Abstract
Objective: The aim of this study was to compare the moisturising efficacy and acceptability of physical characteristics of two commonly prescribed emollients licenced in the UK, Doublebase Dayleve gel (DELP) and Diprobase cream (DIPC). Methods: The study was a double-blind, concurrent bi-lateral comparison in female eczema subjects with dry skin. Results: In Part 1, comparing the area under the curve (AUC) change from baseline corneometer readings over 24 h following single applications of the emollients to the volar forearms of 34 subjects, the AUC for DELP was more than three times that seen for DIPC (p < 0.0001). In Part 2, comparing the same outcome measured over 5 days of twice daily applications to the lower legs in 36 subjects, the AUC for DELP was approximately five times that for DIPC (p < 0.0001). 69% of subjects “Like Slightly” or “Like Strongly” DELP compared to 33% for DIPC (p = 0.025). 72% indicated they would use DELP again compared to 33% for DIPC (p = 0.033). 75% of subjects preferred DELP, 17% preferred DIPC and 8% expressed no preference (p = 0.0004).
Introduction
Emollient therapy is the mainstay for treating dry skin conditions including atopic eczema (AE), psoriasis and elderly pruritus (Citation1–3). Emollients work chiefly by maintaining increased skin water content, particularly in the outermost stratum corneum layer.
Prescribers recommend emollient products based primarily on patient preference because treatment concordance depends on patient satisfaction with the product’s physical characteristics. The comparative effectiveness of different emollient products tends to be overlooked because there are few published studies performed under conditions mimicking normal clinical use, and even fewer comparative studies (Citation1,3–6). National Institute for Health and Clinical Excellence has called for more research in this area.
The aim of this study was to compare two commonly UK prescribed licenced emollients, Doublebase Dayleve gel (DELP) and Diprobase cream (DIPC). Their skin moisturising effects are compared using corneometry, a well-established, non-invasive method for accurate determination of skin hydration by measuring changes in electrical capacitance of the stratum corneum (Citation7–10). Physical acceptability is compared by patients’ subjective assessments.
Materials and methods
The study design was a double-blind, randomised, bilateral, concurrent comparisons of DELP gel (Dermal Laboratories Ltd, Hitchin, UK) and DIPC cream (Merck Sharp & Dohme, Hoddesdon, UK) applied to areas of dry skin (without significant flare) on the forearms (Part Citation1) and the lower legs (Part Citation2) of eczema sufferers, all between 18 and 65 years of age. Dry skin was defined, for study purposes, as having baseline corneometer readings of less than 45 units, and to be eligible for participation differed by no more than 6 units between the left and right arms/legs. Because excessive hair interferes with corneometry measurements, participation was restricted to females only. Eligible subjects also committed to following a sedentary lifestyle for the duration of their involvement (in order to avoid more frequent washing/bathing than permitted). The two parts of the study were performed approximately a week apart using essentially the same panel of subjects. The study was conducted with full ethics (Reading Independent Ethics Committee, Reading, UK) and regulatory approvals, in compliance with the principles of the Declaration of Helsinki and in accordance with Good Clinical Practice. Written informed consents were obtained from all subjects.
Exclusion criteria were: significant concurrent illness or skin disease currently involving the test sites; history of allergy relevant to the test products or their ingredients; use of any topical or systemic treatment likely to affect skin response; use of oral and topical steroids for any condition within the previous 4 weeks; visible skin abnormality or excessive hair growth likely to interfere with instrumental measurements; irritation, tattoos, scars or birthmarks at the test measurement sites; participation in any other study presently or within the past 3 months; breastfeeding and pregnancy. Also, removal of leg hair was not allowed within 48 h prior to, or during participation in Part Citation2. Employees of either Dermal Laboratories or RSSL Pharma, or their immediate family members, were not allowed to participate.
Commencing 1 week prior to participation, and continuing for the duration of the study, eligible subjects were asked to use only the supplied Simple® soap for washing and were asked not to apply moisturising products to their arms or legs, or to use depilatory products or shave these areas.
Part Citation1 – skin hydration following single application
This part of the study compared skin hydration over a 24-h period following single applications of DELP and DIPC emollients. Thirty-four subjects took part in two cohorts.
Two test sites, each measuring 20 cm2, were demarcated on both volar (inside) aspects of subjects’ forearms, adjacent to the wrist and flexure, and baseline measurements of skin hydration were performed in triplicate at about 9 am using the Multiprobe Adapter MPA5 with Corneometer CM825 probe (Hydration) (Courage-Khazaka electronic, Germany).
The two test products are white semi-solids, essentially indistinguishable from one another in appearance and texture, and in this part of the study were presented, for blinding purposes, in pre-filled 1 ml syringes. Directly from the syringe, 0.05 ml of DELP and DIPC were applied by a member of the investigator team uninvolved in the corneometry measurements to one test site each (resulting in 2.5 μl of product being applied per cm2) on opposing forearms using a randomisation prepared in advance so that the right/left and wrist/flexure allocation was approximately equal for both products. The second test site on each arm served as an untreated control.
Triplicate corneometry measurements at treated and adjacent untreated skin sites were repeated nominally at hourly intervals for the first 4 h and at 6, 8, 12 and 24 h after application. During this period subjects were not permitted to bath, shower or bathe, and they kept their arms uncovered.
Changes in skin hydration was analysed by measurement of the area under the curve (AUC) of the change from baseline corneometer readings over 24 h. AUC, using the actual corneometer measurement times, was calculated, after checking for normality, using the trapezoidal rule, and treatment effects were estimated using the within subject error term, after adjustment for any effect of arm (right/left). An additional sensitivity analysis for the primary efficacy variable was performed adjusting for the AUC of the untreated controls, but these results are not presented because the conclusions are the same as those for the main analysis.
Part Citation2 – assessment of cumulative skin hydration and product acceptability
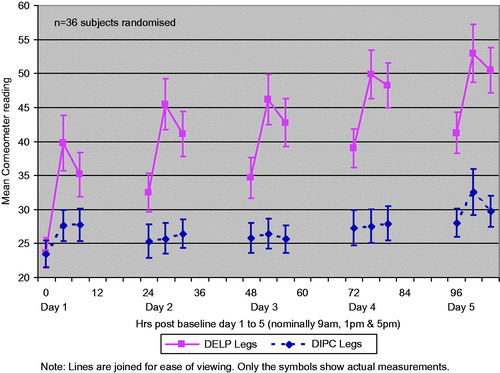
This part of the study compared the skin moisturisation effects and acceptability of the two emollients when applied twice daily for 5 consecutive days. Thirty-six subjects took part (including the 34 who had previously participated in Part Citation1) in two cohorts.
Baseline measurements of skin hydration on both lower legs were performed at about 9 am on day 1 on skin areas measuring approximately 5 cm × 10 cm located in the same position for each subject using templates. Subjects were then given the two emollients, presented in identical 500 g pre-weighed pump containers randomly labelled left and right, to apply to their lower legs twice daily (immediately after the 9 am corneometry measurement and at approximately 9 pm) for the next 4 days and on the morning of day 5. Subjects were instructed to apply enough of each product to treat the whole of the respective lower leg (as a guide, described as being about 1 inch of product pumped from the bottle or a mass about the size of a 20-p piece) using a few gentle strokes to smooth the products across the skin in the direction of hair growth (like stroking a cat or dog). They were asked not to rub the skin vigorously, and if necessary to allow time for the products to soak in before covering with any clothing.
Corneometry measurements were performed three times each day (nominally 9 am, 1 pm and 5 pm), on each occasion after at least 30-min acclimatisation in the clinic. Measurements were performed in triplicate.
Subjects were asked to refrain from bathing, showering or washing their lower legs at all on skin measurement days 1, 3, 5 but were invited to do so during the evening on intervening days 2 and 4. Subjects were not permitted to use any other skin moisturiser on their legs at any time during their participation in the study, nor any other topical or systemic medication considered by the chief investigator to potentially interfere with the study outcome. Details of any medication being used by subjects prior to, and during, the study were recorded.
Change in skin moisturisation was analysed by measurement of the AUC over the 5-day treatment period, as described above for Part Citation1.
Subjects’ opinions on the overall acceptability of the products were recorded on day 5 of the study by asking them to select one of the following options for each product: “Like Strongly”, “Like Slightly”, “Neither Like nor Dislike”, “Dislike Slightly” and “Dislike Strongly”. They were also asked if they would use each product again, and whether they preferred either product. The percentage of subjects selecting either “Like Strongly” or “Like Slightly” for each product, as well as the percentage of subjects reporting that they would use each product again or had a preference for either, were compared within subjects (using Prescott’s test to allow for effect of leg).
In addition, subjects were asked to indicate the extent to which they agreed or disagreed, on a 5-point scale, with 10 statements relating to various product attributes. For each attribute, the number and percentage of subjects giving each response was summarised for descriptive purposes only. Statistical comparison of the products in relation to these attributes was not undertaken.
This study was designed to test superiority. A sample size of 19 subjects was calculated to give at least 80% power to detect a difference in mean AUC between DELP and DIPC of 3.5 units/h assuming a standard deviation (of paired differences) of 5 units/h and using a 5% significance level. Up to 40 subjects were therefore planned in order to allow for possible dropouts – bearing in mind the large number of clinic visits required and the possibility of losing randomised subjects if at the end of the 1-week run in the severity of their dry skin was no longer bilaterally matched.
Results
Forty-six women were screened. 5 failed screening and 3 were unable to attend the required visits, so 38 were randomised to take part and commenced washout. Two of these failed baseline screening in Part Citation1, leaving 36 to take part. 34 of these participated in both Parts 1 and 2, and two participated in Part Citation2 only.
Fourteen subjects used concomitant medication such as contraceptive hormone treatments, anti-depressants, pain relief tablets and asthma inhalers, none of which were considered to interfere with the study outcome. One patient used Aqueous cream on skin other than the study areas.
Three adverse events in three subjects were considered as being possibly related to treatment. They involved minor local skin warmth, rash or tingling reactions to both treatments.
Part Citation1 – skin hydration following single applications
Significant differences were observed between the two cohorts (comprising 19 and 15 subjects), probably owing to differing environmental conditions over their respective treatment days.
For Part Citation1, the primary efficacy parameter was the AUC change from baseline corneometer readings over 24 h. Following single applications, cumulative increases were statistically significantly greater for patients’ arms treated with DELP compared to arms treated with DIPC (). This was true for both cohorts. Overall the estimated treatment difference, DELP minus DIPC, was an increased AUC of 306 units (95% CI: 273–338, p < 0.0001), which represents an increase in skin hydration with DELP of at least three times that seen for DIPC. There was no significant difference between the skin hydration of the untreated area of the DELP arms and the untreated area of the DIPC arms (p = 0.75).
Table 1. Part 1 – 24 h AUC change from baseline corneometer reading.
Since the AUC was measured over a 24-h period, dividing the treatment difference AUC by 24 gives a value which approximates to a “mean corneometer reading”, and corresponds to an estimated treatment difference for DELP over DIPC of more than 12 units (12.2 for the first cohort and 13.7 for the second cohort).
The difference in mean corneometer readings between the DELP and DIPC arms are shown versus time for the treated and untreated sites (), indicating the long-lasting nature of the moisturisation benefit of DELP over DIPC.
Figure 1. Part 1 – mean corneometer readings for the treatment difference (DELP arm minus DIPC arm) vs. untreated arm by cohort.
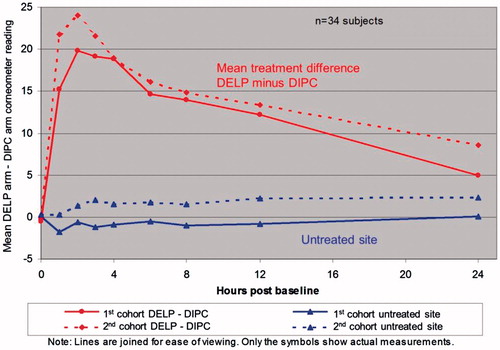
The cumulative increase in change from baseline corneometry readings with DIPC were very modest by comparison, and were statistically significant from zero for the second cohort only ().
Part Citation2 – assessment of cumulative skin hydration and product acceptability
No significant differences were observed between the two cohorts (comprising 21 and 15 subjects). Adherence with the twice daily treatment regimen (as recorded in subjects’ treatment diaries) was good, with only two reported missed applications. There were no significant differences between the amounts of products used on left versus right legs or between products. Subjects typically used between 11 and 22 g of each product (corresponding to 1.2 or 2.5 g per application). Approximately three quarters of the subjects recorded that they bathed their lower legs or showered on both permitted occasions during the evenings of days 2 and 4.
For Part Citation2 the primary efficacy parameter was the AUC change from baseline corneometer readings over the 5-day treatment period (104 h from 09.00 on day 1 to 17.00 on day 5). There were only two missed follow-up appointments (involving different subjects). These missing values were linearly interpolated. Both products significantly improved skin hydration from baseline (). However, DELP performed statistically significantly better than DIPC such that the cumulative increase in skin hydration over the 5 days was estimated to be an increased AUC of 1399 units which represents an increase in skin hydration of approximately five times that seen for DIPC (95% CI: 1180–1618, p < 0.0001). This conclusion is from an intention to treat analysis of all 36 subjects randomised in Part Citation2.
Table 2. Part 2 – 5-day AUC change from baseline corneometer reading.
The improved skin hydration of DELP over DIPC was seen at every time point over the 5-day period. The mean corneometer readings are shown in . The long-lasting and cumulative benefit of DELP over DIPC is particularly illustrated by the morning readings each day (which were typically 12 h after the latest application of the products the day before) which were significantly greater than the baseline reading (day 1, 9 am) and increased step-wise from day 2 to day 5 – even following the washing/bathing permitted during the evenings of days 2 and 4.
Overall product acceptability
69% of subjects selected either “Like Slightly” or “Like Strongly” for DELP compared to 33% for DIPC (). This difference in overall product acceptability was statistically significant (Prescott’s test, p = 0.025). 53% of subjects disliked (“Dislike strongly” or “Dislike slightly”) DIPC compared to 12% disliking DELP.
Table 3. Acceptability of DELP and DIPC.
Willingness to use the products again
One-third of the subjects answered that they would use DIPC again compared to 72% for DELP (). This difference was statistically significant (Prescott’s test p = 0.033).
Table 4. Willingness to use DELP and DIPC again.
Product preference
Three quarters (75%) of subjects preferred DELP, whilst 17% preferred DIPC and the remaining 8% of subjects had no preference (). This difference was highly statistically significant (Prescott’s test p = 0.0004).
Table 5. Preferred treatment option.
Ten product attributes
Subjects were asked to indicate their level of agreement (from five categories: “Disagree Strongly”, “Disagree Slightly”, “Neither agree nor disagree”, “Agree Slightly” or “Agree Strongly”) with each of the 10 statements relating to product attributes, for each leg (). The percentage of subjects ticking one of the top two categories (“Agree Slightly” and “Agree Strongly”) was higher for DELP than for DIPC for all of the 10 attributes studied.
Table 6. Percentage of subjects ticking “Agree Slightly” or “Agree Strongly” for 10 product attributes.
Discussion
AE is a chronic, inflammatory disease affecting children and adults and is associated with abnormalities in skin barrier function (Citation11). Prevailing expert medical advice is that AE patients should apply their emollients generously and frequently in order to maintain the hydration of the stratum corneum, thereby keeping the corneocytes “plumped up”, closing cracks and restoring the natural barrier function of the skin (Citation12). Although patients are normally recommended to re-apply their emollient several times daily in order to achieve the best therapeutic effect, this is not always possible while they are going about their daily routine, and in practice many, particularly patients who attend school or go to work, only manage to apply one or two applications per day – once in the morning before dressing, and again in the evening before retiring to bed. The dosage regimen used in Part Citation2 of study was therefore chosen to enable comparison of the two products when used under conditions more relevant to real life situations. The treatment period of 5 days was considered long enough to establish any differences between the two products in cumulative skin hydration whilst maintaining patients’ cooperation.
In this study, DELP gel exhibited statistically significantly greater and longer-lasting cumulative skin hydration in subjects with dry skin than the comparator DIPC cream. Although a single application of each product was shown to significantly improve skin hydration over a 24-h period (for one cohort only, in the case of DIPC), the statistically significant increase in skin hydration for DELP was more than three times that seen for DIPC. When applied twice daily over a period of 5 days, the statistically significant cumulative increase in skin hydration for DELP was approximately five times that seen for DIPC.
These significant performance differences are unlikely to be solely attributed to the slightly higher oil content of DELP compared to DIPC, 30% vs. 21% (). Other ingredients and the manner in which the products are formulated are also likely contributing factors. For example, an earlier study has indicated that an emollient gel achieved better skin moisturisation than an emollient cream (Citation13). This may be partly explained by their differing substantivities on the skin. In the case of cream formulations, such as DIPC tested in this study, the high oil content is achieved by emulsifying the lipids into the aqueous phase by the addition of standard surfactants. This produces a stable emulsion which can be easily spread to deposit the oils over the skin surface. However, with the subsequent addition of water (e.g. during washing) the residual surfactant serves to effectively remove the oils from the skin. For the emollient gels such as DELP, on the other hand, the oils are emulsified by the incorporation of a polymer carbopol (Citation14,Citation15). The emulsifying properties of this polymer system are destroyed by electrolytes (Citation16), and so the salts present on the surface of the skin cause irreversible separation of the oil and water phases of the gel during application. The oils are then left to form an occlusive barrier over the skin which subsequently is much less readily re-emulsified and dispersed from the skin during washing. Another point of difference is that emollient gels also tend to contain high levels of glycerol, which is a humectant and has the ability to bind and retain water within the entire thickness of the stratum corneum (Citation17–19). In the case of the particular emollient gel tested here, DELP, the formulation also contains a film-former, povidone, which may also improve the formulation’s barrier properties on the skin (Citation12,Citation20). Indeed, there is some evidence that this excipient may improve the water holding capacity of DELP gel by changing the gel’s microstructure (Citation21).
Table 7. Doublebase Dayleve gel and Diprobase cream composition.
The cosmetic acceptability of emollients is very important because patients are unlikely to use formulations with poor cosmetic appeal, resulting in no clinical benefit (Citation11). In this blinded study, the physical characteristics of DELP were rated statistically significantly more favourably than DIPC for all three parameters analysed (likeability, willingness to use again and preference), and were generally superior for the 10 additional attributes listed. These results are consistent with an earlier study showing a strong preference for an emollient gel in comparison with emollient creams and ointments (Citation22).
Although corneometry is an exceedingly well-established measure of skin hydration, a possible limitation of this study is that the measurements may be regarded as a surrogate clinical end point. It may therefore be helpful if future studies compared the effectiveness of different emolients using therapeutic end points. Although the study population was entirely adult females, the results may be reliably extrapolated to all age groups and both sexes because the products work by physical action only.
Conclusion
Although emollients are widely prescribed in the UK, it is not always possible to apply them frequently, and many patients only manage twice daily applications. In this study comparing two commonly prescribed licenced emollients, we have measured highly statistically significant differences in the degree and duration of skin hydration, and patients have reported substantial differences between their physical acceptability. These results confirm that not all emollients are the same (Citation19) and this is something that healthcare professionals should be aware of when prescribing these products.
Declaration of interest
This study was sponsored by Dermal Laboratories Ltd, Hitchin, UK and carried out by RSSL, Reading, UK.
References
- Clark C. How to chose a suitable emollient? Pharm J. 2004;273:351–3
- Cork MJ, Danby S. Skin barrier breakdown: a renaissance in emollient therapy. Br J Nurs. 2009;18:872–7
- NICE. Management of atopic eczema in children from birth up to the age of 12 years. Clinical Guidelines, CG57. London: National Institute for Health and Clinical Excellence, 2007
- Simpson EL. Atopic dermatitis prevention. Dermatol Ther. 2006;19:108–17
- MeRec B. The use of emollients in dry skin conditions. MeReC Bull. 1998;9:45–8
- Lewis-Jones S. Dry skin in childhood and their misery of eczema and its treatments. In: Loden M, Maibach H, eds. Treatment of Dry Skin Syndrome – The Art and Science of Moisturisers. New York: Springer, 2012. p 41–58
- van Neste D. Comparative study of normal and rough human skin hydration in vivo: evaluation with four different instruments. J Dermatol Sci. 1991;2:119–24
- Girard P, Beraud A, Sirvent A. Study of three complementary techniques for measuring cutaneous hydration in vivo in human subjects: NMR spectroscopy, transient thermal transfer and corneometry – application to xerotic skin and cosmetics. Skin Res Technol. 2000;6:205–13
- Loden M, Hagforsen E, Lindberg M. The presence of body hair influences the measurement of skin hydration with the corneometer. Acta Derm Venereol. 1995;75:449–50
- Holm EA, Wulf HC, Thomassen L, Jemec GB. Instrumental assessment of atopic eczema: validation of transepidermal water loss, stratum corneum hydration, erythema, scaling, and edema. J Am Acad Dermatol. 2006;55:772–80
- Cork MJ. The importance of skin barrier function. J Dermatol Treat. 1997;8:s7
- Cork MJ. The importance of complete emollient therapy. Med Matters Primary Care 2007;142:1–8
- Djokic-Gallagher J, Rosher P, Walker J, Hart V. Objective and subjective in vivo comparison of two emollient products. Clin Cosmet Investig Dermatol. 2012;5:85–91
- Lochhead RY, Hemker WJ, Castaneda JY. Creams and lotions documentary. Cosmet Toiletries. 1986;101:125–38
- Wynne A, Whitefield M, Dixon AJ, Anderson S. An effective, cosmetically acceptable, novel hydro-gel emollient for the management of dry skin conditions. J Dermatolog Treat. 2002;13:61–6
- Bremecker KD, Koch B, Krause W, Neuenroth L. Application-triggered drug release from an O/W-emulsion. Die Pharmazeutische Industrie. 1992;54:182–5
- Okamoto T, Inoue H, Anzai S, Nakajima H. Skin-moisturizing effect of polyols and their absorption into human stratum corneum. J Cosmet Sci. 1997;49:39–67
- Fluhr JW, Gloor M, Lehmann L, et al. Glycerol accelerates recovery of barrier function in vivo. Acta Derm Venereol. 1999;79:418–21
- Moncrieff G, Cork M, Lawton S, et al. Use of emollients in dry-skin conditions: consensus statement. Clin Exp Dermatol. 2013;38:231–8
- Moncrieff G. Complete emollient therapy – a personal view. PCDS Bull. 2013;9–14
- Gallagher J, Rosher P, Mohammed D, et al. The role of the excipient povidone in an advanced gel formulation specially designed for the treatment of dry skin conditions. 23rd EADV Congress; Amsterdam, Netherlands, 2014
- Aslam A. Empowering children to select their own emollients. Dermatol Nurs. 2009;8:16–20