Sir, we describe the successful but unlicensed use of ustekinumab to treat severe psoriasis in a 13-year old girl. Our patient was first seen aged 9 with a 9-month history of severe scalp and nail psoriasis with associated pruritus. Her past medical history included peanut allergy, hay fever and frequent epistaxis. There was no history of eczema. She lived with her younger sister and parents, achieved high grades academically and excelled nationally in her chosen sport. Her father had a history of severe psoriasis.
On initial assessment there were thick hyperkeratotic plaques involving the scalp, trunk and lower limbs with marked nail involvement. There was no joint involvement.
Her psoriasis was unresponsive to intensive topical therapy and phototherapy.
A month after her eleventh birthday she developed thick psoriatic plaques on her body, pityriasis amiantacea of the scalp and painful dystrophy of all her nails.
Over the next eighteen months our patient was treated with maximal doses of acitretin (35 mg for 5 months), methotrexate (25 mg for 15 months) and ciclosporin (400 mg for 6 months) with little or no response in her condition.
The teratogenicity effect of acitretin was discussed with our patient and her parents.
Etanercept 50 mg once weekly was commenced in accordance with NICE guidelines. Her weight was 74 kg, baseline PASI 11.3 (whilst still on ciclosporin) and cDLQI 14.
She developed injection site reactions requiring clobetasone propionate locally and prophylactic anti-histamine. She became increasingly anxious about the injections and her parents reported new obsessive behaviour. After 6 months on etanercept her PASI score was 18.6 and cDLQI 18. Her weight was 95 kg. She started to refuse school attendance.
At this time her father died suddenly from a myocardial infarction aged 46 years. His cardiovascular risk factors are not known.
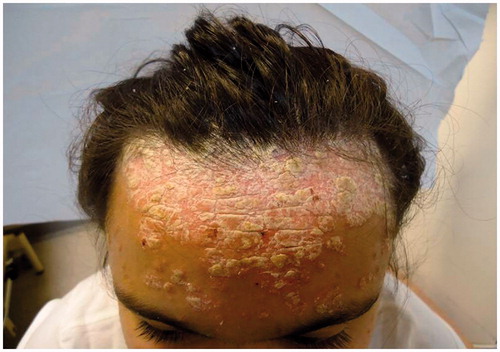
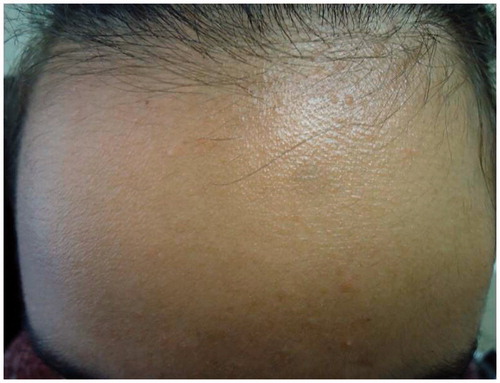
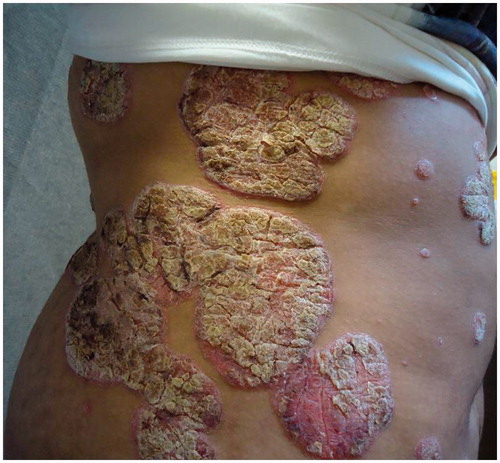
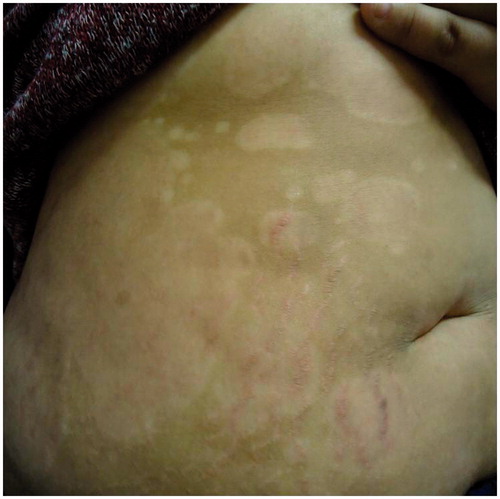
Predictably her psoriasis ( and ) and psychological state deteriorated following this major life event and she became disruptive at home and at school. An urgent individual patient funding request was made to use ustekinumab on an unlicensed basis and this was commenced at a dose of 45 mg given subcutaneously at week 0, 4 and 16. After 16 weeks of treatment ( and ) her PASI was 0.4 and her cDLQI 1. She has now been treated on this dose for 18 months with sustained efficacy and no reported side effects.
Our patient is regularly evaluated for cardiovascular risk factors and thus far the blood pressure and lipids are normal. She is a non-smoker.
In an open letter, Dixit et al. reported a successful outcome with ustekinumab therapy in a 16-year old obese male with severe psoriasis and associated low mood. Complete skin clearance was noted at eight weeks and his PASI score of 0 was maintained for 18 months. He did not report any side-effects. Additionally, the patient lost weight (10 kg) during ustekinumab treatment (Citation1).
Fotiadu et al. reported a dramatic improvement in both cDLQI and PASI from the onset of ustekinumab therapy in a 14-year old male with intractable psoriasis. After one year of therapy he had complete skin clearance and was free of any complications. Similar to our patient, his psoriasis had not responded to standard treatments and etanercept. His parents reported anxiety and non-compliance with entanercept injections because of the lack of improvement and a reluctance to attend school because of bullying (Citation2). Habal et al. observed a good response to ustekinumab in a 4-year old girl with extensive and resistant pustular psoriasis. The patient’s condition had failed to respond to methotrexate, ciclosporin, infliximab and anakinra (Citation3).
Presently, etanercept is the only licensed biological therapy for children aged 8 years and over. The use of ustekinumab in this age group has only been reported in the literature on three occasions prior to our case.
We have found ongoing, well-tolerated and effective results with the unlicensed use of ustekinumab in a teenage girl with severe psoriasis and observed the psychological benefits of proactive treatment in adolescence.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Dixit S, Shumack S, Fischer G. Ustekinumab in the treatment of severe paediatric psoriasis. Aust J Dermatol. 2013;54:147
- Fotiadu C, Lazaridou E, Giannopoulou C, Ioannides D. Ustekinumab for the treatment of an adolescent patient with recalcitrant plaque psoriasis. Eur J Dermatol. 2011;21:117--18
- Habal N, Chen Y, Jordan C, et al. Pathogenesis study of infantile-onset, severe pustular psoriasis reveals a de novo mutation in CARD14 causing psoriasis which responds clinically to IL-12/23 blocking treatment with ustekinumab. Arthritis Rheumat. 2010;63:10