Adherence to biological treatment is influenced by drug efficacy, adverse events, and patients’ satisfaction with treatment. Administration intervals and route may also affect treatment persistence (how long patients stay on treatment). Adalimumab is administered subcutaneously every other week, and injections at hospitals are widely used, requiring frequent visits that may be a burden for patients. We retrospectively analyzed 36 consecutive patients treated with adalimumab for moderate to severe psoriasis to determine persistence with the treatment regimen.Patients were enrolled between February 2010 and April 2013 and were followed until August 2013. Patients for whom adalimumab had been initiated in other hospitals and who were subsequently treated in our department were excluded from the study. We collected information on gender, age, disease type, duration of the disease prior to baseline, Psoriasis Area and Severity Index (PASI) at baseline, side effects, presence or absence of self-injection, and comorbidities relevant to psoriasis. Patients were divided into two groups on the basis of adherence to adalimumab at the end of the observational period: the retention group and the drop-out group. All patients were initiated with hospital-based administration of the adalimumab, but during the follow-up period, some patients switched to home injections when they preferred self-injections regardless of the degree of the improvement or when they agreed to our recommendation for self-injection because of the achievement of PASI 75. Approval for this retrospective study was obtained from Kurume University review boards.
The general characteristics of the retention group and drop-out group were compared, and no statistical differences were found (). The mean probability of drug survival was 20.0 months (median, 18 months; ). Multivariate analysis using the Cox proportional hazard model disclosed that age, PASI at baseline, and gender were not significant predictors for drug survival rate.
Figure 1. Drug survival rate curve for adalimumab, analyzed by the Kaplan–Meier method. Dotted lines show 95% confident intervals. For the multivariate analysis using the Cox proportional hazard model, the data were stratified by gender, age, and PASI score at baseline. p < 0.05 was considered significant.
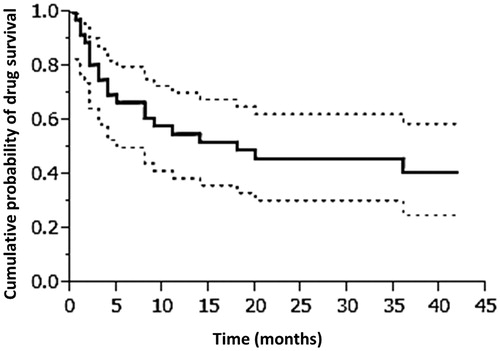
Table 1. Summary of characteristics of the study population.
Persistence to adalimumab treatment was less than that reported in several previous studies () (Citation1–4). This may be because of the burden of frequent visits for injection. No precise description of self-injection was available in many studies regarding drug survival rates of adalimumab as well as other biological agents (Citation1–3,Citation5). However, introduction of an autoinjection pen for adalimumab was reported to increase the percentage of patients self-administering medication from 51 to 84% and decrease the percentage of patients attending primary care for injection from 33 to 2% in Spanish study population including 55 patients (rheumatoid arthritis 29, psoriatic arthritis 17, ankylosing spondykitis 9) (Citation6). The high rate of self-injection in Spanish study population was in contrast to low rate in the present study. It is conceivable that greater use of self-injection may improve persistence to adalimumab.
Table 2. Adalimumab drug survival rate.
Acknowledgements
We gratefully appreciate the secretarial work of Ms. Tomoko Tashima and Ms. Mami Nishida.
Declaration of interest
Authors do not have any conflict of interest
References
- Gniadecki R, Kragballe K, Dam TN, et al. Comparison of drug survival rates for adalimumab, etanercept and infliximab in patients with Psoriasis vulgaris. Br J Dermatol. 2011;164:1091–6
- Esposito M, Gisondi P, Cassano N, et al. Survival rate of antitumour necrosis factor-alpha treatments for psoriasis in routine dermatological practice: a multicentre observational study. Br J Dermatol. 2013;169:666–72
- Lopez-Ferrer A, Vilarrasa E, Gich IJ, et al. Adalimumab for the treatment of psoriasis in real life: a retrospective cohort of 119 patients at a single Spanish centre. Br J Dermatol. 2013;169:1141–7
- Umezawa Y, Nobeyama Y, Hayashi M, et al. Drug survival rates in patients with psoriasis after treatment with biologics. J Dermatol. 2013;40:1008–13
- van den Reek JM, van Lumig PP, Driessen RJ, et al. Determinants of drug survival for etanercept in a long-term daily practice cohort of patients with psoriasis. Br J Dermatol. 2014;170:415–24
- Borras-Blasco J, Gracia-Perez A, Rosique-Robles JD, et al. Acceptability of switching adalimumab from a prefilled syringe to an autoinjection pen. Expert Opin Biol Ther. 2010;10:301–7
