Abstract
Purpose: Quantitative analysis of cancer risk of ionising radiation as a function of dose-rate.
Materials and methods: Non-tumour dose, Dnt, defined as the highest dose of radiation at which no statistically significant tumour increase was observed above the control level, was analysed as a function of dose-rate of radiation.
Results: An inverse correlation was found between Dnt and dose-rate of the radiation. Dnt increased 20-fold with decreasing dose-rate from 1–10−8 Gy/min for whole body irradiation with low linear energy transfer (LET) radiation. Partial body radiation also showed a dose-rate dependence with a 5- to 10-fold larger Dnt as dose rate decreased. The dose-rate effect was also found for high LET radiation but at 10-fold lower Dnt levels.
Conclusions: The cancer risk of ionising radiation varies 1000-fold depending on the dose-rate of radiation and exposure conditions. This analysis explains the discrepancy of cancer risk between A-bomb survivors and radium dial painters.
Introduction
The dose-rate of ionising radiation that humans have been exposed to from natural to accidental radiation sources varies over a wide range from 10−9 to 107 Gy/min. Radiation dose-rate affects the magnitude of cancer risk even for the same total dose, and in addition changes the shape of the dose-response curve. For assessment of cancer risks of ionising radiation resulting from different exposure conditions, ideally, a set of dose response curves is needed for each dose-rate.
Currently, the estimation of human cancer risk from low doses of radiation is an important problem and data have been extensively reviewed (Committee on the Biological Effects of Ionizing Radiation [BEIR]/National Research Council [NRC], United Nations Scientific Committee on the Effects of Atomic Radiation [UNSCEAR] 1986, 2000, National Council on Radiological Protection and Measurements [NCRP] 1980, BEIR V 1990, BEIR VII 2005, National Radiological Protection Board [NRPB] 1995, Duport 2003). The dose and dose-rate effectiveness factor (DDREF) for cancer risk was determined as 2–10 depending on the target organ (NCRP Citation1980, International Commission on Radiological Protection [ICRP] 1991, UNSCEAR 1993, NRPB 1995). The application of the linear non-threshold (LNT) model, based on the apparently linear dose-response relation of cancer mortality obtained from extremely high dose-rate cases of A-bomb survivors, was recommended for the estimation of the cancer risk of low dose radiation for protection purposes (NCRP Citation2001, Brenner et al. Citation2003, BEIR VII Citation2005, ICRP Citation2006); however, the LNT model was questioned for its validity from experimental and epidemiological evidence (Kondo Citation1993, Académie des Sciences Citation1997, Tanooka Citation2001, Tubiana et al. Citation2006, Feinendegen et al. Citation2007). The history of the LNT model explains how the idea of a tolerance dose was changed to the linearity concept by incorporating the view of the geneticist (Calabrese Citation2009). However, a recent review of new biological and epidemiological data still adopted the LNT model (Mullenders et al. Citation2009). Whatever the model, there exists both linear and threshold type dose-response relations for radiation-induced cancers in experimental and epidemiological data. For example, the shape of the dose-response curve for cancer incidence may conform to a linear type for leukemia and solid cancers in A-bomb survivors (Chomentowski et al. Citation2000), while it is non-linear, or even threshold-like, for bone tumours in radium dial painters (Rowland et al. Citation1978) and liver tumours in thorotrast-injected patients (Anderson and Storm Citation1992). This discrepancy remained still to be explained.
In a previous study, non-tumour dose, Dnt, was defined as the highest dose at which no statistically significant tumour increase was observed above the control level. It was proposed as a measure of the upper limit of radiation dose for non-detectable cancer and Dnt values were surveyed for in the literature. The results showed that Dnt depended on exposure conditions, i.e., acute, protracted, and chronic exposures for whole body and partial body radiation for either low linear energy transfer (LET) or high LET radiation, respectively, with an inverse correlation between Dnt and dose-rate (Tanooka Citation2001). The present study aimed to show the dose-rate dependence of Dnt more quantitatively as a function of the dose-rate of radiation.
Data base
Dose-response data covering ionising radiation exposures from non-tumour to tumour-inducing doses were surveyed in the literature and are listed in . These include Dnt values, and corresponding dose-rates of radiation in mice, rats, dogs, and humans with different tumour types obtained under different exposure conditions. Data in the previous study (Tanooka Citation2001) and additional data were used for the present quantitative analysis. The data numbers in the previous study were unchanged for the convenience of comparison.
Table I. Dose-rate of radiation and non-tumour dose, Dnt.
Estimation of dose-rate
The values for the dose-rate were obtained from each published paper. For external radiation, the dose-rate was clearly presented in the literature either for whole body or partial body exposures. However, for internal radiation from radioactive nuclides, the estimation of dose-rate required assumptions and calculations depending on whether internal radioactive nuclides were distributed in the whole body or deposited partially in the target organ. Moreover, the radioactivity decayed with time and the radioactive nuclide was cleared from the body. In the present analysis, an average dose-rate was estimated from the total dose divided by the exposure time or, when a decay curve was available, an average dose-rate over the 70% decay time was taken. This calculation may have resulted in a lower estimate of dose-rate and a higher estimate of Dnt, provided that the radiation dose given only in the first half of the exposure time was effective for tumour induction. However, correction for this gave little change in the plot of Dnt versus dose-rate on a bi-logarithmic scale.
Results and discussion
Numerical values for Dnt and corresponding dose-rates obtained from various tumour systems are listed in . These values were divided into four groups, i.e., whole body irradiation with low LET and high LET radiation and partial body irradiation with low LET and high LET radiation, respectively. shows a plot of Dnt against dose-rate on a bi-logarithmic scale and regression lines fitted to the data for dose-rates below 1 Gy/min. A clear dose-rate dependence of Dnt is seen for the four exposure patterns.
Figure 1. Non-tumour dose, Dnt, plotted as a function of the dose-rate of radiation. (a) Whole body radiation. (b) Partial body radiation. Block symbols, low LET; open symbols, high LET. Mouse (, ○); rat (▴, ▵); dog (▪, □); human, whole-body low LET (H); and human, partial body high LET (h). Arrows indicate Dnt higher. Numbers affixed to each point are data numbers (see ).
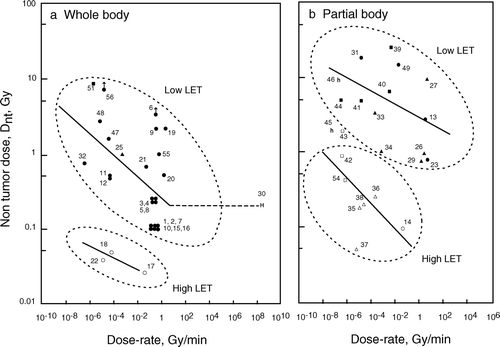
For whole body irradiation with low LET radiation, Dnt increased when lowering the dose-rate below 1 Gy/min and became 20-fold higher at 10−8 Gy/min (). Only one point for humans was available for the high dose-rate 107 Gy/min, based on the assumption that the A-bomb radiation was delivered in 1 μsec. It appeared that Dnt is constant for dose-rates between 1 and 107 Gy/min, as shown by the horizontal line in . For high LET irradiation of the whole body, there were few data available, but the dose-rate dependence of Dnt was seen at a level about 10- to 20-fold lower than for low LET radiation, although high LET radiation has been considered to have no dose-rate effect.
For partial body irradiation, the dose-rate dependence of Dnt was again seen for both low LET and high LET radiation (). Dose-response data for dose-rates higher than 10 Gy/min were not available in the literature. The Dnt level of partial body radiation was about 5- to 10-fold higher for low LET radiations and 3- to 5-fold higher for high LET radiations than those for whole body radiation.
At an extremely high dose-rate for whole body radiation, A-bomb survivor data (Shimizu et al. Citation1990) gave a Dnt of 0.2 Gy for leukemia mortality; while mouse data from nuclear detonation experiments at similar dose-rates showed a significant increase in pituitary and Harderian gland tumours at the same dose, 0.2 Gy (Furth et al. Citation1954). Consequently, humans seem to be more tolerant to radiation than mice and the regression lines drawn from animal data may under-estimate Dnt for humans. Dnt values, for partial body high-LET radiation to radium dial painters (Rowland et al. Citation1973, Citation1978) and thorotrast-injected patients (Anderson and Storm Citation1992), were much larger than those for experimental animals (), again indicating a higher radiation tolerance of humans. The other extreme case is the absence of thymic lymphoma induction in mice irradiated at 2 × 10−5 mGy/min with a total whole body dose of 7.2 Gy; whereas, acute radiation given in four fractions with the same total dose yielded a 90% tumour incidence (Ina et al. Citation2005), as was originally found in the early experiments of Kaplan and Brown (Citation1952).
Fractionation of radiation dose at a fixed dose-rate within a defined time interval lowers cancer incidence, as shown in the induction of skin tumours by local irradiation in rats (Burns et al. Citation1973, Citation1975, Citation1993). However, fractionation necessarily involves repetitive irradiations, which results in a tumour-enhancing effect as seen for mouse thymic lymphoma induction (Kaplan and Brown Citation1952) and also in mouse skin tumour induction (Ootsuyama and Tanooka Citation1991). It should be noted that the repetitive treatment is efficient for chemical induction of tumours. This contradictory effect should be considered in analysing the dose-rate effect.
summarises the regression lines for the four exposure patterns. These four lines are thought to cover all possible radiation exposure cases and hopefully to serve as a measure of cancer risk for any exposure situation in the human environment. Total whole body radiation doses received over 70 years from the natural environment high background radiation areas in Kerala, India (Nair et al. Citation1999) and Yanjiang, China (Chen and Wei Citation1991) are much smaller than Dnt for the respective dose-rates in each district (). The radiation dose to astronauts in space (Horneck et al. Citation2003) is also shown in , indicating a value close to Dnt even with a radiation shield. The cancer risk of medical examination with computer tomography (CT) has been analysed on the basis of whole-body data of A-bomb survivors (Berrington de Gonzalez and Darby Citation2004); however, this risk should have been analysed on the basis of partial body data. The highest possible dose for CT was still far lower than the corresponding Dnt. Recently, Tubiana et al. (in press) reported the dose response of second cancer incidence after radiation therapy with a Dnt of about 1 Gy based on a large number of patients. This study provides important data on human exposure to partial body low LET radiation.
Figure 2. Summary of regression lines for non-tumour dose, Dnt, versus dose-rate of radiation. Regression lines for dose-rate range from 10−8 to 1 Gy/min: whole body low LET, Y = 0.258 X−0.141, R2 = 0.320; whole body high LET, Y = 0.0207 X−0.0733, R2 = 0.781; partial body low LET, Y = 2.69 X−0.0857, R2 = 0.147; partial body high LET; Y = 0.0439 X−0.167, R2 = 0.303. Bars: radiation doses received by residents in natural (NB) and high background areas in Kerala, India, and Yanjiang, China, over 70 years. CT: possible highest dose to patients under CT examination. Space: possible highest dose in space using a 10 g/cm2 shield for six months. Dotted vertical lines indicate the difference between exposure dose and corresponding Dnt value.
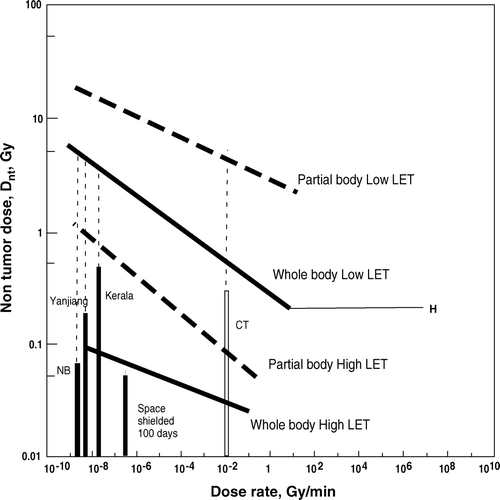
There are differences in the radiation sensitivity of tumour induction, depending on the type of tumour and host sensitivity. Dnt is much smaller in repair-deficient mice compared to wild-type mice (Ishii-Ohba et al. Citation2007), indicating that the regression lines represent the wild-type character of the hosts. Currently, a large scale life-time exposure of mice to external γ rays with graded dose-rates from 1–800 mGy per 22 h a day (dose-rate: 7.5 × 10−6 − 6 × 10−3 Gy/min, total dose for 3 years: 1.1 – 876 Gy) together with control mice is being conducted and chromosome aberration data have been reported (Tanaka et al. Citation2009). Such experiments will give more accurate data for the effect of dose-rate on tumour induction. Further data will be needed to cover the whole dose-rate range for tumour induction.
Summary
Meta-analysis of the non-tumour dose, Dnt, of ionising radiation showed a clear dependence on dose-rate over a wide range for four exposure conditions, i.e., whole body irradiation with low LET or high LET radiation and partial body irradiation with low LET or high LET radiation. From the regression lines for the relation between dose-rate and Dnt, a cancer risk or tolerance level of radiation could be estimated for a variety of exposure conditions. An apparent discrepancy in radiation-induced tumour data could be explained in terms of dose-rate.
Acknowledgements
I thank Dr Kouichi Tatsumi, Radiation Effects Association, Dr Toshihiko Sado, National Institute of Radiological Sciences, and Dr Tomotaka Sobue, National Cancer Center, for useful discussions and suggestions, Dr Takahiro Ochiya, National Cancer Center, for support of this work, Dr Gerda Horneck, Institute of Aerospace Medicine, German Aeropace Center, Germany, for information on the radiation dose in space, Dr Bruce B. Boecker, Loveless Respiratory Research Institute, USA, for information on internal emitters, and Dr Maurice Tubiana, University of Paris, France, for providing me with valuable data prior to publishing.
Declaration of interest: The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Académie des Sciences, France. 1997. Problems associated with the effects of low doses of ionizing radiations. Report 38. Amsterdam: Elsevier.
- Albert RE, Burns FJ, Bennet P. 1972. Radiation-induced hair-follicle damage and tumour formation in mouse and rat skin. Journal of National Cancer Institute 49:1131–1137.
- Anderson M, Storm HH. 1992. Cancer incidence among Danish thorotrast-exposed patients. Journal of National Cancer Institute 84:1318–1325.
- Bartsra RW, Bentvelzen PAJ, Zoetelief J, Mulder AH, Broerse JJ, van Bekkum DW. 2000. The effects of fractionated gamma irradiation on induction of mammary carcinoma in normal and estrogen-treated rats. Radiation Research 153:557–569.
- Berrington de Gonzalez A, Darby S. 2004. Risk of cancer from diagnostic X-rays: Estimates for the UK and 14 other countries. The Lancet 363:345–351.
- Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, Lubin JH, Preston DL, Preston RJ, Puskin JS, Ron E, Sachs RK, Samet JM, Setlow RB, Zaider M. 2003. Cancer risks attributable to low doses of ionizing radiation: Assessing what we really know. Proceedings of National Academy of Sciences of the USA 100:13761–13766.
- Burns FJ, Albert RE, Sinclair IP, Bennett P. 1973. The effect of fractionation on tumour induction and hair follicle damage in rat skin. Radiation Research 53:235–240.
- Burns FJ, Albert RE, Sinclair IP, Vanderlaan M. 1975. The effect of a 24-hour fractionation interval on the induction of rat skin tumours by electron radiation. Radiation Research 62:478–487.
- Burns FJ, Jin Y, Koenig KL, Hosselet S. 1993. The low carcinogenicity of electron radiation relative to argon ions in rat skin. Radiation Research 135:178–188.
- Burns FJ, Strickland P, Vanderlaan M, Albert RE. 1978. Rat skin tumour incidence following single and fractionated exposures to proton radiation. Radiation Research 74:152–158.
- Calabrese EK. 2009. The road to linearity: Why linearity at low doses became the basis for carcinogen risk assessment. Archives of Toxicology 83:203–225.
- Chen D, Wei LX. 1991. Chromosome aberration, cancer mortality and hormetic phenomena among inhabitants in areas of high background radiation in China. Journal of Radiation Research 32(Suppl. 2):46–53.
- Chomentowski M, Kelleler AM, Pierce D. 2000. Radiation dose dependence in the atomic bomb survivor cancer mortality data: A model-free visualization. Radiation Research 153:289–294.
- Committee on the Biological Effects of Ionizing Radiation (BEIR V). 1990. National Research Council, USA. 1990. Health effects of exposure to low levels of ionizing radiation. Washington, DC: National Academy Press.
- Committee on the Biological Effects of Ionizing Radiation (BEIR VII). 2005. National Research Council, USA. 2005. Health risk from exposure to low levels of ionizing radiation. Washington, DC: National Academy Press.
- Covelli V, Coppola M, Di Majo V, Rebessi S, Bassani B. 1988. Tumour induction and life shortening in BC3F1 female mice at low doses of fast neutrons and X rays. Radiation Research 113:362–374.
- Di Majo V, Coppola M, Rebessi S, Bassani B, Alati T, Saran A, Bangrazi C, Covelli V. 1986. Radiation-induced mouse liver neoplasms and hepatocyte survival. Journal of National Cancer Institute 77:933–939.
- Dudoignon N, Guezinar-Liebard F, Guilet K, L'Hullier I, Monchaux G, Fritsch P. 1999. Lung carcinogenesis in rats after inhalation exposure to 237NpO2. Radiation Research 152:S31–33.
- Duport P. 2003. A data base of cancer induction by low dose radiation in mammals: Overview and initial observations. International Journal of Low Radiation 1:120–131.
- Feinendegen LE, Pollycove M, Neuman RD. 2007. Whole body responses to low-level radiation exposure. New concepts in mammalian radiobiology. Experimental Hematology 35:37–46.
- Finkel MD, Biskis BO, Scriber GM. 1959. The influence of strontium-90 upon life span and neoplasms of mice. In: Progress of nuclear energy, series VIvol. 2. London: Pergamon; pp 199–209.
- Furth J, Upton AC, Christenberry KW, Benedict WH, Moshman J. 1954. Some late effects in mice of ionizing radiation from an experimental nuclear detonation. Radiology 4:562–570.
- Hahn FF, Muggenburg BA, Guilmette RA, Boecker BB. 1999. Comparative stochastic effects of inhaled alpha- and beta-particle-emitting radionuclides in beagle dogs. Radiation Research 152:S19–22.
- Horneck G, Facius R, Reichert M, Rettberg P. 2003. HUMEX: A study on the survivability and adaptation of humans to long-duration exploratory missions. Noodwijk, The Netherlands: ESA Publications Division.
- Hulse EV, Lewkowicz SJ, Batchelor AL, Papworth DG. 1983. Incidence of radiation-induced skin tumours in mice and variations with dose-rate. International Journal of Radiation Biology 57:797–808.
- Hulse EV, Mole RH. 1969. Skin tumour incidence in CBA mice given fractionated exposures to low energy beta particles. British Journal of Cancer 23:452–463.
- Ina Y, Tanooka H, Yamada T, Sakai K. 2005. Suppression of thymic lymphoma induction by life-long low dose-rate irradiation accompanied by immune activation in C57Bl/6 mice. Radiation Research 163:153–158.
- International Commission on Radiological Protection (ICRP). 1991. Publication 60: 1990. Recommendations of the international commission on radiological protection. Oxford: Pergamon.
- International Commission on Radiological Protection (ICRP). 2006. Publication 99: Low-dose extrapolation of radiation related cancer risk. Oxford: Elsevier.
- Ishii-Ohba H, Kobayashi S, Nishimura M, Shimada Y, Tsuji H, Sado T, Ogiu T. 2007. Existence of a threshold-like dose for γ-ray induction of thymic lymphomas and no susceptibility to radiation-induced solid tumours in SCID mice. Mutation Research 619:124–133.
- Kaplan HS, Brown MB. 1952. A quantitative dose-response study of lymphoid-tumour development in irradiated C57 black mice. Journal of National Cancer Institute 13:185–208.
- Kondo S. 1993. Health effects of low-level radiation. Osaka: Kinki University Press and Madison: Medical Physics Publishing.
- Lee W, Chiacchierini RP, Shleien B, Telles NC. 1982. Thyroid tumours following 131I or localized X irradiation to the thyroid and pituitary glands in rats. Radiation Research 92:307–319.
- Maisin JR, Wambersie GB, Gerber GB, Mattelin G, Lambiet-Collier M, Gueulette J. 1983. The effects of a fractionated gamma irradiation on life shortening and disease incidence in BALB/c mice. Radiation Research 94:359–373.
- Mays CW, Finkel MP. 1980. RBE of α-particles vs. β-particles in bone sarcoma induction. In: Radiation protection: A systematic approach to safety. Proc. 5th Congress of International Radiation Protection AssociationOxford: Pergamon Press; pp 661–665.
- Morlier JP, Morin M, Monchaux G, Fritsch P, Pineau JF, Chameaud J, Lafuma J, Masse R. 1994. Lung cancer incidence after exposure of rats to low doses of radon: Influence of dose-rate. Radiation Protection and Dosimetry 56:93–97.
- Mullenders L, Atkinson M, Paretzke H, Sabatier L, Bouffler S. 2009. Assessing cancer risks of low-dose radiation. Nature Reviews Cancer 9:596–604.
- Nair MK, Nambi KSV, Amma NS, Gangadharan P, Jayalekshmi P, Jayadevan S, Cherian V, Reghuram KN. 1999. Population study in the high natural background radiation area in Kerala, India. Radiation Research 152:S145–148.
- National Council on Radiological Protection and Measurements (NCRP). 1980. Report 64: Influence of dose and its distribution in time on dose-response relationships for low LET radiations. Bethesda, MD: National Council on Radiological Protection and Measurements.
- National Council on Radiological Protection and Measurements (NCRP). 2001. Report 136: Evaluation of the linear non-threshold dose-response model for ionizing radiation. Bethesda, MD: National Council on Radiological Protection and Measurements.
- National Radiological Protection Board (NRPB). UK. 1995. Risk of radiation-induced cancer at low doses and low dose rates for radiation protection purposes. Report. Vol. 6, No. 1.
- Ootsuyama A, Tanooka H. 1991. Threshold-like dose of local β-irradiation repeated throughout the life span of mice for induction of skin and bone tumours. Radiation Research 125:98–101.
- Ootsuyama A, Tanooka H. 1993. Zero tumour incidence in mice after repeated lifetime exposures to 0.5 Gy of beta radiation. Radiation Research 134:244–246.
- Raabe OG. 1984. Comparison of the carcinogenicity of radium and bone-seeking actinides. Health Physics 46:1241–1258.
- Rowland RE, Keane AT, Lucas HF. 1973. A preliminary comparison of the carcinogeniceity of 226Ra and 228Ra in man. Radionuclide carcinogenesisIn: Sanders CL, Busch RH, Ballou JE, Mahlum DD, editors. Springfield: US Department of Commerce; pp 406–420.
- Rowland RE, Stehney AF, Lucas HF. 1978. Dose-response relationships for female radium dial workers. Radiation Research 76:368–383.
- Sanders CL, Dagle GE, Cannon WC, Powers GJ, Meier DM. 1977. Inhalation carcinogenesis of high-fired 238PuO2 in rats. Radiation Research 71:528–546.
- Sanders CL, Mahaffey JA. 1978. Inhalation carcinogenesis of high-fired 244CmO2 in rats. Radiation Research 76:384–401.
- Shimizu Y, Kato H, Schull WJ. 1990. Studies of the mortality of A-bomb survivors. 9. Mortality, 1950–1985: Part 2: Cancer mortality based on the recently revised doses (DS86). Radiation Research 121:120–141.
- Tanaka K, Kohda A, Satoh K, Toyokawa T, Ichinoe K, Ohtaki M, Oghiso Y. 2009. Dose-rate effectiveness for unstable-type chromosome aberrations detected in mice after continuous irradiation with low-dose-rate γ rays. Radiation Research 171:290–301.
- Tanooka H. 2001. Threshold dose-response in radiation carcinogenesis: An approach from chronic β-irradiation experiments and a review of non-tumour doses. International Journal of Radiation Biology 77:541–551.
- Thompson RC. 1989. Life-span effects of ionizing radiation in the beagle dog. A summary account of four decades of research funded by the US Department of Energy and its predecessor agencies. Pacific Northwest Laboratory Report PNL-6822 UC-408. pp 1–323.
- Tubiana M, Aurengo A, Averbeck D, Masse R. 2006. Recent reports on the effect of low doses of ionizing radiation and its dose-effect relationship. Radiation and Environmental Biophysics 44:245–251.
- Tubiana M, Diallo I, Chavaudra J, Lefkopoulos D, Bourhis J, Girinsky T, Brider A, Hawkins M, Haddy N, El-Fayech C, Adjadj E, Clero E, de Vathaire F. 2010. A new method of assessing the dose-carcinogenic effect relationship in patients exposed to ionizing radiation. A concise presentation of preliminary data. Health Physics, in press.
- Ullrich RL. 1983. Tumour induction in BALB/c mice after fission neutron or γ-irradiation. Radiation Research 93:506–515.
- Ullrich RL. 1984. Tumour induction in BALB/c mice after fractionated or protracted exposures to fission-spectrum neutrons. Radiation Research 93:587–597.
- Ullrich RL, Jernigan MC, Adams LM. 1979. Induction of tumours in RFM mice after localized exposure to X-rays or neutrons. Radiation Research 80:464–473.
- Ullrich RL, Jernigan MC, Cosgrove GE, Satterfield LC, Bowles ND, Storer JB. 1976. The influence of dose and dose-rate on the incidence of neoplastic disease in RFM mice after neutron irradiation. Radiation Research 68:115–131.
- Ullrich RL, Storer JB. 1979a. Influence of γ-irradiation on the development of neoplastic disease in mice. I. Reticular tissue tumours. Radiation Research 80:303–316.
- Ullrich RL, Storer JB. 1979b. Influence of γ-irradiation on the development of neoplastic disease in mice. II. Solid tumours. Radiation Research 80:317–324.
- Ullrich RL, Storer JB. 1979c. Influence of γ-irradiation on the development of neoplastic disease in mice. III. Dose-rate effects. Radiation Research 80:325–342.
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNCEAR). 1986. Dose-response relationships for radiation-induced cancer. In: Sources and effects of ionizing radiation: Genetic and somatic effects of ionizing radiation. Report to the General AssemblyNew York: United Nations.
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNCEAR). 1993. Influence of dose and dose-rate on stochastic effects of radiation. In: Sources and effects of ionizing radiation. Report to the General Assembly, Annex FNew York: United Nations.
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNCEAR). 2000. Biological effects at low radiation doses: Models, mechanisms and uncertainties. In: Sources and effects of ionizing radiation: genetic and somatic effects of ionizing radiation. Report to the General Assembly, Annex INew York: United Nations.
- Upton AC, Randolph ML, Conklin JW. 1970. Late effects of fast neutrons and gamma-rays in mice as influenced by the dose-rate of irradiation: Induction of neoplasia. Radiation Research 41:467–491.
- White RG, Raabe OG, Culbertson MR, Parks NJ, Samuels SJ, Rosenblatt LS. 1993. Bone sarcoma characteristics and distribution in beagles fed strontium-90. Radiation Research 136:178–189.
- White RG, Raabe OG, Culbertson MR, Parks NJ, Samuels SJ, Rosenblatt LS. 1994. Bone sarcoma characteristics and distribution in beagles injected with radium-226. Radiation Research 137:361–370.
- Yamamoto O, Seyama T, Itoh H, Fujimoto N. 1998. Oral administration of tritiated water (HTO) in mouse. III. Low dose-rate irradiation and threshold dose-rate for radiation risk. International Journal of Radiation Biology 73:535–541.
