Abstract
Background: A ready to use betamethasone valerate 0.1% (BMV) dressing was effective and well-tolerated by patients receiving chronic plaque psoriasis treatment. Objective: Collect data related to BMV dressing used in the context of market authorization. Methods: An observational, prospective study, including 258 patients with a maximum 4-weeks-treatment of inflammatory dermatosis with BMV 2.25 mg plaster was performed. The prescription pattern was described and the disease severity assessed using a Physician Global Assessment (PGA). Patient satisfaction as well as their quality of life (DLQI) were evaluated. Clinical evaluation was performed before and after the treatment. Results: The DLQI scores improved from 10.0 ± 5.4 to 3.5 ± 3.5 points (p < 0.0001) and PGA decreased from 12.5 ± 3.1 to 4.2 ± 3.0 points (p < 0.0001). The highest DLQI and PGA improvement was reported for the eczema group. Subjects reported the test dressing was better than prior therapies with 93.5% very satisfied and 90.4% satisfied. Conclusions: The BMV dressing is well-tolerated and effective in the treatment of inflammatory dermatoses, improving both the objective signs of the disease as well as patients’ quality of life.
Introduction
Inflammatory skin diseases are frequent in routine practice and can impair the health and the quality of life of the patients. Topical corticosteroids are the first-line treatment of steroid-responsive inflammatory skin diseases, such as chronic plaque psoriasis and eczema, because of their anti-inflammatory, immunosuppressive, anti-proliferative and vasoconstrictive properties (Citation1). A ready-to-use, self-adhering medicated plaster containing the active ingredient betamethasone 17-valerate (2.25 mg BMV (0.1%)) has been developed. Trimmed to exactly cover the plaques, the BMV plaster offers advantages over usual local dermocorticoids (e.g. creams, ointments, etc.) such as delivering a uniform concentration of active ingredients to the affected area and acting as a barrier preventing further damage of the plaque from trauma or scratching (Citation2). Previous trials, studying the treatment of psoriasis, showed that it was safe and efficient (Citation2,Citation3). These clinical studies were the basis for granting market authorization to treat “inflammatory dermatoses”, namely chronic plaque psoriasis, atopic dermatitis, contact dermatitis, lichenifications, lichen planus, granuloma annulare and palmo-plantar pustulosis. Most data available on BMV plaster safety and efficacy originate from clinical studies on chronic plaque psoriasis treatment. Thus, little is known on BMV plaster effects on other usual topical steroids-sensitive dermatoses, especially in the context of routine dermatological care. This post-marketing observational study presents the description of the prescription patterns of a new corticosteroid plaster by both general practitioners (GPs) and dermatologists in the routine management of outpatients with inflammatory skin diseases cited in its marketing authorization. The study also included observations related to the safety and clinical effectiveness of BMV plasters, as well as their impact on patient quality of life (DLQI).
Patients and methods
Study design
This prospective multicentric open-label observational study was performed in France by 132 investigators, including 46 dermatologists and 86 GPs which were recruited from a representative panel through phone calls.
This work was approved by the CCTIRS, the French Advisory Committee on Information processing in the matter of Research in the field of Health and by the CNIL, the French Data Protection Authority. Patients were informed by the investigators on the purpose and course of the study and gave their consent to have their data recorded.
Inclusion and exclusion criteria
Eligible patients were adults with any inflammatory skin disease not exceeding 5% of the body surface and for whom the physician had previously decided to prescribe the BMV plaster (BETESIL®) according to its approved indications and limitations of use. Patients who participated in any other study during the month prior to their enrolment were not eligible.
Treatment and outcome measures
All the patients were prescribed to apply up to 6 BMV plasters per day during a treatment period not exceeding a maximum of 30 days. Patient assessment and data collection were organized through two visits: at the inclusion (visit 1) and at the end of the study (visit 2). The mean interval between the first and second visit is 32.7 ± 20.9 days.
The following data were recorded: patient demographic characteristics; skin disease type, localization, extension, prior therapies and reason for trying the new BMV plaster; investigator-assessed global disease severity using the Physician Global Assessment (PGA) on a 20-points scale (0 = no disease; 1–5 = minimal severity, 6–10 = mild severity, 11–15 = moderate severity, 16–20 = severe to very severe disease) calculated as the sum of severity scores of 4 individual components (erythema; crusting; thickening; itching) on a semi-quantitative 5-points scale [0 = absent, 1 = minimal, 2 = mild, 3 = moderate, 4 = moderate to severe, 5 = severe]; treatment tolerability and safety by patient having reported adverse effects and inconveniences; QoL assessed by the Dermatology Life Quality Index (DLQI; (Citation4,Citation5)) and severity of its impairment. In addition, on the second visit, the global satisfaction with the treatment was assessed by the physicians (Yes/No question), and by the patients (4-items scale ranging from very satisfied to very unsatisfied); patients also assessed the global efficacy of BMV plasters versus. prior treatments (Yes/No question) and its convenience (ease of use, rapidity of use, galenic form and tolerability; 5-points scale ranging from poor to excellent).
Data management and statistical analysis
Data from questionnaires were double entered in SQL via SAISORG. Statistical analyses and data processing were performed using the software SAS 9.3 (SAS Institute Inc., Cary, NC). All the patients were included in the analysis. Quantitative variables were described by the number of documented and missing data, the mean, standard deviation, extreme values and the median. Categorical variables were described by the amount of missing data, frequency and percentage of each category. During the follow-up, changes in PGA and DLQI were analyzed using the Student t-test for paired variables (p < 0.05).
Results
Study course and patient characteristics
The 132 participating physicians (86 GPs and 46 dermatologists) included 258 patients () from August 2013 to March 2014; 126 patients were enrolled by GPs and 132 by dermatologists.
Table 1. Patient baseline characteristics.
Protocol violations were observed for six patients only: five were retrospectively enrolled since they were already treated by BMV plasters on the first visit; one patient had his questionnaire returned at the first visit.
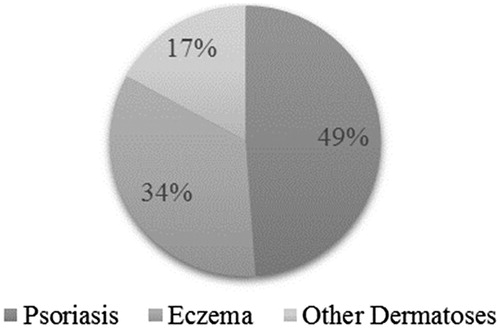
Overall, psoriasis was the most frequent cause for prescribing BMV plasters, accounting for nearly half of included patients (). However, indications differed between dermatologists and GPs, the latter targeting various forms of “eczema” more often than psoriasis (). Other dermatoses treated by BMV plasters (41/45) were almost exclusively restricted to dermatologists. The vast majority of included dermatoses were previously treated (). Thus, the BMV plaster was the first topical treatment ever received in only 9.1 % of psoriatic patients. The prescription duration was 22.7 (±8.8) days, varying from a maximum of 26.5 (± 8.0) days in “other dermatoses” to 18.7 (±9.1) days in “eczema”. The number of prescribed plasters was 2.2 (±1.3)/day, and was independent of the severity of the disease (). The most common prescription was of 1–2 plasters per day (68.6 % of the patients) and a maximum of 5 or 6 daily plasters were needed very rarely.
Table 2. Proportion of treated dermatoses according the medical specialty of physicians.
Table 3. Prescription of prior therapies according to disease type.
Table 4. Prescription duration and posology of BMV plaster according to skin disease.
Clinical evaluation
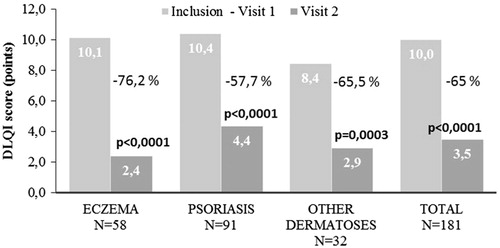
During the study period, patient-scored QoL was improved (): the DLQI scores were reduced from 10.0 ± 5.4 to 3.5 ± 3.5 points (p < 0.0001), reflecting a 65.0% relative improvement. Absolute score reductions ranged from −7.8 ± 4.7 in patients with eczema to −5.6 ± 5.0 in patients with “other dermatoses”. In psoriasis patients, the mean absolute reduction was −6.1 ± 5.1. Thus, during the course of the study the disease-related QoL impairment fell from strong to mild in eczema and psoriasis patients, and from moderate to mild in patients with other dermatoses.
Figure 2. Changes in mean DLQI scores in response to BMV plaster treatment at baseline (visit 1) and at the end of the treatment (visit 2). Improvement is expressed in percentage for the inflammatory dermatoses investigated. Changes in lesion score were evaluated using Student’s t-test for paired values. A p-value < 0.05 was considered statistically significant.
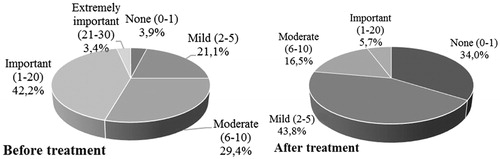
The impact of the disease on the QoL declined through the treatment period, quoted from “moderate” or “important” at the start of the study by 71.6% of the patients to “none” or “mild” at the end of the treatment by 77.8% of the patients (). Whatever the disease, patients quoted the ease of use (75.3%), the design of the treatment (70.9%) and the rapidity of the application (75.2%) as good or very good. Finally, the patients judged the tolerability from “very good” (44.5%) to “excellent” (28.2%) (results not shown).
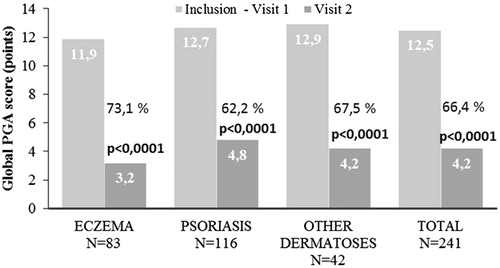
PGA decreased from 12.5 ± 3.1 (“moderate”) to 4.2 ± 3.0 points (“mild”) during the treatment period (p < 0.0001), which is independent of the disease type (). The highest PGA improvement was reported in patients with eczema (−8.91 ± 2.79).
Figure 4. Changes in mean PGA scores in response to BMV plaster treatment at baseline (visit 1) and at the end of the treatment (visit 2). Improvement is expressed in percentage for the inflammatory dermatoses investigated. Changes in lesion score were evaluated using Student’s t-test for paired values. A p-value < 0.05 was considered statistically significant.
Overall, 90.4% of the patients quoted the BMV plaster treatment as more effective than prior treatments. Both physicians and patients were satisfied by the treatment (95.1% and 93.5% respectively) with the highest satisfaction in the eczema group (98.8% and 95.2% respectively) (results not shown).
Out of the twenty-six adverse events reported (), 21 were related to BMV plasters, whatever was the disease, and were characterized mainly by itching and redness. No serious adverse event (SAE) was reported in this study. All AEs are already known and listed in the SPC as redness and itching.
Table 5. Adverse events.
Discussion
We report that BMV dressing statistically reduced the overall PGA score and improved the DLQI score whatever be the type of disease and associated severity. Comparable improvements were reported in other observational studies in the treatment of psoriasis by topical steroids of the same reference class (Citation6). In addition, this study showed that psoriasis was the inflammatory dermatoses the most frequently treated by the BMV plaster (48.6%), followed by eczema (33.9%) and other dermatoses (17.5%).
Neither the prescribed dose nor the prescription period were correlated to the type of the disease or its baseline severity. Due to its galenic form, the number of the prescribed plasters per day was most likely linked to the area or the duration of the disease rather to its severity. However, this explanation is speculative since these parameters were not recorded in this study.
Finally, the BMV plaster is labelled as first-line therapy of psoriasis and second-line therapy of other “inflammatory dermatoses”. Our study shows that 84.5% of the patients were already treated before the enrolment, including 90.9% of the patients with psoriasis. Thus, at the time of the study, BMV plaster was not yet prescribed as the first-line therapy of psoriasis most likely due to the recent reimbursement of the product in France.
Acknowledgements
The author wishes to thank all the patients and investigators involved in the study and all project contributors. He also wishes to thank Dr Sophie Richier for editorial support.
Declaration of interest
F.M. was in charge of the study design, protocol drafting and article revision as a member of the coordination committee and he received fees for its contribution. This study was funded by the Laboratoires Genévrier.
References
- Del RJ, Friedlander SF. Corticosteroids: options in the era of steroid-sparing therapy. J Am Acad Dermatol. 2005;53:S50–8
- Naldi L, Yawalkar N, Kaszuba A, et al. Efficacy and safety of the betamethasone valerate 0.1% plaster in mild-to-moderate chronic plaque psoriasis: a randomized, parallel-group, active-controlled, phase III study. Am J Clin Dermatol. 2011;12:191–201
- Ortonne JP, Esposito M, Chimenti S, et al. Betamethasone valerate dressing is non-inferior to calcipotriol-betamethasone dipropionate ointment in the treatment of patients with mild-to-moderate chronic plaque psoriasis: results of a randomized assessor-blinded multicentre trial. J Eur Acad Dermatol Venereol. 2014;28:1226–34
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–16
- Hongbo Y, Thomas CL, Harrison MA, et al. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol. 2005;125:659–64
- Sticherling M, Eicke C, Anger T. Practicability of combined treatment with calcipotriol/betamethasone gel (Daivobet(R) Gel) and improvement of quality of life in patients with psoriasis. J Dtsch Dermatol Ges. 2013;11:420–7