Abstract
Purpose: This paper reviews and reassesses the internationally accepted niches or ‘targets’ in bone marrow that are sensitive to the induction of leukaemia and primary bone cancer by radiation.
Conclusions: The hypoxic conditions of the 10 μm thick endosteal/osteoblastic niche where preleukemic stem cells and hematopoietic stem cells (HSC) reside provides a radioprotective microenvironment that is 2- to 3-fold less radiosensitive than vascular niches. This supports partitioning the whole marrow target between the low haematological cancer risk of irradiating HSC in the endosteum and the vascular niches within central marrow. There is a greater risk of induced bone cancer when irradiating a 50 μm thick peripheral marrow adjacent to the remodelling/reforming portion of the trabecular bone surface, rather than marrow next to the quiescent bone surface. This choice of partitioned bone cancer target is substantiated by the greater radiosensitivity of: (i) Bone with high remodelling rates, (ii) the young, (iii) individuals with hypermetabolic benign diseases of bone, and (iv) the epidemiology of alpha-emitting exposures. Evidence is given to show that the absence of excess bone-cancer in atomic-bomb survivors may be partially related to the extremely low prevalence among Japanese of Paget's disease of bone. Radiation-induced fibrosis and the wound healing response may be implicated in not only radiogenic bone cancers but also leukaemia. A novel biological mechanism for adaptive response, and possibility of dynamic targets, is advocated whereby stem cells migrate from vascular niches to stress-mitigated, hypoxic niches.
Introduction
To assess the potential risk of radiation-induced cancer, there is a need to calculate the absorbed dose to radiosensitive tissues or ‘targets’ and to compare this dose with the results of epidemiological studies. Leukaemia and bone cancer contribute to the total risk of cancer and hereditary disease estimated by the committed effective dose as recommended in Publication 103 of the International Commission on Radiological Protection (ICRP 103 2007). The long-time ICRP target tissue for radiogenic bone cancer is the 10 μm thick “endosteal layer” (ICRP 30 1979/81) adjacent to trabecular and cortical bone surfaces (). This target was based on quiescent osteoblasts/bone lining cells that are no longer considered as possessing substantial carcinogenic potential (ICRP 11 1968). In 2007, the ICRP considered changing the thickness of the primary bone cancer target adjacent to bone surface from 10–50 μm (Bolch et al. Citation2007): This new thicker target will be referred to as “peripheral marrow”. Bone cancer risk is assessed from the dose to peripheral marrow found in trabecular bone cavities and medullary (long bone) cavities in cortical bone, now excluding cortical Haversian canals. The whole red marrow including the endosteum continues to be the ICRP's target tissue for calculating dose and risk of radiation-induced leukaemia.
Table I. Marrow niche characteristics of normal and cancer stem cells.
The historical emphasis of the ICRP's dosimetry has been primarily for adults. This paper reviews skeletal targets and proposes new ones suitable for all ages of human development. Richardson (Citation2009a) assessed the risk of excess fatal cancers from internal radionuclide exposures as the product of three factors: the radiation hazard (excess mortality Sv−1), exogenous exposure (Bq) and internal exposure dose coefficient (Sv Bq−1). The National Research Council publication Report V on the Biological Effects of Ionising Radiations, i.e., BEIR V (1990), estimated children were around 10 times more vulnerable per unit dose (Sv−1) to fatal, radiation-induced solid cancers than middle-aged adults: leukaemia exhibited less variation with age.
Children take in smaller amounts of contaminated air and food than adults. However, in smaller bodies, radioactivity is more concentrated, turned over more rapidly due to a higher metabolism and uptake in organs elevated due to growth. The selection of appropriate radiation targets for children that have been internally exposed to bone-seeking radionuclides is important, as the infant:adult ratio of the total fatal cancer risk per unit inhaled activity (Bq−1) is around 10:1 (Richardson Citation2009a). Similarly, the infant:adult risk per Bq ingested activity is ∼100:1. The ICRP in 1989 extended radiation dosimetry from adults to children and infants, and later added the embryo/foetus (ICRP 56 1989, ICRP 88 2001).
The ICRP's skeletal targets, developed for adults, were recommended for assessing the radiation risk to non-adults. However, unlike some soft tissue organs, not only are the dimensions of the skeleton changed by growth in childhood but there are also profound age-related changes in skeletal physiology and morphology.
Since 1979, when the endosteum and whole red marrow were chosen as ICRP targets, there have been significant advances in the understanding of radiation health effects and the biology of stem cells, cancer stem cells, fibrosis, inflammation and carcinogenesis. This paper was written to meet the challenge set in 2007 by the ICRP Task Groups on Internal Dosimetry (INDOS) and Dose Calculation (DOCAL) to provide supporting biological and epidemiological evidence for proposed partitioned bone cancer and leukaemia targets. Nie and Richardson (Citation2009) modeled the dose to stem/progenitor cells adjacent to the trabecular bone remodelling compartment and found this site to be a more likely candidate target for radiation-induced bone cancers, especially in childhood, than the ICRP target (i.e., the peripheral marrow adjacent to quiescent bone surfaces). This new target is based on the risk of bone cancer being higher when bone turnover is elevated: this is the case in the young rather than the old, in trabecular rather than cortical bone, and in association with hyper-metabolic pathology such as Paget's disease (osteitis deformans or osteodystrophia deformans).
The following dosimetry targets for ‘marrow neoplasms’ (i.e., bone cancer and leukaemia) are proposed as better representatives of radiation-induced risk to the third trimester foetus and at all post-natal ages ():
Figure 1. Schematic diagrams (not to scale) depict trabecular marrow cavities that in reality are usually interconnecting. Sub-figure (A) names the anatomical parts of a marrow cavity and shows the partitioned hematopoietic targets for leukemia, consisting of the endosteum and central hematopoietic marrow and (B) shows the partitioned peripheral marrow targets for bone cancer consisting of quiescent surface peripheral marrow (QSPM) and forming surface peripheral marrow (FSPM): the latter target could include the bone remodelling compartment (BRC).
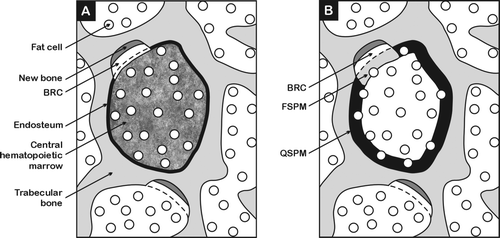
Leukaemia ‘partitioned hematopoietic targets’ – instead of the ICRP's whole red marrow, trabecular hematopoietic tissue is partitioned into two weighted parts: The endosteum/endosteal niche (reduced weighting due to hypoxia) and the remainder central cavity marrow.
Bone cancer ‘partitioned peripheral marrow targets’ – the ICRP's whole peripheral marrow is partitioned into two weighted parts: First, peripheral marrow associated with quiescent bone surfaces, and second, peripheral marrow associated with forming bone surfaces.
Both partitioned targets exclude marrow fat cells that have low carcinogenic potential. The dose associated with skeletal growth, remodelling and forming surfaces can be weighted to represent the greater cancer risk to children.
The contemporary knowledge on stem cells, including their niches and oxygen levels, is reviewed to determine the choice of marrow targets and their radiosensitivity, especially taking infants and children into account. It would be ideal if the calculated radiation dose to targets always predicted risk, but there are factors other than dose – such as cell proliferation, genomic instability and the prevalence of cell killing or pre-existing lesions – that affect the risk of spontaneous and radiogenic neoplasms in different populations (Streffer Citation2009). The influence of pre-existing lesions on the occurrence of adult radiogenic bone cancer was formulated a decade ago (Gössner Citation1999). Since that time, the knowledge of biological mechanisms of malignancy transformation has advanced and may further explain the development of radiation-induced neoplasms. Finally, existing and proposed marrow targets both have static locations; but in future dynamic targets could be an option as stem cells migrate in some circumstances.
Skeletal cancer targets lie primarily in trabecular bone marrow
(1) Spontaneous and radiation-induced leukaemia
Leukaemia is the most common childhood cancer (), representing ∼31% of cancer cases in US children under 15 years of age (Smith et al. Citation1999). In US adults over 65 years old, leukaemia has ∼12 times higher incidence than in children, but makes up only about one-fortieth of all cancers reported (Ries et al. Citation2007). Acute myeloid leukaemia (AML), chronic myeloid leukaemia (CML) and chronic lymphocytic leukaemia (CLL) are primarily cancers of old age, whereas acute lymphocytic leukaemia (ALL) is particularly common in childhood. Most leukaemias originate from precursor cells in bone marrow (including B-cell lymphocytic leukaemias), a notable exception being thymic T-cell ALL which represents about 15–20% (mostly in children) of cases of ALL in Western countries. An advisory group of the National Radiological Protection Board (NRPB 2003) tasked with estimating the incidence of different types of cancers for the UK population reported that myeloid leukaemias made up just over a third of spontaneous leukaemias, whereas radiation-induced myeloid leukaemias, especially AML, were over-represented compared to their lymphoid counterparts (). Thorotrast-administered patients irradiated with high-linear-energy-transfer (high-LET) 232Th alpha-radiation exhibit even greater domination by AML, ∼4/5ths of the leukaemia cases. An excess risk of leukaemia is also associated with the higher doses prescribed in radiotherapy for cancer (Boice et al. Citation1987, Curtis et al. Citation1994) and X-ray treatment of ankylosing spondylitis (Weiss et al. Citation1995). Thorotrast, radiotherapy and atomic-bomb survivor studies all reported no radiation-induced CLL (Preston et al. Citation1994). However, CLL's rarity among Asian populations, prolonged latency (perhaps 20 years compared with ∼5 years for other leukeamias), and misdiagnosis may have erroneously designated CLL as a non-radiogenic form of cancer (Richardson et al. Citation2005).
Table II. Characteristics of different types of leukaemia, namely their relative incidence levels of spontaneous occurrence at different stages of human development and the stem or progenitor cells from which the leukaemic types originate (Ries et al. Citation2007). Note that the Philadelphia chromosome is not only found in children with ALL but also found in adults with ALL, and occasionally those with AML (Jamieson et al. Citation2004)a.
Table III. Comparison of the percentage incidence rates for spontaneous and radiation-induced leukaemia (assuming 0% radiation-induced CLL). The spontaneous incidence rates are those of England and Wales from NRPB (2003)a as given in their Table 1.1. The low-LET incidence rates are those of the UK population, also from NRPB (2003)a as evaluated in their Table 4.3 based on the BEIR V (1990) risk model. The high-LET incidence rates are those of Danish Thorotrast patients (Visfeldt and Andersson Citation1995)b.
The excess relative risk of leukaemia in children or adults exposed to the radiation dose from atomic bombs dominated by low-LET high-energy gamma rays is more than other cancers (Preston et al. Citation1994). The evidence for lymphoma and multiple myeloma in atomic-bomb survivors is weak or non-existent. Brenner et al. (Citation2003) found significant excesses of leukaemia in children reported for different public and occupational populations exposed to ionising radiation doses as low as a few mSv. The Oxford survey is the largest case-controlled study of childhood cancers associated with obstetric X-rays, mean dose 10–20 mGy (Bithell and Stewart Citation1975). This study found, in common with other smaller case-control studies, a proportional increase in risk of ∼40% for childhood cancer (about half being leukaemia) associated with prenatal irradiation (Doll and Wakeford Citation1997). However, cohort studies of medical or atom bomb in-utero irradiation provides limited evidence of increased risk of cancer (ICRP 90 2003).
(2) Spontaneous and radiation-induced bone cancers
Sarcomas of the bone and cartilage are relatively rare comprising about 0.2% of all malignancies in the total US population (but a higher proportion of childhood cancers, ∼6%) – an incidence rate similar to other developed countries (Gurney et al. Citation1999, Ghadirian et al. Citation2001, Ries et al. Citation2007). Chrondrosarcomas, derived from a mesenchymal cartilage progenitor, are the spontaneous bone cancers most frequent in adults; however, they occur about five times less commonly as a fraction of radiation-induced bone cancers (). Osteosarcomas, an osteoblastic neoplasm, are the most common form of spontaneous and radiation-induced bone cancer in a population, and are particularly prevalent in children ( and ). A risk factor for osteosarcoma is the rapid bone growth in adolescence. The intramedullary form of osteosarcoma is most common (∼75%), the periosteum form makes up 4–10% of cases, while intracortical osteosarcoma is rare (<1%) (Murphey et al. Citation1997). The remainder of osteosarcomas is more defined by histopathology than their location in bone.
There is a low percentage of the spontaneous primary bone tumours occurring in the cortical midshaft of long bones, being about 10% of osteosarcomas, 10% of chondrosarcomas and 14% of malignant fibrous histiocytomas, but these occurence drop to 2–6% for post-irradiation bone sarcomas, including those 226Ra-induced (Pool et al. Citation1983, Huvos Citation1991, Unni Citation1996). Spiers and Beddoe (Citation1983) found the frequency of occurrence of spontaneous and radiation-associated osteogenic sarcomas in the long bones of humans to be more associated with the trabecular bone surface area rather than the cortical surfaces.
Table IV. Characteristics of different types of spontaneous and radiation-induced bone tumours (Ewing's sarcoma is not associated with radiationa) including their relative incidence levels of spontaneous occurrence at different stages of human development, the stem or progenitor cells from which the bone tumour types originate and their location in the skeleton (Gurney et al. Citation1999).
Table V. Comparison of the percentage bone tumour incidence rates that occur spontaneously or are radiation-induced, including malignant fibrous histiocytoma (MFH). Other types of spontaneous malignant bone tumours than are named here make up 14% of tumour types examined in ‘all population’ but only 3% of those aged 0–20 y (Unni Citation1996)a. The Paget's disease study was by Schajowicz et al. (Citation1983)b, the radium values were reported by Gössner (Citation1999)c, the external irradiation of adults was based on the combined studies of Huvos (Citation1991) and Unni (Citation1996)d. Tucker et al. (Citation1987)e assessed the bone cancers developed after the radiotherapy treatment of childhood cancer.
In addition, Priest et al. (Citation1995) showed that it is rare for osteosarcomas to be induced in animals following intakes of 226Ra and 241Am, both alpha-emitters that deposit in locations of low bone turnover (cortical or trabecular quiescent bone surfaces). However, intakes of 224Ra and 239Pu are much more toxic as they preferentially deposit in, and irradiate, sites of high bone turnover, in particular trabecular forming surfaces. In summary, trabecular bone is an important potential bone cancer site, whereas cortical bone is a minor target for radiation bone cancer.
Niches and oxygen levels of stem cells and progenitor cells at risk
There are two major types of multipotent stem cells found in the skeleton. First, hematopoietic stem cells (HSC) form progenitors of both the myeloid and lymphoid lineages including blood cells, immune cells and osteoclasts that excavate bone. Second, mesenchymal stem cells (MSC) produce bone (osteoblasts), cartilage (chrondroblasts), fat and stromal cells. Many, but probably not all, solid and hematopoietic neoplasms arise from cancer stem cells that are dysfunctional versions of a normal stem cell. These cancer stem cells can self-renew and differentiate into some or all cell types of the tumour (Kelly et al. Citation2007). The concept of the cancer-stem cell was first developed in leukaemia (AML), but has also been characterised in some solid cancers, including bone tumours that arise from aberrant MSC, e.g., osteosarcoma and chondrosarcoma, (Hope et al. Citation2004, Gibbs et al. Citation2005). AML (arising from aberrant HSC), but also CML and ALL have been shown to be maintained by cancer stem cells (Reya et al. Citation2001). Childhood ALL has been found to be initiated in fetuses by primitive cells of the HSC lineage acquiring characteristic chromosome translocations; these rare mutated cells largely remain covert and quiescent, with only some of them being promoted postnatally to full, overt leukaemia (Greaves et al. Citation2003). There is still much to learn about radiation's role in the origin of leukemic stem cells, which can involve HSC that became abnormal as a result of accumulated mutations or alternatively, arise via progenitors that regain the stem cell property of self-renewal (Passegué et al. Citation2003). Normal and cancer stem cells remain quiescent in hypoxic niches, but migrate to or create vascular niches in order to proliferate (). This section provides evidence for partitioning the ICRP's leukaemia and bone cancer targets based primarily on the age-dependent locations of normal and cancer stem/progenitor cells in marrow, and to a lesser extent on the oxygen-dependent radiosensitivity of their niches.
(1) Leukaemia: Age, cellularity and skeletal targets
Bone marrow is the major site of hematopoiesis in the third trimester fetus, infants, children and throughout adulthood (ICRP 70 1995). HSC are found in medullary cavities in bone, especially in trabecular marrow with high hematopoietic cellularity and little fat. The fetal marrow consists solely of hematopoietic tissue, which is replaced by adipocytes as the individual ages, dropping to an average skeletal cellularity of ∼46% at 70 years old (Richardson and Dubeau Citation2003). The loss of hematopoiesis in the marrow of long bone cavities is faster in general than in the rest of the skeleton: long bone medullary cavities lack cellularity and hematopoiesis from around 20 years onwards (Cristy Citation1981).
Acute and chronic leukaemia () is characterised by unregulated growth of blood cells and their precursors, with acute leukaemia involving relatively immature cells (blasts) compared to the more mature cell types of chronic leukaemia. A CML blast crisis occurs in granulocyte-macrophage progenitor cells that acquire self-renewal capability (Jamieson et al. Citation2004). CML cases usually are present in a chronic phase before progressing after about three years on average, to an acute form of leukaemia. The initial development of AML appears to involve HSC, while subsequent mutations in progenitors are necessary for full transformation (Hope et al. Citation2004).
It is generally accepted that HSC and their progenitors are located in two distinct microenvironments or ‘niches’: Firstly, the endosteum or so-called, osteoblastic niche and secondly, the sinusoidal perivascular areas or vascular/endothelial niche (Schofield Citation1978, Arai and Suda Citation2007, Lo Celso et al. Citation2009). Kiel et al. (Citation2005) found that 14% of HSC in murine bone marrow are associated with the endosteum, while most HSC are in contact with sinusoidal endothelia. Experimental evidence generally shows that quiescent HSC (slow cell turnover, with most in the G0/G1 mitotic cell cycle phase), other primitive hematopoietic progenitor cell types, and AML stem cells, have their highest concentrations close to bone surfaces (Lord Citation1990, Nagasawa Citation2006, Colmone et al. Citation2008, Ishikawa et al. Citation2007). The 10 μm thick endosteum accommodates small, 3–7 μm diameter, spore-like stem cells (Vacanti Citation2004) accompanied by 1 μm thin osteoblasts that are bone lining cells. Quiescent HSC may reside in an osteoblastic niche in close contact with N-cadherin+ pre-osteoblastic cells and endothelial cells that are MSC progeny, whereas active HSC prefer peri-vascular niches containing mesenchymal reticular cells (Arai and Suda Citation2007, Kiel et al. Citation2007).
Less primitive progenitors, pre-leukemic cells and malignant metastasising cells migrate towards the vascular sinusoids located in the central part of marrow in bone cavities (Colmone et al. Citation2008). Perivascular sites provide nutrients and vascular access to mitotically active cancer stem cells, as well as benefiting HSC/MSC and their differentiated progenitors that replenish the blood system (Sipkins et al. Citation2005, Kopp et al. Citation2005). The presence of endosteal and vascular niches supports the proposed partitioning of ICRP's whole marrow target for leukaemia, especially considering that these locations have differing radiosensitivity as described in subsection (3) below.
(2) Bone cancer: Age, cellularity and skeletal targets
During the third month after conception, a primary then secondary ossification centre is formed in long bones; both ossification centres produce cartilage cavities that fill with marrow. The epiphyseal growth plate produces longitudinal bone growth, while the periosteum provides appositional growth. This skeletal growth is active in the infant, peaks in the adolescent and ends in the young adult: Bone remodelling activity mirrors that of growth but continuing at a low level into old age. In childhood, the spontaneous and radiation-induced bone cancer incidences shows a similar trend to bone growth. The proportions of trabecular bone and cortical bone vary with age, being predominantly trabecular/cancellous bone in the fetus and newborn when skeletal remodelling and proliferative activity is at its maximum (ICRP 70 1995). Cortical bone, which dominates in adults, comprises 80% of the skeletal mass and has a turnover six times slower than trabecular bone. In infants (when cortical bone is scarce), forming surfaces consist of up to 50% of trabecular bone surfaces, while in adults this figure drops to only 3% of cortical bone surfaces and 6% of trabecular bone surfaces.
Human MSC that differentiate into connective tissue cells with osteogenic and chrondrogenic potential are resident in many sites including bone growth plates, the outer cambian layer of the periosteum, and within Haversian canals of cortical bone (Muschler et al. Citation2003). MSC, originally referred to as colony-forming unit-fibroblasts, support hematopoiesis and osteogenesis, but unlike HSC do not exhibit all stem-cell criteria as they have a more limited capacity for self-renewal and cannot regenerate a whole tissue. Like HSC, MSC seem to occupy two distinct marrow niches (Bianco et al. Citation2001). Active MSC occupy vascular niches in association with endothelial cells and quiescent MSC are found in close proximity to trabeculae bone surfaces. The number of osteoblastic stem cells, osteoblastic progenitors and osteoblasts in marrow markedly decreases with age after skeletal maturation (Nishida et al. Citation1999, Muschler et al. Citation2001). Osteoblast and MSC concentrations, like bone turnover, were observed to be inversely associated to the adiposity of marrow (Moerman et al. Citation2004). In children, spontaneous and radiation-induced bone cancers are primarily metaphyseal and diaphyseal intramedullary lesions, although benign tumours (e.g., chondroblastoma) are commonly associated with growth plates (). In adults, spontaneous and radiation-induced bone cancers also usually originate in trabecular bone with red marrow, e.g. proximal/distal ends of long bones (Spiers and Beddoe Citation1983). Trabecular cavity marrow generally exhibits high cellularity and MSC levels, which result in a higher proliferative capacity than cortical bone. The skeletal location of MSC and radiogenic bone cancers support the selection of a radiation target for bone cancer that is associated with remodelling bone found on trabecular bone surfaces.
(3) Oxygen effect and radioprotection of quiescent stem cells
The preferred microenvironments of differentiated and undifferentiated cells in bone marrow have radically different oxygen tensions and oxygen consumption levels. In a hypoxic environment, stem cells have a low metabolism and a long lifespan. However, stem cells and their shorter-lived progenitors proliferate under higher oxygen pressures. The presence of the hypoxic marker pimonidazole demonstrates that HSC in bone marrow are mostly located in the endosteum at 1% O2 or less, i.e., <1 kPa or 7.6 torr (Parmar et al. Citation2007). The self-renewal of HSC or MSC, and their maintenance in an undifferentiated state, is enhanced in hypoxic conditions existing close to bone surfaces compared with normoxic conditions found elsewhere in marrow (Grayson et al. Citation2006). Reactive oxygen species (ROS) arise from exogenous radiation sources, but mostly originate from endogenous mitochondria. The transcription factor, hypoxia-inducible factor-1, (HIF-1) adapts cells to survive the stress of low oxygen conditions (Papandreou et al. Citation2006). In hypoxic regions, glycolysis is stimulated, oxidative phosphorylation becomes more efficient, and mitochondrial proton leakage and cell death are repressed (Gnaiger et al. Citation2000). Therefore, hypoxia and the ‘oxygen effect’ protect normal stem cells from ROS, thereby reducing the consequential DNA damage and apoptosis (Moore and Lemischka Citation2006, Arai and Suda Citation2007). Similarly, quiescent leukemic stem cells residing in the hypoxic endosteum are resistant to radio- and chemo-therapy and treatment-induced ROS (Ishikawa et al. Citation2007).
The oxygen enhancement ratio (OER) value is defined as the ratio of the hypoxic and normoxic doses which lead to the same biological effect, such as cell death (Thoday and Read Citation1949). Radiosensitivity varies most rapidly with oxygen tensions below about 2% O2. The maximum OER value increases with decreasing LET (particle keV lost per μm of track). For example, the OER value utilised by Richardson (Citation2008) for low LET X-rays, was 2.6 in air (21% O2). The maximum OER value is 1.3 for high-LET 4.0 MeV alpha particles from 232Th decay, compared to a maximum OER of 1.7 for 224Ra alpha particles of 5.7 MeV. Therefore, any deleterious radiation effects to MSC, HSC or cancer stem cells residing in the radioprotective microenvironment of the endosteum, compared with radiosensitive vascular niches, will be mitigated by a factor of, at most, two for high LET alpha-radiation and three for low LET beta-, gamma- and X-rays (). The radioresistance of the narrow endosteum will have a minor influence on the overall higher radiosensitivity of the 50 μm thick peripheral marrow that encompasses it.
The match/mismatch between dosimetry and epidemiological leukaemia incidence
When selecting radiation targets one important aim is for the target dose (Gy) to correspond with the cancer incidence risk obtained from epidemiological studies. This is particularly difficult to achieve for low doses and low dose rates. The choice of target is complicated by factors other than radiation dose that affect the risk of fatal cancer. This section reviews two biological mechanisms that may influence risk: First, cell killing, which has been known for decades to decrease high dose response, and second, recent research on radiation-associated migration of HSC that is hypothesised to be a form of adaptive response.
(1) Leukaemia risk is reduced by high dose cell killing
Low-LET studies of medical irradiation for Japanese atomic-bomb survivors show a curvilinear dose response for leukaemia, with comparatively lower risk at doses below ∼1 Gy, and cell sterilisation from 3 Gy upwards (Pierce et al. Citation1996, Little et al. Citation1999). The leukemic risk and cell killing from this low-LET acute irradiation is compared next with observations from two studies where patients were administered radium and thorium radionuclides and received internal, high dose, high-LET exposures.
German patients, mostly adults, during the 1940s and 50s were treated with multiple injections of bone-seeking radium alpha-emitters for various conditions (Nekolla et al. Citation2005, Wick and Nekolla Citation2005). Excess leukaemia was induced when low doses of short-lived 224Ra were administered to ankylosing spondylitis patients (Harrison and Muirhead Citation2003). However, there were lower incidences of leukaemia than expected observed in high dose studies of long-lived 226Ra alpha-emitters, including radium dial painters (Spiers et al. Citation1983). Thorotrast, a thorium dioxide colloid containing 232Th and its progeny, was injected mainly into adults as a radiographic contrast medium in the 1930s and 40s. The alpha-radiation exposure of the liver, spleen and bone marrow was long term and probably homogeneously distributed within these organs and tissues. Harrison and Muirhead (Citation2003) compared the risk of radiation-induced leukaemia in patients that received Thorotrast alpha-irradiation with atomic-bomb survivors that were exposed to high-energy gamma rays. When the ICRP value of 20 is employed as the alpha-radiation weighting factor, the hematopoietic malignancies of the Thorotrast exposures were 10 times lower than expected (van Kaick and Wesch 2005). These high alpha-radiation doses sterilised hematopoietic progenitors reducing malignancies (Gössner Citation2003).
Cardis et al. (Citation1995) conducted the largest international combined study of nuclear industry workers assessing the health risks associated with low dose, mainly low-LET external exposures. The review of these occupational exposures by BEIR VII (2006) concluded that although the risk estimates for leukaemia varied considerably, they were consistent with those on which current radiation protection recommendations are based. Therefore, the current ICRP whole-marrow target for radiation-induced leukaemia provides a reasonable estimate of the haematological cancer risk for low-LET external exposures of adults. Whereas, dosimetric evaluations for high-dose, short-range, high-LET, internal irradiations, based on the same target, appear to over-emphasise the risk. This poor assessment of cancer risk is primarily due to cell killing. But could an adaptive response also be playing a role by reducing the biological effects of the internal, chronic irradiation?
(2) Stem cells migrate after a radiation-insult as an adaptive response?
There is a higher excess relative risk of leukaemia after whole body irradiation of atomic-bomb survivors compared to the risk of leukaemia as a second cancer in patients treated for cervical cancer and uterine cancer with partial-body external beam radiation (Boice et al. Citation1987, Curtis et al. Citation1994, Little Citation2001). Shuryak et al. (Citation2006) contend that the lower leukemogenic risk for partial-irradiation is due to HSC migrating through the blood stream, from niches in bone marrow and spleen that received low doses, to repopulate high dose sites where stem cells have been inactivated. Medical irradiations, often concurrent with chemotherapy, generally involve multiple acute external beam exposures or chronic brachytherapy exposures. It is hypothesised that an early adaptation to the ongoing stress of multiple exposures may reduce the risk of a second cancer.
Real-time imaging has been recently employed to show the localisation of HSC and progenitor cells in medullary cavities of femurs from un-irradiated and irradiated mice. Xie et al. (Citation2009) found HSC randomly distributed in the marrow within 50 μm of the trabecular bone surface of irradiated mice. Controversially, Lo Celso et al. (Citation2009), like some others before them, contend that HSC are normally sparsely localised, if at all, within 30 μm of the endosteum (Kiel et al. Citation2007). In both studies, other mice were also imaged after receiving around 10 Gy before bone marrow transplantation. HSC were then injected into the irradiated recipients and they localised closer to the endosteal surface than in the unirradiated mice: of the HSC, 47% were now within 15 μm of the endosteal surface according to Lo Celso et al. (Citation2009).
There appears to be good biological reasons to support marrow stem cells being normally located in the vascular niche, but migrating to the endosteal niche in times of physiological stress. A vascularised and well-oxygenated environment provides the essential nutrients for production of progeny and DNA repair by homologous recombination, the latter an energetically demanding but virtually error-free process. Under stress, stem cells that migrate to the hypoxic endosteum will experience the advantages of less free-radical damage due to reduced endogenous and exogenous ROS, as well as increased self-renewal and longevity (Eliasson and Jönsson Citation2010). Ionising radiation, especially alpha-particles, produce comparatively more DNA double strand breaks than mitochondrial proton leakage. A downside of hypoxia is slow HSC turnover (∼1 year in non-human primates) and reduced DNA repair (Mahmud et al. Citation2001). This leads to a greater reliance on double-strand breaks being repaired by error-prone replication-independent processes (Rodríguez-Jiménez et al. Citation2008). No matter what, the long-term stress associated with ageing will result in stem cells, even if quiescent, accumulating mutations and exhibiting shorter telomeres, as well as accelerated stem cell exhaustion and senescence (Nijnik et al. Citation2007; Richardson Citation2009b).
Several biological mechanisms – including DNA-repair processes, apoptosis, and the immune response – have been expounded in the literature to explain the adaptive response of somatic cells to stress, such as heat or radiation (Streffer Citation2009). In many situations a protective adaptive response by radiation is demonstrated by a low acute dose of 5–200 mGy, given 4–24 h before a higher challenging dose of 1 to several Gy. The mice in the experiments of Lo Celso et al. (Citation2009) and Xie et al. (Citation2009) were irradiated at higher levels than for adaptive response experiments in general. After Xie et al. (Citation2009) exposed their mice to 10 Gy, and in response to the resulting bone marrow damage, HSC migrated into the radioprotective endosteum, which they then occupied for >10 months. It would be of interest to learn if this migratory behaviour of stem cells from a vascular niche to a hypoxic one occurs for shorter times at the lower doses usually employed to demonstrate adaptive response; and in addition, to also determine if this adaptation is responsible for reducing the late effects of multiple or chronic radiation doses compared with acute exposure.
Biological mechanisms linking radiogenic fibrosis and marrow cancers
Although the targets proposed were selected to allow a prediction of excess cancers attributable to the radiation dose, there are other factors besides dose that influences the risk. This section reviews the evidence that fibrosis and pre-existing lesions are associated with aging and radiation-induced bone cancer. Conversely, it is suggested that where population groups display a rarity of these lesions, as for all children and Asians adults, this will modify the risk of this lesion-associated, late-effect neoplastic development. Therefore, the bone cancer targets of children and adults will differ as different factors such as pre-existing lesions and growth influence their risk. Lastly, this section reviews the recently published research on biological mechanisms which may suggest a link between radiation, inflammation, fibrosis and leukaemia.
(1) Pre-existing lesions, fibrosis and radiation-induced bone cancer in adults
Several researchers have observed that following a radium intake a mainly acellular, fibrotic layer forms on bone surfaces. Lloyd and Henning (Citation1983) contended that this 8–50 μm thick microenvironment found in the neighbourhood of a bone tumour predisposed the site to radiation-induced bone cancers. Gössner (Citation1999) hypothesised that pre-existing lesions (e.g., Paget's disease, bone infarct, fibrous dysplasia), or radiation-induced bone lesions (e.g., radiation osteitis), and associated fibrosis might be critical for the induction of bone sarcomas of fibroblastic and fibrohistiocytic origin. The case is made in the next subsection that this hypothesis is more relevant to adults than children. Gössner's observation was made on the evidence that adults receiving radium or external beam radiotherapy showed the same spectrum of bone tumour types as adults with pre-existing bone lesions (rows 4, 5, 6; ). For example, in patients with Paget's disease Schajowicz et al. (Citation1983) reported a high fraction (27%) of fibrosarcomas and malignant fibrous histiocytomas (row 4, ). In a population comprising of mainly adults, fibrosarcoma and malignant fibrous histiocytoma are about three times more prevalent as a radiation-induced tumour than as a spontaneous tumour (rows 2, 5, 6; ).
Similar to radiation-induced cataracts, alpha particle irradiation of the skeleton appears to have a deterministic effect by inducing premature aging, probably by increased inflammation and fibrosis, the depletion of stem cells in marrow, and increased mutations of the remainder stem cells (Richardson Citation2009b). The average age of female US radium-dial painters at first exposure was just 20 ± 5 years old (Rowland et al. Citation1983). The appearance time of the 226,228Ra-induced bone sarcomas was long, on average 27 ± 14 years. The latent period was shortened dependent on the average skeletal dose. With the characteristics of a dose threshold, at lower doses the aging effect in these young women was small and inconsequential, as the cancer induction period was likely greater than the human life span (Evans et al. Citation1969).
Unlike leukaemia, bone sarcomas are rarely induced below skeletal doses less than 10 Gy (Brenner et al. Citation2003). This applies to adult radium-dial painters (BEIR IV 1988), Mayak workers exposed to plutonium (Shilnikova et al. Citation2003), ankylosing spondylitis patients treated with 224Ra (Wick and Nekolla Citation2005), and children receiving radiotherapy (Tucker et al. Citation1987). The high dose threshold for radiogenic bone cancer may be related to the finding that at low or moderate doses most tissues/organs studied express more fibrosis cytokine than bone marrow (Chang et al. Citation1995, Martin et al. Citation2000): the possible involvement of this biological mechanism is explored further in subsection (4) below. In light of the association of radiogenic bone cancer and fibrosis, Lloyd and Henning (Citation1983), supported by Gössner (Citation2003), proposed increasing the thickness of the ICRP radiation target from the endosteum of 10 μm thickness to a 50 μm marrow layer adjacent to the bone surface, where fibrosis associated with spontaneous or radiation-induced bone lesions is commonly located.
(2) Higher bone cancer rates in children are not associated with pre-existing lesions
Children and adults receiving high medicinal doses of 224Ra had a statistically significant increase in bone cancer risk with decreasing age at exposure (Nekolla et al. Citation2005). In patients treated for tuberculosis and other diseases by an injectant containing 224Ra, the incidence of bone sarcoma was about 14 times higher in the 1- to 5-year-olds, compared to adults >20 years old (Spiess Citation1969). Children receiving radiotherapy had an increased risk of bone sarcomas above doses of 10 Gy (Tucker et al. Citation1987). The mean period from the treated first cancer to the secondary bone cancer was around 10 years, a shorter latency than for adults receiving similar therapy.
Pre-existing lesions of bone are more common in adults, whereas benign tumours are more common in children. Around half of spontaneous bone tumours in American children are non-malignant (Unni Citation1996), although the transformation from benign to malignant form is rare. Children receiving radiotherapy developed malignant bone cancers of a similar type to those arising in a population spontaneously, but with a lower incidence of Ewing's sarcoma (rows 3, 7; ). Irradiated children developed fewer bone sarcomas of fibroblastic and fibrohistiocytic origins (Tucker et al. Citation1987); these sarcomas are relatively common among the radiation-induced bone cancers of adults (see previous subsection). The ICRP justifies the employment of a peripheral marrow target on the basis that radiogenic cancers are associated with fibrosis or pre-existing lesions. Although, Gössner's (Citation1999) observation is important for adults, it has little relevance to irradiated infants and children for whom pre-existing lesions and fibrosarcoma/malignant fibrous histiocytoma are rare. Instead, rapid skeletal remodelling and growth are probably the dominant factors linked with both spontaneous and radiogenic bone cancers of children and adolescents, thereby supporting the partitioning (and perhaps age-dependent weighting) of the peripheral marrow target into that associated with quiescent and forming bone surfaces.
(3) Rarity of Paget's disease and lack of bone cancer in Japanese atomic-bomb survivors.
Paget's disease of bone has a strong genetic component (Albagha et al. Citation2010) and is very common in some elderly Caucasian populations in Europe and US; it is less common in Scandanavia and extremely rare in Asia and Africa (Hashimoto et al. Citation2006). Paget's disease is characterised, similar to radiation osteitis, by abnormal and rapid osseous remodelling. It is rarely found in adults under 45 years old and becomes more common with ageing (Cooper et al. Citation2006). The disease is a benign condition with the potential for malignant transformation. Those with Paget's disease have an approximately 10-fold higher risk than the general population in developing bone sarcomas (Wick et al. Citation1981, Ghadirian et al. Citation2001). In most countries, Paget's disease is estimated to occur in 0.1–5% of individuals. However, in Japan, spontaneous bone cancer rates, including those in childhood, are relatively low when compared to international values (Parkin et al. Citation1993). In particular, there are low incidences of osteosarcoma in Japanese and Chinese adults aged over 50 years, which could be related to the lack of Paget's disease (Guo et al. Citation1999).
While a significant increase in bone-cancer mortality has been seen in adult radium dial painters in Europe and the US, bone cancer is among the few exceptions to the full spectrum of spontaneous cancers reported in excess among adult atomic-bomb survivors exposed to relatively low doses (Brenner et al. Citation2003, Preston et al. Citation2007, Richardson Citation2009b). It is acknowledged that the characteristics of these two radiation exposures were very different in terms of homogeneity and duration of the exposures, the mean dose, LET and relative biological effectiveness (RBE). Nevertheless, Little (Citation2001) found a strong increasing trend of bone cancers with atomic-bomb dose for those exposed as children (excess relative risk, 16.5), even though there was no significant association for adult-exposed survivors (excess relative risk, −0.3).
One reason for the relative lack of excess bone cancers in atomic-bomb survivors could be due to only 323 of the 86,661 exposed survivors having whole body (life-threatening) doses over 4 Gy (Pierce et al. Citation2008) and possibly none exceeding a 10 Gy skeletal dose threshold. Even if radiation can induce bone cancers at doses <10 Gy, a second factor may be the extremely low prevalence of Paget's disease in Japan, estimated at just 2.8 cases per million capita (Hashimoto et al. Citation2006). Follow-up studies of patients administered alpha-emitting Thorotrast (Mori et al. Citation2005, van Kaick and Wesch 2005) have exhibited excess bone cancers at significantly higher incidences (p < 0.05) in at least four of the six countries studied. Further analysis may provide the opportunity to statistically determine if irradiated adults from countries where Paget's disease of bone is very common (i.e., Germany, Portugal and the US) have a higher predilection to develop excess bone sarcomas than citizens from countries where Paget's disease is uncommon or extremely rare (i.e., Denmark, Sweden and Japan). Therefore, it appears the ICRP's present bone cancer target, the peripheral marrow adjacent to quiescent surfaces, has limited relevance to Asian and Nordic adults, and children globally, for whom pre-existing bone lesions are very rare.
(4) Involvement of fibrosis and the wound model in radiation-induced marrow neoplasms
One could speculate from recent research that radiation inflammation, observed as a low to high dose-dependent effect in atomic-bomb survivors (Hayashi et al. Citation2008), and associated fibrosis, may be important factors not only for radiogenic bone cancers but also in the induction of leukamia. The fat cell content of adult marrow increases with aging and irradiation, and probably contributes to increased inflammation due to hormonal effects of the ‘fat hormone’ leptin (Iikuni et al. Citation2008). Myelodysplastic syndrome (MDS), sometimes called preleukaemia, is a clonal disorder of HSC (Kuramoto et al. Citation2002). MDS is also associated with both aged and irradiated marrow. MDS patients present more angiogenesis in bone marrow than normal (Medinger and Mross Citation2010). The resulting increase in radiosensitivity may be a factor in the MDS excess relative risk being ∼13 Gy−1 in atomic-bomb survivors, which is much higher than the risk of leukaemia, 3.8 Gy−1 and all other cancers, 0.3 Gy−1 (Pierce et al. Citation1996, Tsukasaki et al. Citation2007). Higher occurrences of fibrosis and MDS are also prominent in Thorotrast administered to patients (Visfeldt and Andersson Citation1995, Ishikawa et al. Citation2001). The ineffective hematopoiesis and marked reticulin fibrosis displayed by MDS and caused by high radiation doses or chemotherapy can lead to AML/erythroleukaemia or marrow failure. As shown earlier (), the AML incidence as a percentage of the total leukaemias is enhanced in atomic-bomb survivors (2-fold) and Thorotrast patients (3-fold) compared with the spontaneous incidence rate. A quarter of the AML cases were erythroleukaemias in Danish Thorotrast patients, whereas only a few percent occur spontaneously (Visfeldt and Andersson Citation1995).
Transforming growth factor β1 (TGF-β1) is elevated in plasma during radiotherapy and involved in the proliferation of MSC, which lead to reticulin and collagen fibrosis in bone marrow by fibroblasts (Martin et al. Citation2000). TGF-β1 is a cytokine that regulates defence response to injury. Latent TGF-β1 is activated by ROS from radiation and other sources/stresses such as chemicals or heat (Jobling et al. Citation2006). Progenitor cells from patients with MDS-derived AML display a mixed response to TGF-β, with AML cells in only four of seven cases escaping TGF-β mediated growth inhibition (Koschmieder et al. Citation2001). The involvement of TGF-β1 is more transparent in transforming pre-leukeamic cells with the TEL-AML1 chimeric fusion gene into childhood ALL (Ford et al. Citation2009). The biological mechanism transforming MDS to AML/erythroleukaemia is complex and as yet unclear. However, recent research suggests that radiation's ability to produce inflammation and fibrosis may be important factors in the induction of not only bone cancer but also leukaemia.
Discussion
The former ICRP target, the 10 μm thin endosteum may be a questionable size for a target as a measure of radiation dose and risk, because of the genetic instability observed in bystander non-irradiated marrow stem cells and progeny (Kadhim et al. Citation2004): this could be due to a radiation-induced inflammatory response (Lorimore et al. Citation2005). Cell-to-cell signalling regulates, in part, cell growth, differentiation and apoptosis. It is conceivable that radiation has an influence on the niche cells that regulate stem cell fates by affecting signaling molecules, the extracellular matrix, and cell adhesion molecules (Arai and Suda Citation2007). Therefore, irradiating support cells of stem- or progenitor-cells may provoke long-range detrimental biological effects due to disruption of the support cell's communication.
To be accurate, it is only the haematopoitic tissue which constitutes a leukaemia target rather than whole red bone marrow that ICRP (ICRP 23 1975) describes as consisting of 40% fat cells in addition to hematopoietic tissue in adults. The age-dependent alpha-particle dosimetry of hematopoietic marrow, rather than red marrow, was first carried out employing analytical methods (Richardson et al. Citation1991), whereas more sophisticated Monte Carlo simulations enable alpha- and beta-radiation dosimetric analyses of the skeleton, both in the quiescent (Eckerman and Stabin Citation2000, Richardson and Dubeau Citation2003, Watchman et al. 2005) and remodelling states (Richardson et al. Citation2007, Nie and Richardson Citation2009). For a minority of radionuclides and compounds, the dose to hematopoietic marrow and adipocytes can be markedly different and at variance with the average dose to homogeneous red marrow. This dose variation can occur for short-range radiations and radionuclides of elements or compounds that have higher activities in either adipocytes e.g. carbon, radon (high gaseous solubility), or alternatively, higher activities in hematopoietic tissue, such as plutonium (Leggett Citation1985) as well as the lean tissue seekers, potassium and polonium. A representative dose for the whole skeleton by short range irradiation of bone cancer and leukemic targets can be obtained by summing dose to these targets in various bones, weighted by a factor involving the trabecular or cortical bone's cellularity, an indicator of proliferative capacity (Richardson et al. Citation1991). If this weighting process is applied to the proposed ICRP target, the cortical medullary endosteum, in essence, has virtually no influence on the radiation dose to the bone cancer target at any age.
Increasing the peripheral marrow layer from 10 μm to 50 μm will affect the alpha particle dose to the bone cancer target from activity located in trabecular bone or marrow (Nie and Richardson Citation2009). This is due to the limited maximum range of alpha particles, e.g., 10 μm in soft tissue for 144Nd, 1.8 MeV particles and 90 μm for 212Po, 8.8 MeV. The same is true for low energy, shorter-range beta-emitters, such as 14C with a mean range of 40 μm in water. Alpha-particle activity was modeled by Nie and Richardson (Citation2009) as a uniform source in bone marrow, bone volume and on bone surface. The ICRP's decision to move to a 50 μm marrow layer results in a relatively small increase in dose to the bone cancer target from alpha-emitters deposited in marrow, e.g., Thorotrast. Conversely, a relatively large decrease in target dose results from alpha-emitters homogeneously deposited in bone volume, and particularly for alpha-emitters on bone surface. The ratio comparing the 50 μm peripheral marrow dose to the 10 μm endosteum dose is around 1:3.4 for 226Ra, 224Ra and 239Pu on bone surface, and 1:4.3 for 232Th. The ratio of the doses is 1:5 for alpha- or beta-particles with a range equal to or less than the width of the endosteum, e.g., 144Nd. Therefore, by employing the 50-μm target over the 10 μm one, the ICRP will effectively reduce by as much as 5-fold the alpha-particle target dose from bone-volume and bone-surface seekers, which in turn lessens the predicted risk of bone cancer (and conversely, increases the RBE more in line with ICRP recommendations).
Fractional weighting of the endosteum and central marrow regions could be chosen based on the calculated leukemic target dose and risk, and compared with the corresponding excess leukaemia observed in epidemiological studies. A 10 μm thick endosteum takes up only about 4% of the trabecular marrow cavity volume of an adult; however this rises to ∼9% for the newborn. These values are derived assuming the dimensions and morphology of a “spherical” trabecular cavity, as evaluated by Richardson and Dubeau (Citation2003). The proposed ICRP target of average dose to trabecular red bone marrow in effect weighs the dose to the endosteum and central marrow regions by a ratio of about 1:24 and 1:10 for adults and newborn, respectively. However, taking account of the radioprotection of the hypoxic endosteum and a maximum OER of 3 and 2 for low-and high-LET irradiation, the 10-μm endosteum to central marrow dose ratio would be weighted by about 1:48 and 1:20 for adults and newborn exposed to high-LET radiation. This places less emphasis on the dose to the endosteum and for bone-seeking alpha-emitters, e.g., 226Ra, would lessen the overall dose to the leukemic target and go some way, besides cell killing, to explaining the virtual lack of excess leukaemias in radium studies (Spiers et al. Citation1983, BEIR IV 1988). It is worth noting that modified weighting of dual non-overlapping, hematopoietic targets does not greatly change the leukemic dose and risk assessment for homogeneous irradiation of whole marrow by external or internal sources, e.g., atomic-bomb gamma rays or Thorotrast.
The use of the marrow targets proposed here will require considerable changes to dosimetric methods presently employed by ICRP to ascertain radiation dose and risk. Firstly, new dosimetric methods are needed to assess the radiation dose to the peripheral marrow associated with both the quiescent bone surface and bone remodelling. Nie and Richardson (Citation2009) conducted a dynamic simulation that studies the temporal changes of short-range radionuclides incorporated in a trabecular bone remodelling compartment. This compartment consists of a well-oxygenated vascular microenvironment located within a canopy of bone-lining cells. Angiogenesis, and consequently the radiosensitivity of marrow, is enhanced in the vicinity of bone remodelling. The stem/progenitor cells participating in bone remodelling are in close proximity to radioactivity incorporated in new bone. Secondly, biokinetic models provide the temporal variation in radioactivity from an internal exposure. The models currently employed by the ICRP do not have compartments specifically assigned to marrow targets associated with bone-remodelling. A physiological skeletal biokinetic model has been developed that differentiates between quiescent and forming bone surfaces (Richardson, 2010). This modification is essential for meaningful risk assessment of marrow neoplasms arising from irradiating infants and children.
The development of cancer is a multistage process; animal studies have shown radiation to induce more initiation than promotion events (BEIR VII 2006). It is well known that radiation has an enhanced ability, compared to mitochondrial-generated ROS, to cause DNA double-strand breaks and presumably, cancer-initiating chromosome translocations. The evidence points to obstetric X-rays initiating childhood leukaemia in utero with no dose threshold (Bithell and Stewart Citation1975). But there again, radiation-induced inflammation and fibrosis appears to have a mainly promotional role in adult bone cancers. Pre-existing lesions play a similar role. Paget's disease of bone is present in around 2% of individuals of European ancestry and has an age-threshold of ∼45 years. The average skeletal dose threshold of about 10 Gy evident for the induction of bone tumours in radium dial painters (Evans et al. Citation1969) may be partly due to premature aging, advancing Paget's disease and the shortening of the cancer latent period in these young women of mainly European descent. However, even this strong case for a dose threshold for bone cancer incidence after ingestion of radium by dial painters is disputed by Leenhouts and Brugmans (Citation2000) who employ a two-mutation carcinogenesis model that supports a linear-quadratic dose-effect relationship with lowered risk at low dose.
Conclusion
Partitioning the ICRP dosimetry targets of trabecular whole bone marrow (into central marrow and endosteum) and peripheral marrow (into quiescent and forming bone surfaces) will improve the risk assessments for radiation-induced leukaemia and primary bone cancer, especially for children. A summary of the main points:
Rarity in Japanese of Paget's disease of bone, characterised by hypervascular marrow and focal increases in bone turnover, is a factor in the lack of excess bone cancers in adult atomic-bomb survivors.
Pre-existing lesions are not an important prerequisite for radiogenic bone cancer in children whereas growth is, therefore supporting the additional target of peripheral marrow adjacent to forming bone surfaces.
Quiescent, normal stem cells and cancer-initiating cells reside in a hypoxic niche located in the endosteum layer and have approximately a 2- to 3-fold greater degree of radioprotection compared to vascular niches.
HSC and MSC migrate to microvessels to repopulate the blood cells and remodel bone: Cancer stem cells similarly occupy or create vascular niches to produce malignant progeny and metastasise. Morphological and epidemiological evidence, as well as variable niche radiosensitivity, supports the need to partition the ICRP's whole marrow and peripheral marrow targets.
It is suggested that stem cells in central marrow migrate to the hypoxic endosteum after a radiation exposure as an adaptive response, thereby showing temporal variation in target location.
Radiation-induced inflammation, fibrosis and consequent progression in pathogenesis from a non-malignant to malignant form support a ‘radiation wound to malignancy’ model for bone cancer and perhaps leukaemia.
To assess the risk of bone cancer, especially in children, requires new biokinetic compartmental models to calculate the radiation dose associated with forming bone surfaces and growth plates.
There are indications that radiation at low doses can induce chromosome translocations and at higher doses accelerate aging by inflammation, shorten the cancer latent period, and advance fibrosis/MDS and stem cell senescence.
The aim of this paper is to propose radiation dose targets, based on current knowledge, for assessing the risk of inducing marrow neoplasms, especially for the third trimester fetus, infants and children. However, there are many uncertainties, such as the exact origin of cancer stem cells and radiation's contribution to the carcinogenic process, especially at low doses. Therefore, the proposed hematological targets have a speculative element until there is better clarification of radiation's role in the cancer stem cell process which may well differ for childhood and adult cancers. Consequently, like the ICRP targets, the targets proposed here will need future modification as biological and epidemiological knowledge advances.
Acknowledgements
I thank Dr Takesaburo Mori for helpful observations and his survey results of death from Paget's disease of bone in Japan. Drs David Allen (Ottawa Hospital, Canada), John Harrison (HPA, UK), Nick Priest (AECL, Canada) and Christian Streffer (University-Clinics, Essen, Germany) reviewed the paper at version stages and are thanked for providing salient comments.
Declaration of interest: The author reports no conflicts of interest. The author alone is responsible for the content and writing of this paper.
References
- Albagha OM, Visconti MR, Alonso N, Langston AL, Cundy T, Dargie R, Dunlop MG, Fraser WD, Hooper MJ, Isaia G, Nicholson GC, del Pino Montes J, Gonzalez-Sarmiento R, di Stefano M, Tenesa A, Walsh JP, Ralston SH. 2010. Genome-wide association study identifies variants at CSF1, OPTN and TNFRSF11A as genetic risk factors for Paget's disease of bone. Nature Genetics 42:520–524.
- Arai F, Suda T. 2007. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Annals of the New York Academy of Sciences 1106:41–53.
- Committee on the Biological Effects of Ionizing Radiation (BEIR IV). 1988. Health effects of radon and other internally deposited alpha-emitters. Committee on the Biological Effects of Ionizing Radiation, National Research Council. Washington, DC: National Academy Press;
- Committee on the Biological Effects of Ionizing Radiation (BEIR V). 1990. Health effects of exposure to low levels of ionizing radiation. Committee on the Biological Effects of Ionizing Radiation, National Research Council. Washington, DC: National Academy Press;
- Committee on the Biological Effects of Ionizing Radiation (BEIR VII). 2006. Health risks from exposure to low levels of ionizing radiation, Phase 2. Committee on the Biological Effects of Ionizing Radiation, National Research Council. Washington, DC: National Academy Press;
- Bianco P, Riminucci M, Gronthos S, Robey PG. 2001. Bone marrow stromal stem cells: Nature, biology, and potential applications. Stem Cells 19:180–192.
- Bithell JF, Stewart AM. 1975. Prenatal irradiation and childhood malignancy: A review of British data from the Oxford survey. British Journal of Cancer 31:271–307.
- Boice JD Jr, Blettner M, Kleinerman RA, Stovall M, Moloney WC, Engholm G, Austin DF, Bosch A, Cookfair DL, Krementz ET, Latourette HB, Peters LJ, Schulz MD, Lundell M, Pettersson F, Storm HH, Bell CMJ, Coleman MP, Fraser P, Palmer M. 1987. Radiation dose and leukemia risk in patients treated for cancer of the cervix. Journal of the National Cancer Institute 79:1295–1311.
- Bolch WE, Shah AP, Watchman CJ, Jokisch DW, Patton PW, Rajon DA, Zankl M, Petoussi-Henss N, Eckerman KF. 2007. Skeletal absorbed fractions for electrons in the adult male: Considerations of a revised 50-microm definition of the bone endosteum. Radiation Protection Dosimetry 127(1–4):169–173.
- Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, Lubin JH, Preston DL, Preston, RJ, Puskin JS, Ron E, Sachs RK, Samet JM, Setlow RB, Zaider M. 2003. Cancer risks attributable to low doses of ionizing radiation: Assessing what we really know. Proceedings of the National Academy of Sciences of the USA 100:13761–13766.
- Cardis E, Gilbert ES, Carpenter L, Howe G, Kato I, Armstrong BK, Beral V, Cowper G, Douglas A, Fix J, Fry SA, Kaldor J, Lavé C, Salmon L, Smith PG, Voelz GL, Wiggs LD. 1995. Effects of low doses and low dose rates of external ionizing radiation: Cancer mortality among nuclear industry workers in three countries. Radiation Research 142:117–132.
- Chang CM, Limanni A, Baker WH, Dobson ME, Kalinich JF, Jackson W, Patchen ML. 1995. Bone marrow and splenic granulocyte-macrophage colony-stimulating factor and transforming growth factor-beta mRNA levels in irradiated mice. Blood 86:2130–2136.
- Colmone A. Amorim M. Pontier AL, Wang S. Jablonski E. Sipkins DA. 2008. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science 322:1861–1865.
- Cooper C, Harvey NC, Dennison EM, van Staa TP. 2006. Update on the epidemiology of Paget's disease of bone. Journal of Bone and Mineral Research 21(Suppl. 2):3–8.
- Cristy M. 1981. Active bone marrow distribution as a function of age in humans. Physics in Medicine and Biology 26:389–400.
- Curtis RE, Boice JD Jr, Stovall M, Bernstein L, Holowaty E, Karjalainen S, Langmark F, Nasca PC, Schwartz AG, Schymura MJ, Storm HH, Toogood P, Weyer P, Moloney WC. 1994. Relationship of leukemia risk to radiation dose following cancer of the uterine corpus. Journal of the National Cancer Institute 86:1315–1324.
- Doll R, Wakeford R. 1997. Risk of childhood cancer from fetal irradiation. The British Journal of Radiology 70:130–139.
- Eckerman KF, Stabin MG. 2000. Electron absorbed fractions and dose conversion factors for marrow and bone by skeletal regions. Health Physics 78:199–214.
- Eliasson P, Jönsson J-I. 2010. The hematopoietic stem cell niche: Low in oxygen but a nice place to be. Journal of Cell Physiology 222:17–22.
- Evans RD, Finkel AJ, Hasterlik RJ, Keane AT, Kolenkow RJ, Neal WR, Shanahan MM. 1969. Radiogenic tumors in the radium and mesothorium cases studied at M.I.T. Delayed effects of boneseeking radionuclidesIn: Mays CW, et al. editors. Salt Lake City: University of Utah Press; pp 157–194.
- Ford AM, Palmi C, Bueno C, Hong D, Cardus P, Knight D, Cazzaniga G, Enver T, Greaves M. 2009. The TEL-AML1 leukemia fusion gene dysregulates the TGF-ß pathway in early B lineage progenitor cells. The Journal of Clinical Investigation 119:826–836.
- Ghadirian P, Fathie K, Emard JF. 2001. Epidemiology of bone cancer: An overview. Journal of Neurological and Orthopaedic Medicine and Surgery 21:8–16.
- Gibbs CP, Kukekov VG, Reith JD, Tchigrinova O, Suslov ON, Scott EW, Ghivizzani SC, Ignatova TN, Steindler DA. 2005. Stem-like cells in bone sarcomas: Implications for tumorigenesis. Neoplasia 7:967–976.
- Gnaiger E, Méndez G, Hand SC. 2000. High phosphorylation efficiency and depression of uncoupled respiration in mitochondria under hypoxia. Proceedings of the National Academy of Sciences of the USA 97:11080–11085.
- Gössner W. 1999. Pathology of radium-induced bone tumors: New aspects of histopathology and histogenesis. Radiation Research 152(Suppl.):12–15.
- Gössner W. 2003. Target cells in internal dosimetry. Radiation Protection Dosimetry 105:39–42.
- Grayson WL, Zhao F, Izadpanah R, Bunnell B, Ma T. 2006. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. Journal of Cellular Physiology 207:331–339.
- Greaves MF, Maia AT, Wiemels JL, Ford AM. 2003. Leukemia in twins: Lessons in natural history. Blood 102:2321–2333.
- Guo W, Xu W, Huvos AG, Healey JH, Feng C. 1999. Comparative frequency of bone sarcomas among different racial groups. Chinese Medical Journal (English) 112:1101–1104.
- Gurney JG, Swensen AR, Bulterys M. 1999. Malignant bone tumours. Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995In: Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR, editors. NIH Pub. No. 99–4649. Bethesda, MD: National Cancer Institute; pp 17–34.
- Harrison JS. 2002. Oxygen saturation in the bone marrow of healthy volunteers. Blood 99:394.
- Harrison JD, Muirhead CR. 2003. Quantitative comparisons of cancer induction in humans by internally deposited radionuclides and external radiation. International Journal of Radiation Biology 79:1–13.
- Hashimoto J, Ohno I, Nakatsuka K, Yoshimura N, Takata S, Zamma M, Yabe H, Abe S, Terada M, Yoh K, Fukunaga M, Cooper C, Morii H, Yoshikawa H. 2006. Prevalence and clinical features of Paget's disease of bone in Japan. Journal of Bone and Mineral Metabolism 24:186–190.
- Hayashi T, Kusunoki Y, Morishita Y, Nagamura H, Maki M, Kubo Y, Yamaoka M, Hayashi I, Yoshida K, Nakachi K. 2008. Acceleration of aging-associated increase in inflammatory markers and attenuation of the immune system among atomic-bomb survivors. Cytokine 43:255–256.
- Hope KJ, Jin L, Dick JE. 2004. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nature Immunology 5:738–743.
- Huvos AG. 1991. Bone tumors: Diagnosis, treatment and prognosis. Philadelphia: WB Saunders Co;
- International Commission on Radiological Protection (ICRP Publication 11). 1968. A review of the radiosensitivity of the tissues in bone. Oxford: Elsevier;
- International Commission on Radiological Protection (ICRP Publication 23). 1975. Reference man: Anatomical, physiological and metabolic characteristics. Oxford: Elsevier;
- International Commission on Radiological Protection (ICRP Publication 30). 1979/81. Limits for intakes of radionuclides by workers. Oxford: Elsevier;
- International Commission on Radiological Protection (ICRP Publication 56). 1989. Age-dependent doses to members of the public from intake of radionuclides. Oxford: Elsevier;
- International Commission on Radiological Protection (ICRP Publication 70). 1995. Basic anatomical and physiological data for use in radiological protection: The skeleton. Oxford: Elsevier;
- International Commission on Radiological Protection (ICRP Publication 88). 2001. Doses to the embryo and fetus from intakes of radionuclides by the mother ICRP. Oxford: Elsevier;
- International Commission on Radiological Protection (ICRP Publication 90). 2003. Biological effects after prenatal irradiation (embryo and fetus). Oxford: Elsevier;
- International Commission on Radiological Protection (ICRP Publication 103). 2007. Recommendations of the ICRP. Oxford: Elsevier;
- Iikuni N, Kwan Lam QL, Lu L, Matarese G, Cava ALA. 2008. Leptin and inflammation. Current Immunology Reviews 10:70–79.
- Ishikawa Y, Wada I, Fukumoto F. 2001. Alpha-particle carcinogenesis in Thorotrast patients: Epidemiology, dosimetry, pathology, and molecular analysis. Journal of Environmental Pathology, Toxicology and Oncology 20:311–315.
- Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, Nakamura R, Tanaka T, Tomiyama H, Saito N, Fukata M, Miyamoto T, Lyons B, Ohshima K, Uchida N, Taniguchi S, Ohara O, Akashi K, Harada M, Shultz LD. 2007. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nature Biotechnology 25:1315–1321.
- Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, Sawyers CL, Weissman IL. 2004. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. The New England Journal of Medicine 351:657–667.
- Jobling MF, Mott JD, Finnegan MT, Jurukovski V, Erickson AC, Walian PJ, Taylor SE, Ledbetter S, Lawrence CM, Rifkin DB, Barcellos-Hoff MH. 2006. Isoform-specific activation of latent transforming growth factor beta (LTGF-beta) by reactive oxygen species. Radiation Research 166:839–848.
- Kadhim MA, Moore SR, Goodwin EH. 2004. Interrelationships amongst radiation-induced genomic instability, bystander effects, and the adaptive response. Mutation Research 568:21–32.
- Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. 2007. Tumor growth need not be driven by rare cancer stem cells. Science 317:337.
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. 2005. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121:1109–1121.
- Kiel MJ, Radice GL, Morrison SJ. 2007. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell 1:127–129.
- Koschmieder S, Hofmann WK, Kunert J, Wagner S, Ballas K, Seipelt G, Hoelzer D, Ottmann OG, Kalina U. 2001. TGF beta-induced SMAD2 phosphorylation predicts inhibition of thymidine incorporation in CD34+ cells from healthy donors, but not from patients with AML after MDS. Leukemia 15:942–949.
- Kopp HG, Avecilla ST, Hooper AT, Rafii S. 2005. The bone marrow vascular niche: Home of HSC differentiation and mobilization. Physiology (Bethesda) 20:349–356.
- Kuramoto K, Ban S, Oda K, Tanaka H, Kimura A, Suzuki G. 2002. Chromosomal instability and radiosensitivity in myelodyspastic cells. Leukemia 16:2253–2258.
- Leenhouts HP, Brugmans MJP. 2000. An analysis of bone and head sinus cancers in radium dial painters using a two-mutation carcinogenesis model. Journal of Radiological Protection 20:169–188.
- Leggett RW. 1985. A model of the retention, translocation and excretion of systemic Pu. Health Physics 49:1115–1137.
- Little MP. 2001. Comparison of the risks of cancer incidence and mortality following radiation therapy for benign and malignant disease with the cancer risks observed in the Japanese Atomic bomb survivors. International Journal of Radiation Biology 77:431–464.
- Little MP, Weiss HA, Boice JD, Darby SC, Day NE, Muirhead CR. 1999. Risks of leukemia in Japanese atomic bomb survivors, in women treated for cervical cancer, and in patients treated for ankylosing spondylitis. Radiation Research 152:280–292.
- Lloyd EL, Henning CB. 1983. Cells at risk for the production of bone tumors in radium exposed individuals: An electron microscope study. Health Physics 44 (Suppl. 1):135–148.
- Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, Côté D, Rowe DW, Lin CP, Scadden DT. 2009. Live-animal tracking of individual hematopoietic stem/progenitor cells in their niche. Nature 457:92–96.
- Lord BI. 1990. The architecture of bone marrow cell populations. The International Journal of Cell Cloning 8:317–331.
- Lorimore SA, McIlrath JM, Coates PJ, Wright EG. 2005. Chromosomal instability in unirradiated hemopoietic cells resulting from a delayed in vivo bystander effect of gamma radiation. Cancer Research 65:5668–5673.
- Mahmud N, Devine SM, Weller KP, Parmar S, Sturgeon C, Nelson MC, Hewett T, Hoffman R. 2001. The relative quiescence of hematopoietic stem cells in nonhuman primates. Blood 97:3061–3068.
- Martin M, Lefaix J, Delanian S. 2000. TGF-β1 and radiation fibrosis: A master switch and a specific therapeutic target? International Journal of Radiation Oncology Biology Physics 47:277–290.
- Medinger M, Mross K. 2010. Clinical trials with anti-angiogenic agents in hematological malignancies. Journal of Angiogenesis Research. 2:10 (doi:10.1186/2040-2384-2-10).
- Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. 2004. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-γ2 transcription factor and TGF-β/BMP signaling pathways. Aging Cell 3:379–389.
- Moore KA, Lemischka IR. 2006. Stem cells and their niches. Science 311:1880–1885.
- Mori T, Machinami R, Hatakeyama S, Fuktomi K, Kato Y, Akahi M, Fukmoto M, Aoki I. 2005. The 2002 results of the first series of follow-up studies on Japanese Thorotrast patients and their relationships to autopsy series. Health effects of internally deposited radionuclides: Emphasis on radium, thorium, uranium and their daughter productsIn: Oeh U, Roth P, Paretzke HG, editors. Proc. HEIR 2004 Neuherberg: GSF – Forschungszentrum. pp 31–46.
- Murphey MD, Robbin MR, McRae GA, Flemming DJ, Temple HT, Kransdorf MJ. 1997. The many faces of osteosarcoma. Radiographics 17:1205–1231.
- Muschler GF, Nitto HJ, Boehm CA, Easley KA. 2001. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. Journal of Orthopaedic Research 19:117–125.
- Muschler GF, Midura RJ, Nakamoto C. 2003. Practical modeling concepts for connective tissue stem cell and progenitor compartment kinetics. Journal of Biomedicine and Biotechnology 2003:170–193.
- Nagasawa T. 2006. Microenvironmental niches in the bone marrow required for B-cell development. Nature Reviews Immunology 6:107–116.
- Nekolla EA, Walsh L, Schottenhammer G, Spiess H. 2005. Malignancies in patients treated with high doses of 224Ra. Health effects of internally deposited radionuclides: Emphasis on radium, thorium, uranium and their daughter productsIn: Oeh U, Roth P, Paretzke HG, editors. Proc. HEIR 2004 Neuherberg: GSF – Forschungszentrum; pp 67–74.
- Nie H, Richardson RB. 2009. Radiation dose to trabecular bone marrow stem cells from 3H, 14C, and selected α-emitters incorporated in bone remodelling compartment. Physics in Medicine and Biology 54:963–979.
- Nijnik A, Woodbine L, Marchetti C, Dawson S, Lambe T, Liu C, Rodrigues NP, Crockford TL, Cabuy E, Vindigni A, Enver T, Bell JI, Slijepcevic P, Goodnow CC, Jeggo PA. Cornall RJ. 2007. DNA repair is limiting for hematopoietic stem cells during ageing. Nature 447:686–690.
- Nishida S, Endo N, Yamagiwa H, Tanizawa T, Takahashi HE. 1999. Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. Journal of Bone and Mineral Metabolism 17:171–177.
- National Radiological Protection Board (NRPB). 2003. Risk of leukemia and related malignancies following radiation exposure: Estimated for the UK population Documents of the NRPB, 14,1 119S. Chilton, Didcot: NRPB;
- Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. 2006. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metabolism 3:187–197.
- Parkin DM, Stiller CA, Nectoux J. 1993. International variations in the incidence of childhood bone tumours. International Journal of Cancer 53:371–376.
- Parmar K, Mauch P, Vergilio JA, Sackstein R, Brown JD. 2007. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proceedings of the National Academy of Sciences of the USA 104:5431–5436.
- Passegué E, Jamieson CH, Ailles LE, Weissman IL. 2003. Normal and leukemic hematopoiesis: Are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proceedings of the National Academy of Sciences of the USA 100 (Suppl. 1):11842–11849.
- Pierce DA, Vaeth M, Cologne JB. 2008. Allowance for random dose estimation errors in atomic bomb survivor studies: A revision. Radiation Research 170:118–126.
- Pierce DA, Shimizu Y, Preston DL, Vaeth M, Mabuchi K. 1996. Studies of the mortality of atomic bomb survivors. Report 12, Part I. Cancer: 1950–1990. Radiation Research 146:1–27.
- Pool RR, Morgan JP, Parks NJ, Farnham JE, Littman MS. 1983. Comparative pathogenesis of radium-induced intracortical bone lesions in humans and beagles. Health Physics 44(Suppl. 1):155–177.
- Preston DL, Kusumi S, Tomonaga M, Izumi S, Ron E, Kuramoto A, Kamada N, Dohy H, Matsuo T, Nonaka H, Thompson DE, Soda M, Mabuchi K. 1994. Cancer incidence in atomic bomb survivors. Part III. Leukemia, lymphoma and multiple myeloma, 1950–1987. Radiation Research 137(Suppl. 2):S68–97; Erratum: Radiation Research 139:129.
- Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama K. 2007. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiation Research 168:1–64.
- Priest ND, Freemont A, Humphreys JA, Kathren RL. 1995. Histopathology and 241Am microdistribution in skeletal USTER Case 246. Health Physics 69:330–337.
- Reya T, Morrison SJ, Clarke MF, Weissman IL. 2001. Stem cells, cancer, and cancer stem cells. Nature 414:105–111.
- Richardson RB, Eatough JP, Henshaw DL. 1991. Dose to red bone marrow from natural radon and thoron exposure. The British Journal of Radiology 64:608–624.
- Richardson RB, Dubeau J. 2003. Monte Carlo determination of age-dependent steady-state dose to red bone marrow and bone from 14C exposure. Radiation Protection Dosimetry 103:5–18.
- Richardson RB, Nie HL, Chettle DR. 2007. Monte Carlo simulation of trabecular bone remodelling and absorbed dose coefficients for tritium and 14C. Radiation Protection Dosimetry 127:158–162.
- Richardson RB. 2008. Age-dependent radiation effects to normal and diseased coronary arteries – part 3: Oxygen effect and its implications on high- and low-LET dose. International Journal of Radiation Biology 84:858–865.
- Richardson RB. 2009a. Factors that elevate the internal radionuclide and chemical retention, dose and health risks to infants and children in a radiological-nuclear emergency. Radiation Protection Dosimetry 134:167–180.
- Richardson RB. 2009b. Ionizing radiation and aging: Rejuvenating an old idea. Aging 1:887–902.
- Richardson RB. 2010. A physiological skeletal model for radionuclide and stable element biokinetics in children and adults. Health Physics; 99: 471–482.
- Richardson DB, Wing S, Schroeder J, Schmitz-Feuerhake I, Hoffmann W. 2005. Ionizing radiation and chronic lymphocytic leukemia. Environmental Health Perspectives 113:1–5.
- Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Horner MJ, Howlader N, Eisner MP, Reichman M, Edwards BK. 2007. SEER Cancer Statistics Review, 1975–2004. Bethesda, MD: National Cancer Institute; Accessed 10 October 2007 from the website: http://seer.cancer.gov/csr/1975_2004/
- Rodríguez-Jiménez FJ, Moreno-Manzano V, Lucas-Dominguez R, Sánchez-Puelles JM. 2008. Hypoxia causes downregulation of mismatch repair system and genomic instability in stem cells. Stem Cells 26:2052–2062.
- Rowland RE, Stehney AF, Lucas HF. 1983. Dose-response relationships for radium-induced sarcomas. Health Physics 44(Suppl. 1):15–31.
- Schajowicz F, Santini Araujo E, Berenstein M. 1983. Sarcoma complicating Paget's disease of bone. A clinicopathological study of 62 cases. Journal of Bone and Joint Surgery – British Volume 65:299–307.
- Schofield R. 1978. The relationship between the spleen colony-forming cell and the hemopoietic stem cells. A hypothesis. Blood Cells 4:7–25.
- Shilnikova NS, Preston DL, Ron E, Gilbert ES, Vassilenko EK, Romanov SA, Kuznetsova IS, Sokolnikov ME, Okatenko PV, Kreslov VV, Koshurnikova NA. 2003. Cancer mortality among workers at the Mayak nuclear complex. Radiation Research 159:787–798.
- Shuryak I, Sachs RK, Hlatky L, Hlatky L, Little MP, Hahnfeldt P, Brenner DJ. 2006. Radiation-induced leukemia at doses relevant to radiation therapy: Modeling mechanisms and estimating risks. Journal of the National Cancer Institute 98:1794–1806.
- Sipkins DA, Wei X, Wu JW, Runnels JM, Côté D, Means TK, Luster AD, Scadden DT, Lin CP. 2005. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 435:969–973.
- Smith MA, Ries LAG, Gurney JG, Ross JA. 1999. Leukemia. Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995In: Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR, editors. NIH Pub. No. 99–4649 Bethesda, MD: National Cancer Institute; pp 17–34.
- Spiers FW, Beddoe AH. 1983. Sites of incidence of osteosarcoma in the long bones of man and the beagle. Health Physics 44(Suppl. 1):49–64.
- Spiers FW, Lucas HF, Rundo J, Anast GA. 1983. Leukemia incidence in the U.S. dial workers. Health Physics 44(Suppl. 1):65–72.
- Spiess H. 1969. 224Ra-induced tumors in children and adults. Delayed effects of bone-seeking radionuclidesIn: Mays CW, Jee WSS, Lloyd RD, Stover BJ, Dougherty JH, Taylor GN editors. Salt Lake City: University of Utah Press; pp 227–243.
- Streffer C. 2009. Radiological protection: Challenges and fascination of biological research. Strahlenschutzpraxis 2: (ISSN 0947–434 X).
- Sugiyama T, Kohara H, Noda M, Nagasawa T. 2006. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25:977–988.
- Thoday JM, Read J. 1949. Effect of oxygen on the frequency of chromosomal aberrantions produced by X-rays. Nature 160:608–609.
- Tsukasaki K, Iwanaga M, Tomonaga M. 2007. Late haematological effects in atomic bomb survivors. International Congress Series 1299:67–72.
- Tucker MA, D'Angio GJ, Boice JD Jr, Strong LC, Li FP, Stovall M, Stone BJ, Green DM, Lombardi F, Newton W, Hoover RN, Fraumeni JF. 1987. Bone sarcomas linked to radiotherapy and chemotherapy in children. The New England Journal of Medicine 317:588–593.
- Unni KK. 1996. Dahlin's bone tumours. General aspects and data on 11,087 cases. 5th ed. Philadelphia, New York: Lippincott-Raven;
- Vacanti C. 2004. Cells for building. The Scientist22–23.
- van Kaick G, Wesch H. 2005. The national Thorotrast studies in comparison. Health effects of internally deposited radionuclides: Emphasis on radium, thorium, uranium and their daughter productsIn: Oeh U, Roth P, Paretzke HG, editors. Proc. HEIR 2004 Neuherberg: GSF – Forschungszentrum; pp 17–20.
- Visfeldt J, Andersson M. 1995. Pathoanatomical aspects of malignant haematological disorders among Danish patients exposed to thorium dioxide. Acta Pathologica, Microbiologica et Immunologica Scandinavica 103:29–36.
- Weiss HA, Darby SC, Fearn T, Doll R. 1995. Leukemia mortality after X-ray treatment for ankylosing spondylitis. Radiation Research 142:1–11.
- Wick MR, Siegal GP, Unni KK, Mcleod RA, Greditzer HG. 1981. Sarcomas of bone complicating osteitis deformans (Paget's disease), 50 years experience. The American Journal of Surgical Pathology 5:47–59.
- Wick RR, Nekolla EA. 2005. Long term investigation of late effects in ankylosing spodylitis patients treated with 224Ra. Health effects of internally deposited radionuclides: Emphasis on radium, thorium, uranium and their daughter productsIn: Oeh U, Roth P, Paretzke HG, editors. Proc. HEIR 2004 Neuherberg: GSF – Forschungszentrum; pp 75–81.
- Xie Y, Yin T, Wiegraebe W, He XC, Miller D, Stark D, Perko K, Alexander R, Schwartz J, Grindley JC, Park J, Haug JS, Wunderlich JP, Li H, Zhang S, Johnson T, Feldman RA, Li L. 2009. Detection of functional hematopoietic stem cell niche using real-time imaging. Nature 457:97–101.