and scaling. In contrast, pruritus is typically assessed through direct patient report.
Here, data from the phase IIb study were used to perform mediation modeling of the ISS to explicate the inter-relationships among pruritus and the clinical signs of psoriasis (erythema, induration and scaling), as well as the underlying mechanisms by which tofacitinib affects pruritus in patients with moderate-to-severe chronic plaque psoriasis. In addition, psychometric assessments were performed on the ISS.
Methods
Study design and patients
Data are from a previously reported phase IIb, randomized, double-blind, parallel-group, placebo-controlled study conducted in the United States and Canada (Citation10). The trial is registered with ClinicalTrials.gov (NCT00678210). All patients provided informed, written consent before participation in any study procedures. The final protocol, amendments and informed consent documentation were reviewed and approved by the Institutional Review Board and the Independent Ethics Committee of the investigational centers.
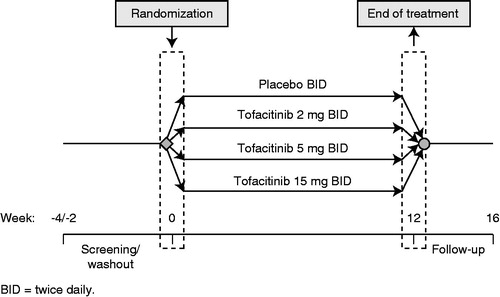
Full details of the trial design, inclusion and exclusion criteria and patient population have been described previously (Citation10). Briefly, eligible patients were aged ≥18 years with a diagnosis of stable plaque-type psoriasis (for ≥6 months prior to first dose of study drug) covering at least 15% of their total body surface area, and had a PASI score ≥13 at the time of first study dose. Patients were randomized to receive tofacitinib 2 mg, 5 mg, 15 mg BID, or placebo (). All patients were treated for 12 weeks, with a 4-week follow-up observational period.
The primary endpoint was the proportion of patients achieving ≥75% reduction in PASI score relative to baseline at week 12. The proportion of patients with a PGA of psoriasis of “clear” or “almost clear” was a key secondary endpoint. Patient-reported outcomes data were also recorded and are reported elsewhere (Citation11).
Measurement of the ISS
The severity of pruritus was assessed using the ISS, a single item that asked patients to rate their pruritus on a 0 (“no itching”) to 10 (“worst possible itching”) numeric rating scale, with a recall period of the past 24 h. Patients completed the ISS daily, at the same time as they took their morning dose of medication, for the first 2 weeks of treatment using a patient diary, and also at study visits.
Other clinical outcomes
Other assessments included the Dermatology Life Quality Index (DLQI), PGA and Patient’s Global Assessment (PtGA).
The DLQI was used to assess patients’ perception of the impact of a chronic skin disease on different aspects of HRQoL over the previous week using a 4-point Likert scale: “not at all/not relevant” [0], “a little” [1], “a lot” [2], and “very much” [3] with higher scores indicating greater impairment of HRQoL (Citation13). Of particular relevance is question 1, which assesses “how itchy, sore, burning or stinging” the skin has been.
The PGA was used to measure the severity of psoriasis, specifically erythema, induration and scaling of psoriasis plaques across the whole body (Citation14). Assessments were conducted by physicians at every study visit using a 5-category scale: “clear” [0], “almost clear” [1], “mild” [2], “moderate” [3] and “severe” [4]. Separate ratings were made for erythema, induration and scaling using the 0–5 scale based on the appropriate morphologic descriptors. The average of the three severity ratings was rounded to the nearest whole number to determine the overall PGA score.
The PtGA is a single-item, patient-completed assessment that is used to evaluate the overall extent of psoriasis-related cutaneous disease at a particular point in time using a 5-category scale: “clear” (no psoriasis), “almost clear”, “mild”, “moderate” and “severe”. The PtGA used the same categories as the PGA; however, unlike the PGA, there were no morphologic descriptors of erythema, induration and scaling.
Test–retest reliability
Test–retest reliability was assessed by estimating the intraclass correlation coefficient (ICC) using daily ISS measurements during the first 2 weeks from all treatment groups. All data were post-treatment and only patients who did not change on the PGA during the first 2 weeks (i.e. PGA at baseline was equal to PGA at week 2) were used to estimate the ICC values. The ISS ICC for a single measurement was estimated based on the estimations of the between-patient error variance (e1) to the within-patient error variance (e2) using the formula: ICC = e1/(e1 + e2), with ICC values larger than 0.7 indicating acceptable reliability.
Correlation analyses
Based on data from all treatment groups at post-baseline (weeks 2, 4, 8 and 12) and at follow-up visits (weeks 14 and 16), Pearson’s correlation coefficients were calculated between the ISS and the following variables: PASI, DLQI, PGA and PtGA. Evidence for convergent validity was based on a Pearson’s correlation coefficient of ≥0.40, consistent with a meaningful correlation (Citation15). Correlation coefficients of <0.30 were taken as evidence for divergent validity (Citation16). Correlation coefficients between 0.30 and 0.40 were interpreted as no evidence to reject either convergent validity or divergent validity (Citation17). Based on the content of the different instruments, we hypothesized that the ISS would show evidence of convergent validity with the PASI, DLQI, PGA and PtGA.
Mediation modeling
Mediation modeling (Citation18,Citation19) was used to prospectively determine postulated inter-relationships among erythema, induration, and scaling (as measured by PGA), pruritus (as measured by ISS) and treatment groups to investigate whether tofacitinib affected pruritus directly or whether improvement in pruritus should be attributed to indirect effects of treatment via improvements in erythema, induration and scaling. The mediation model included ISS as the outcome variable (average score from study visits during weeks 2–12 for each patient), PGA as the mediator (average score during weeks 2–12 for each patient) and treatment groups as independent variables (tofacitinib 2, 5 and 15 mg BID [represented by 3 binary variables] versus placebo).
Implementation
All of the statistical analyses reported in this paper were performed using SAS/STAT® version 9.2 (SAS Institute Inc., Cary, NC; 2008).
Results
Patient demography and baseline characteristics
Patient demographics and baseline characteristics have been reported previously and were similar across the treatment groups (Citation10). Of 197 patients randomized, most were male (n = 125; 63.5%) and white (n = 159; 80.7%), with a mean (standard deviation) age of 44.3 (13.3) years. Mean (standard deviation) body surface area involvement at baseline ranged from 29.8% (13.4%) to 31.9% (18.8%) across treatment groups, and baseline mean (standard deviation) PASI scores ranged from 21.2 (8.1) to 22.6 (10.3) across treatment groups. Baseline means (standard deviations) for the ISS in the tofacitinib 2, 5 and 15 mg BID, and placebo groups were 7.04 (2.65), 6.98 (2.27), 6.96 (2.23) and 6.78 (2.77), respectively, indicating a moderate to high level of pruritus. Only 1% of patients (n = 2) reported a score of 0 (“no itching”) at baseline.
Test–retest reliability
Using daily ISS measurements from baseline to day 14, the ICC value was 0.76, indicating that daily ISS measurements had acceptable reliability.
Correlation analyses
Correlations between the ISS and selected endpoints are presented in . The ISS showed evidence of convergent validity (Pearson’s correlation coefficient ≥0.40) with the DLQI (0.70–0.73), PtGA (0.61–0.71) and a tendency toward convergent validity with PASI (0.32–0.55) and PGA (0.33–0.52). When the ISS was correlated with the first item of the DLQI, which asks patients “Over the last week, how itchy, sore, painful, or stinging has your skin been?”, the correlations were 0.82 and 0.83 at week 4 and week 12, respectively (both p < 0.0001).
Table 1. Post-baseline correlations between ISS and other endpoints.
Mediation modeling
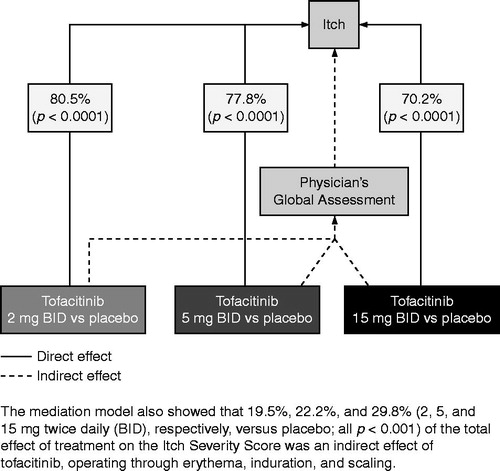
The mediation model showed that 80.5%, 77.8% and 70.2% (2, 5 and 15 mg BID, respectively, versus placebo; all p < 0.0001) of the total effect of treatment on pruritus (as measured by ISS) was a direct effect of tofacitinib, independent of improvement in erythema, induration and scaling, as measured by the PGA (). Conversely, the mediation model also showed that only 19.5%, 22.2% and 29.8% (2, 5 and 15 mg BID, respectively; all p < 0.0001 versus placebo) of the total effect of treatment on the ISS was an indirect effect of tofacitinib, operating through erythema, induration and scaling.
Discussion
Pruritus is listed in medical descriptions as a key symptom of psoriasis (Citation20) and, from a patient’s perspective, is one of the most bothersome features of the disease (Citation7). Indeed, the high frequency with which pruritus was reported in this patient population with moderate-to-severe psoriasis (99% of patients reporting ISS ≥1) indicates the extent to which pruritus affects this population.
It has been suggested that if the visible symptoms of psoriasis improve (e.g. erythema, induration and scaling), then pruritus should also improve. However, the mediation modeling revealed that 70.2–80.5% of the total effect of treatment on patient-reported pruritus was a direct effect of tofacitinib and was independent of improvements in erythema, induration and scaling, as assessed by the physician. This suggests that an improvement in erythema, induration and scaling is not necessarily associated with an improvement in pruritus (and vice versa). The pathogenesis of pruritus may be different from that of erythema, induration and scaling.
The ISS demonstrated acceptable test–retest reliability, and correlation analyses supported the validity of the ISS as a measurement of pruritus in this population. The moderate correlations of the ISS with the PGA and PASI provide additional evidence to support the hypothesis that the variables measure related yet distinct concepts.
Overall, the findings in this study suggest that pruritus should be viewed as an independent manifestation of psoriasis that is distinct from physician assessment of the clinical signs of disease (i.e. erythema, induration and scaling). Furthermore, the results suggest that tofacitinib acts directly to improve itch severity, which is independent from improvements in erythema, induration and scaling, as measured by a physician’s assessment. In order to obtain a more complete understanding of the impact of psoriasis on the patient, as well as treatment effectiveness, physicians should ask their patients about the presence and severity of pruritus and not rely solely on clinical symptoms, such as erythema, induration and scaling, when assessing disease severity.
Acknowledgements
The authors would like to thank the Study A3921047 investigators, patients and study team.
Investigators
Canada: Dr. Robert Bissonnette, Dr. Marc Bourcier, Dr. Jerry K. L. Tan, Dr. Yves Poulin, Dr. Kim Papp, Dr. Wayne D. Carey, Dr. Wayne P. Gulliver, Dr. Aditya K. Gupta, Dr. Vincent Ho, Dr. Rodion A. Kunynetz, Dr. Richard Langley, Dr. Catherine H. Maari, Dr. Leslie Rosoph, Dr. Darryl Toth. USA: Dr. Jerry Bagel, Dr. Diane R. Baker, Dr. Alicia R. Barba, Dr. Charles A. Birbara, Dr. Marie F. Bruyneel, Dr. Lesly S. Davidson, Dr. Jonathan S. Dosik, Dr. Frank E. Dunlap, Dr. Joel M. Gelfand, Dr. Paul Getz, Dr. Paul S. Gillum, Dr. Alice B. Gottlieb, Dr. John M. Humeniuk, Dr. Jonathan Kantor, Dr. Alexandra B. Kimball, Dr. Neil J. Korman, Dr. Gerald G. Krueger, Dr. Patricia C. Lee, Dr. Craig L. Leonardi, Dr. Robert T. Matheson, Dr. Martin A. Menter, Dr. David M. Pariser, Dr. Phoebe P. Rich, Dr. Patricia N. Speelman, Dr. David M. Spencer, Dr. Bruce E. Strober, Dr. John H. Tu, Dr. Stephen K. Tyring
Declaration of interest
All authors are employees of Pfizer Inc and hold stock/stock options in Pfizer Inc. This research was sponsored by Pfizer Inc. Medical writing support was provided by Karen Irving of Complete Medical Communications and was funded by Pfizer Inc.
References
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451–85
- Chang SE, Han SS, Jung HJ, Choi JH. Neuropeptides and their receptors in psoriatic skin in relation to pruritus. Br J Dermatol. 2007;156:1272–7
- Gupta MA, Gupta AK, Kirkby S, et al. Pruritus in psoriasis. A prospective study of some psychiatric and dermatologic correlates. Arch Dermatol. 1988;124:1052–7
- Reich A, Orda A, Wisnicka B, Szepietowski JC. Plasma neuropeptides and perception of pruritus in psoriasis. Acta Derm Venereol. 2007;87:299–304
- Szepietowski JC, Reich A, Wisnicka B. Itching in patients suffering from psoriasis. Acta Dermatovenerol Croat 2002;10:221–6
- Yosipovitch G, Goon A, Wee J, et al. The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. Br J Dermatol. 2000;143:969–73
- Globe D, Bayliss MS, Harrison DJ. The impact of itch symptoms in psoriasis: results from physician interviews and patient focus groups. Health Qual Life Outcomes 2009;7:62
- Dawn A, Yosipovitch G. Treating itch in psoriasis. Dermatol Nurs. 2006;18:227–33
- Prignano F, Ricceri F, Pescitelli L, Lotti T. Itch in psoriasis: epidemiology, clinical aspects and treatment options. Clin Cosmet Investig Dermatol. 2009;2:9–13
- Papp K, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a Phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167:668–77
- Mamolo C, Harness J, Tan H, Menter A. Tofacitinib (CP-690,550), an oral Janus kinase inhibitor, improves patient-reported outcomes in a phase 2b, randomized, double-blind, placebo-controlled study in patients with moderate-to-severe psoriasis. J Eur Acad Dermatol Venereol. 2013 [Epub ahead of print]. doi: 10.1111/jdv.12081
- Mamolo CM, Bushmakin AG, Cappelleri JC, Stewart M. The effect of oral CP-690,550 on pruritus in patients with moderate-to-severe plaque psoriasis. Value Health. 2011;14:A56
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–16
- Cappelleri JC, Bushmakin AG, Harness J, Mamolo C. Psychometric validation of the physician global assessment scale for assessing severity of psoriasis disease activity. Qual Life Res. 2013 [Epub ahead of print]. doi:10.1007/s11136-013-0384-y
- Stevens J. Applied Multivariate Statistics for the Social Sciences. Hillsdale, NJ: Lawrence Erlbaum, 2002
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. New York: Routledge Academic, 1988
- Fayers FM, Machin D. Quality of Life: The Assessment, Analysis, and Interpretation of Patient-Reported Outcomes. 2nd ed. Chichester, England: John Wiley and Sons Ltd, 2007
- Iacobucci D. Mediation Analysis. Thousand Oaks, CA: SAGE Publications, 2008
- MacKinnon DP. Introduction to Statistical Mediation Analysis. New York, NY: Lawrence Erlbaum Associates, 2008
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826–50