Abstract
Purpose: To translate and perform a cross-cultural adaptation of the Late-Life Function and Disability Instrument (LLFDI) to Swedish, to investigate absolute and relative reliability, concurrent validity, and floor and ceiling effects within a Swedish-speaking sample of community-dwelling older adults with self-reported balance deficits and fear of falling. Method: Translation, reliability and validation study of the LLFDI. Sixty-two community-dwelling, healthy older adults (54 women and 8 men) aged 68–88 years with balance deficits and fear of falling performed the LLFDI twice with an interval of 2 weeks. Results: Test–retest agreement, intra-class correlation coefficient was very good, 0.87–0.91 in the LLFDI function component and 0.82–0.91 in the LLFDI disability component. The standard error of measure was small, 5–9%, and the smallest real difference was 14–24%. Internal consistency (Cronbach’s alpha) was high (0.90–0.96). Correlation with the SF-36 PCS and PF-10 was moderate in both LLFDI function, r = 0.39–0.68 and r = 0.35–0.52, and LLFDI disability, r = 0.40–0.63 and 0.34–0.57, respectively. There was no floor or ceiling effects. Conclusion: The Swedish version of the LLFDI is a highly reliable and valid instrument for assessing function and disability in community-dwelling older women with self-reported balance deficits and fear of falling.
The Swedish LLFDI is a highly reliable and valid instrument for assessing function and disability in older women with self-reported balance deficits and fear of falling.
The instrument may be used both in clinical settings and in research.
The instrument is sensitive to change and a reasonably small improvement is enough to detect changes in a group or a single individual.
Implications for Rehabilitation
Introduction
Older adults are the most rapidly growing part of the population worldwide. With advancing age, there is an increased vulnerability to various chronic conditions, functional limitations, disability, and comorbidity, often resulting in compromised physical, social, and psychological well-being as well as reduced quality of life.
Assessing function and disability in late life is crucial for estimating the impact of aging and diseases on activities in daily life, establishing care and rehabilitation services, as well as measure the effects of different interventions. Consequently, physical functioning and disability assessment have become standards in the evaluation of older persons in geriatric research. Self-report measures are found to be the most valid and cost-effective methods of obtaining function and disability information in older adults [Citation1].
The Late-Life Function and Disability Instrument (LLFDI), launched in 2002, is a self-report questionnaire designed to achieve a comprehensive assessment of physical function and disability in community-dwelling older adults [Citation2–6]. The questionnaire consists of two components, a 32-item function component assessing a person’s ability to do discrete actions or activities, and a 16-item disability component assessing a person’s performance in socially defined life tasks. The disability component assesses two dimensions, frequency and limitations, responded to successively, allowing older persons to respond differently to questions of what they actually do in daily life versus what they are capable of doing [Citation7].
The LLFDI is composed of a comprehensive battery of items drawn from a variety of existing batteries. The instrument is designed to assess and respond to changes over time [Citation2,Citation8]. The LLFDI has been validated against established self-report instruments and clinical tests and is found to be a more complete and precise instrument than the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) and the London Handicap Scale [Citation5]. It has been shown to be a substitute for physical performance tests when self-report is a preferred data collection method [Citation6]. The LLFDI has been translated and validated successfully into several languages [Citation9,Citation10], and its utility has been demonstrated in research, clinical practice, and public health [Citation11–13].
The aim of this study was to translate and perform a cross-cultural adaptation of the LLFDI to Swedish, and to investigate absolute and relative reliability, concurrent validity, floor and ceiling effects within a Swedish-speaking sample of community-dwelling older adults with self-reported balance deficits and fear of falling.
Method
The late-life function and disability instrument
The function component of LLFDI evaluates self-reported difficulties in performing 32 physical activities comprised of three dimensions: (1) upper extremity, (2) basic lower extremity, and (3) advanced lower extremity. Questions are phrased, “How much difficulty do you have doing a particular activity without the help of someone else and without the use of assistive devices?” Response options are on a five-graded scale from “none” to “cannot do” [Citation2]. The disability component evaluates self-reported limitations in and frequency of performing 16 tasks in daily life. Limitation questions are phrased, “To what extent do you feel limited in doing a particular task?” with five response options from “not at all” to “completely”. Frequency questions are phrased. “How often do you do a particular activity?” with response options from “very often” to “never” [Citation4]. Each subscale of the function component and the disability component are re-calculated to a 0 to 100 scale, with higher scores indicating better ability [Citation2,Citation4].
Translation and cross-cultural adaptation
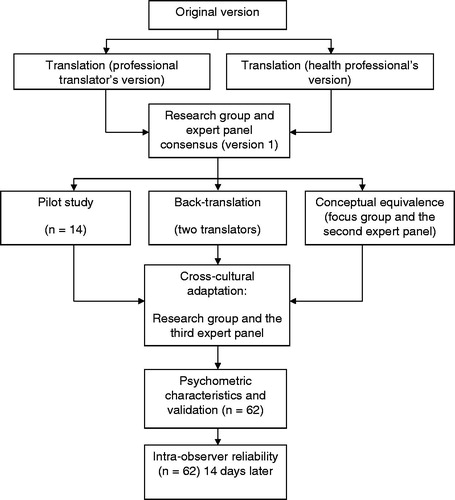
The LLFDI was translated and back-translated according to guidelines for cross-cultural adaptation of self-report instruments [Citation14], see . Initially, a professional translator and a panel of three bilingual health professionals familiar with the terminology, consisting of a physiotherapist (PT), a physician, and an occupational therapist, separately translated the LLFDI from English to Swedish. The translations emphasized conceptual and cultural rather than literal translations. There was a high interdisciplinary agreement between the three professional versions. Thereafter, an expert panel comprising four PT’s with clinical and/or research expertise in the area of aging and elderly care, met to compare the versions. A summary of recommendations was made and inadequate expressions and concepts of the translation were identified and resolved before consensus on the first version was reached. To reach conceptual equivalence, a group discussion consisting of three experienced PT’s and four elderly persons representing the target group, lead by an experienced interviewer, were used to gather comments on the first version. The elderly representatives were two men and two women without cognitive difficulties. The discussion was taped and later transcribed. Simultaneously, the first version of the LLFDI was tested in a pilot study with a convenience sample of 14 geriatric patients. In parallel to the pretesting, two independent translators performed the Swedish–English back-translation of the first version. As in the initial translation, emphasis in the back-translation was on conceptual and cultural equivalence and not linguistic equivalence. Thereafter, the back-translation, the group discussions and the pilot test all revealed difficulty in interpreting nine specific different questions: F4 Run one half-mile, F10 Reach overhead while standing, F16 Remove wrapping with hands only, F18 Get into and out of car, F23 Make bed, D3 Provide assistance to others, D5 Work at a volunteer job, D6 Participate in active recreation, and D10 Take part in an exercise program. The questions were adapted to the habits of Swedish older adults and reworded to facilitate interpretation. Finally, different interpretations, modifications, and discrepancies were discussed in an expert panel of 10 health professionals, to reach cross-cultural adaptation and a satisfactory final version. All the involved PT’s had profound knowledge in Swedish language and culture, had long clinical and/or research experience in older adults and were familiar with the instrument.
Reliability, validity and practicability
To assess absolute and relative reliability and concurrent validity, the final Swedish version of the LLFDI was administered by postal survey to a convenience sample of 62 elderly people (aged 68–88 years, mean 76 years; 54 women and 8 men) volunteering to participate in an on-going study (BETA-study; NCT01417598, ClinicalTrials.gov) on balance training at the Karolinska Institutet, Stockholm, Sweden. They were familiar with the instrument, and had a Mini Mental State Examination of at least 24 points [Citation15,Citation16]. However, the participants did not receive any intervention while participating in this study. Co-morbidities in the group were osteoporosis, heart disease, peripheral vascular disease, chronic obstructive pulmonary disease, diabetes, cancer, visual deficits, hearing deficits, and musculoskeletal problems. See for demographic characteristics. The study protocol complied with the Helsinki rules on human research and was approved by the Regional Ethics Review Board in Stockholm, Sweden (Dnr: 2006/151-31 and Dnr: 2009/819-32). All participants gave their written informed consent before participation.
Table 1. Characteristics of the study sample (n = 62).
To determine the test–retest reliability, the participants answered the LLFDI one more time, also by a postal survey, 2 weeks after the first survey. To control for bias during the period between the two postal surveys the participants answered a complementary question about whether anything had happen, that could influence the outcome of the study since they had answered the first survey.
To validate the LLFDI, the SF-36 Physical Component Score (SF-36 PCS) and the 10-item Physical Functioning scale (PF-10) was assessed at the same time. Practicability was evaluated by observing the characteristics of missing data in the questionnaires.
The SF-36 Physical Component Score (SF-36 PCS) is one of the two main subscales of SF-36 and reflects health in areas like physical functioning, role – physical, bodily pain, general health, vitality, mental health, role – emotional, and social functioning [Citation17]. SF-36 PCS has been used in previous studies as a comparison to LLFDI [Citation18]. The scale has good internal consistency and test–retest reliability [Citation17,Citation19].
The 10-item Physical Functioning scale (PF-10) used in the SF-36 Health Survey [Citation15] is an index designed to sample three main attributes of physical functioning: (1) self-care, (2) mobility, and (3) other physical and body movements, such as lifting and bending. The PF-10 has been used in previous studies as a comparison to LLFDI function component [Citation2,Citation4–6,Citation18] and has shown good reliability and validity with older adults [Citation20,Citation21].
Missing items in the LLFDI were replaced with the mean value if the number of values missed by the respondent was less than 4 in the 32-items sum score (overall function score), less than 3 in the 16-items sum scores (disability frequency and disability limitations), and less than 2 in the 7-to-11-items sum scores (upper and lower extremity function).
Statistical analysis
All statistical analysis was performed using PASW Statistics, version 20.0 (SPSS Inc., Chicago, IL). To establish the relative reliability, the ICC2.1 was used. A one-way repeated measure analysis of variance (ANOVA) was used to calculate the ICC values. Strength of agreement for ICC values was classified according to Bland and Altman [Citation22], that is, <0.20 poor, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 good, 0.81–1.00 very good. In addition to relative reliability, absolute reliability was analyzed both by the standard error of measure (SEM) and by smallest real difference (SRD). SEM denotes the smallest change that indicates a real difference for a group of subjects, while SRD represents the smallest change that indicates a real improvement for a single subject, and the variance of SEM, SEM%, and SRD, SRD%, was calculated [Citation22,Citation23]. Internal consistency was measured using Cronbach’s alpha, which was interpreted as follows for analysis on group level: excellent = >0.9, good = >0.8, acceptable = >0.7, questionable = >0.6, poor = >0.5, unacceptable = <0.5 [Citation24]. For the analysis of convergent validity, Spearman’s rank correlation coefficient (rs) was calculated. Consistent with the criteria reported by McHorney et al. [Citation25] 0.70–1.0 was considered high, 0.30–0.69 moderate, and less than 0.30 low. For the analysis of ceiling or floor effects, the percentage of individuals’ scores at maximum and minimum of each LLFDI subscale was calculated. A conservative estimate is that scales that score greater than 15% of the sample at the floor and ceiling may suggest difficulty discriminating between subjects [Citation26].
Results
The results of the reliability analyses are shown in . The test–retest reliability proved to be very good, represented by ICC2.1 values of 0.87–0.91 in the function component and 0.82–0.91 in the disability component. Absolute reliability showed that the measurement error on group level (SEM) was 2.9–5.1 (5–9%) for the function component and 2.6–4.1 (5–6%) for the disability component. The measurement error on individual level (SRD) was 8.0–14.1 (14–24%) for the function component and 7.2–11.4 (14–16%) for the disability component. The internal consistency measured by Cronbach’s alpha was excellent in both LLFDI function (0.94–0.96) and LLFDI disability components (0.90–0.95).
Table 2. Mean value for test–retest, mean difference between test and retest, 95% confidence interval (95% CI), intra-class correlation coefficient (ICC2.1), standard error of measure (SEM), coefficient of variance (SEM%), smallest real difference (SRD), smallest real difference % (SRD%), internal consistency (Cronbach’s alpha), Spearman’s correlations between Late-Life Function and Disability Instrument (LLFDI) and Physical Component Score (SF-36 PCS) and LLFDI and Physical Function scale (PF-10).
There were moderate correlations between the LLFDI and SF-36 PCS and PF-10 in both the function component, rs = 0.39–0.68 and rs = 0.35–0.52, respectively, and the disability component, rs = 0.40–0.63 and rs = 0.34–0.57, respectively. There was no floor or ceiling effect of the LLFDI, as no participants obtained the 15% highest or lowest scores.
All participants answered all items on the LLFDI function component, while five persons missed answering the LLFDI disability limitations subscale and two persons missed answering the last page of the LLFDI disability component.
Discussion
This study showed that the test–retest reliability of the Swedish version of the LLFDI was very good in healthy older adults with balance deficits and fear of falling, for both the function and the disability components, indicating that the questionnaire is stable over time. Internal consistency by Cronbach’s alpha was also high in both components. Moreover, the low SEM and SRD scores revealed that a reasonably small improvement is enough to detect changes in both a group of individuals and a single individual. Results also showed that the concurrent validity between the LLFDI and physical aspects of health-related quality of life was moderate. No floor or ceiling effects were evident.
Our ICC values (0.87–0.91) are comparable to those found for the English version (0.91–0.98) of the LLFDI function component and slightly higher (0.82–0.91) than those found for the LLFDI disability (0.68–0.82) component by Jette et al. [Citation2] and Haley et al. [Citation3] in community-dwelling older adults. The absolute reliability presented with SEM and SRD provides a conceptual framework to assist when interpreting the clinical relevance of the study results at group and individual levels. To our knowledge, this is the first study to report absolute reliability for the LLFDI. The low SEM and SRD scores revealed that a reasonably small improvement is enough to detect changes in a group (∼3 points) or a single individual (∼8 points), showing that the instrument is sensitive to change. In the function component the SEM and SRD scores were higher in the three dimensions compared to the overall score.
The high internal consistency for the LLFDI function component is comparable to that found by Hand et al. [Citation18] in middle-aged community-dwelling adults with chronic health conditions and by Abizanda et al. [Citation27] in Spanish residents aged 70 years and older, as well as slightly higher than in a sample of older community-dwelling women aged 59–84 years [Citation7]. The internal consistency of the LLFDI disability component is comparable to that found by Hand et al. [Citation18] and slightly higher than that found by McAuley et al. [Citation7]
In accordance with Hand et al. [Citation18], we found moderate correlations between the LLFDI disability component and the SF-36 PCS. Our values were lower than Dubuc et al. [Citation5], who found that the LLFDI function component (overall score and lower extremity scales) and PF-10 were highly correlated. One explanation for this discrepancy may be that our population was older and a majority of women. Moreover, in accordance with Dubuc et al. [Citation5] we found no floor or ceiling effect in the LLFDI, as no participants obtained the 15% highest or lowest scores.
There are some limitations in this study. First, the study population, recruited from an on-going study regarding balance training in community-dwelling elderly individuals, were predominantly women and might, therefore, not be representative of all community-dwelling elderly people. Second, the use of postal survey for data collection limited the possibilities for giving feedback and instructions or to ensure satisfactory use of the available LLFDI visual aid. Thus, further refinement of the layout of the LLFDI may improve responsiveness and practicability for self assessment. Third, a shorter period of time between the first and second postal survey than 2 weeks had been preferable since the population studied was elderly and their condition might change over the 2 weeks. On the other hand, Marx et al. [Citation28] showed that there were no significant differences in the ICC values concerning health status instruments when the time interval was 2 days or 2 weeks.
It is an advantage that absolute reliability for the LLFDI is addressed in the present study, providing a conceptual framework to assist when interpreting a clinical relevant difference at group and individual levels.
Conclusion
The Swedish version of the LLFDI is a highly reliable and valid instrument for assessing function and disability in older adults with self-reported balance deficits and fear of falling. The results apply primarily for elderly community-dwelling women.
Declaration of interest
The authors report no declarations of interest. This work was supported by grants through the regional agreement on medical and clinical research (ALF) between Stockholm County Council and Karolinska Institutet; the Swedish Research Council; the Torsten and Ragnar Söderberg Foundation; Johanitterorden; and Health Care Science Postgraduate School at Karolinska Institutet.
Acknowledgements
The authors would like to thank all the participants in the study. A special thanks to Elisabeth Olsson at Karolinska Institutet for conceptualization of the study and participation in the expert panel, to Alan Jette at Boston University for kind permission to translate the questionnaire, to Lars Oddsson at Sister Kenny Research Center for participating in the translation process, to Anna Petterson and Elin Farén and expert panels at Karolinska University Hospital for participation in the cross-cultural adaptation, and to Lisbet Boman at Karolinska Institutet for help and guidance in data collection and statistical calculations.
References
- Crawford SL, Jette AM, Tennstedt SL. Test–retest reliability of self-reported disability measures in older adults. J Amer Geriat Soc 1997;45:338–41
- Jette AM, Haley SM, Ni P, Olarsch S, Moed R. Creating a computer adaptive test version of the Late-Life Function & Disability Instrument. J Gerontol A Biol Sci Med Sci 2008;63:1246–56
- Haley SM, Lidlow LH, Kooyoomjian JT. Extending the range of functional assessment in older adults: development of the Late-Life Function and Disability Instrument. J Aging Phys Act 2002;10:453–65
- Haley SM, Jette AM, Coster WJ, et al. Late Life Function and Disability Instrument: II. Development and evaluation of the function component. J Gerontol A Biol Sci Med Sci 2002;57:M217–22
- Dubuc N, Haley SM, Ni PS, et al. Function and disability in late life: Comparison of the Late-Life Function and Disability Instrument to the Short-Form-36 and the London Disability Scale. Disabil Rehabil 2004;26:362–70
- Sayers S, Jette A, Haley S, et al. Validation of the Late-Life Function and Disability Instrument (LLFDI). J Amer Geriat Soc 2004;52:1–6
- Late–Life FDITM [internet]. Boston: Boston University School of Public Health. Available from http://sph.bu.edu/HDRI/llfdi/menu-id-617771.html [last accessed 15 February 2012]
- McAuley E, Konopack JF, Motl RW, et al. Measuring disability and function in older women: psychometric properties of the Late-Life Function and Disability Instrument. J Gerontol 2005;60:901–9
- Shin KR, Byeon YS, Kang Y, Oak J. A study on physical symptom, activity of daily living, and health-related quality of life (HRQoL) in the community dwelling older adults. J Korean Acad Nurs 2008;38:437–44
- Melzer I, Kurz I, Sarid O, Jette AM. Relationship between self-reported function and disability and balance performance measures in the elderly. J Rehabil Res Dev 2007;44:685–92
- Gibson K, Day L, Hill KD, et al. Screening for pre-clinical disability in different residential settings. BMC Geriatr 2010;10:52
- Lapier TK, Mizner R. Outcome measures in cardiopulmonary physical therapy: Focus on the late life function and disability instrument (LLFDI). Cardiopulm Phys Ther J 2009;20:32–5
- LeBrasseur NK, Sayers SP, Ouellette MM, Fielding RA. Muscle impairments and behavioral factors mediate functional limitations and disability following stroke. Phys Ther 2006;86:1342–50
- Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol 1993;46:1417–32
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98
- Folstein MF, Luria R. Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychol Med 1973;3:479–86
- Ware JE Jr., Kosinski M, Bayliss MS, et al. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care 1995;33:AS264–79
- Hand C, Richardson J, Letts L, Stratford P. Construct validity of the late life function and disability instrument for adults with chronic conditions. Disabil Rehabil 2010;32:50–6
- Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. Br Med J 1992;305:160–4
- McHorney CA, Ware JE Jr, Rogers W, et al. The validity and relative precision of MOS short- and long-form health status scales and Dartmouth COOP charts. Results from the Medical Outcomes Study. Med Care 1992;30:MS253–65
- Stansfeld SA, Roberts R, Foot SP. Assessing the validity of the SF-36 general health survey. Qual Life Res 1997;6:217–24
- Bland JM, Altman DC. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat 2007;17:571–82
- Bland JM, Altman DG. A note on the use of the intraclass correlation coefficient in the evaluation of agreement between two methods of measurement. Comput Biol Med 1990;20:337–40
- George D, Mallery P. SPSS for Windows step by step: a simple guide and reference. 11.0 update, 4th ed. Boston (MA): Allyn & Bacon; 2003
- McHorney CA, Ware JE Jr., Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36). II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993;31:247–63
- McHorney CA, Tarlov AR. Individual-patient monitoring in clinical practice: are available health status surveys adequate? Qual Life Res 1995;4:293–307
- Abizanda P, Lopez-Jiménez M, López-Torres J, et al. Validation of the Spanish version of the Short-Form Late-Life Function and Disability Instrument. J Am Geriatr Soc 2011;59:893–9
- Marx RG, Menezes A, Horovitz L, et al. A comparison of two time intervals for test–retest reliability of health status instruments. J Clin Epidemiol 2003;56:730–5