Abstract
To execute the membrane fusion function, it is necessary for the fusion protein of the virus to penetrate into the hydrophobic milieu of membrane bilayer. Hence identification of the region(s) of the ectodomain of viral fusion proteins involved in the membrane insertion and their interaction with the rest of the fusion protein in the membrane would be important for the mechanistic study of membrane fusion. To this end, we examined membrane activity of the fusion peptide, and the ectodomain protein with or without the fusion peptide domain of HIV-1 gp41 by several biophysical measurements. The results revealed that the ectodomain protein containing the fusion peptide domain had higher membrane-perturbing activity and deeper membrane insertion, while the construct lacking the fusion peptide domain had much lower membrane activity. Strikingly, the N-terminal heptad repeat region was found to be induced deeper into the membrane by the fusion peptide, consistent with the role of the latter in the membrane penetration. We concluded that the fusion peptide is the only stretch of gp41 ectodomain that embeds deeply in the membrane interior in the prefusion stage. The function of fusion peptide in terms of membrane interaction and the implications of its interplay with other domains of gp41 on the membrane fusion cascade were discussed.
| Abbreviations: | ||
| FP, | = | Fusion peptide |
| DMPC, | = | 1,2-Dimyristoyl-sn-glycero-3-phosphocholine |
| DMPG, | = | 1,2-Dimyristoyl sn-glycero-3-phosphoglycerol |
| DOPC, | = | 1,2-Dioleoyl-sn-glycero-3-phosphocholine |
| SPR, | = | Surface plasmon resonance |
| bis-ANS, | = | 4,4′Dianilino-1,1′-binaphthyl- 5,5′-disulfonic acid, dipotassium salt |
| NBD, | = | 4-Chloro-7-nitrobenz-2-oxa-1,3-diazole |
| FRET, | = | Fluorescence resonance energy transfer |
| DPA, | = | Dipicolinic acid |
Introduction
Fusion between enveloped viruses and host cells is mediated by the viral fusion proteins (Cao et al. Citation1993, Hernandez et al. Citation1996, White et al. Citation2008). For HIV-1 virus, the fusion protein gp160 is cleaved into two non-covalently linked subunits, gp120 (the surface SU subunit) and gp41 (the transmembrane TM subunit), thereby generating the N-terminal fusion peptide (FP) of gp41. Sequestered by gp120 in the metastable state, the gp41 TM is composed of heptad repeat domains (abbreviated as NHR and CHR), connecting loop, membrane spanning domain and cytoplasmic tail, in addition to the FP domain. In the sequential fusion model (Maddon et al. Citation1986), fusion is initiated by specific binding of gp120 to CD4 cell surface molecules followed by interaction with co-receptors (Rizzuto et al. Citation1998, Sullivan et al. Citation1998). Conformational changes in the fusion protein of the virus (Sattentau and Moore Citation1991) result in dissociation of gp120 as well as exposure and relocation of the fusion peptide which projects toward the target cell. The membrane activity of FP was considered crucial as it engages the virus to the target cell for the subsequent processes and the mutation within FP results in a complete loss of the fusion function (Freed et al. Citation1990). The conformational alterations in gp41 ectodomain also entail the prehairpin form characterized by exposure of the coiled coil of NHR, which can be probed by the CHR-derived inhibitors such as T-20 (Furuta et al. Citation1998, Bewley et al. Citation2002).
Previous studies have implicated NHR in the membrane interaction of gp41 while suggested FP as a membrane-anchoring segment replaceable by the fatty acid (Lev and Shai Citation2007, Moreno et al. Citation2007). Importantly, the peptides pertaining to the NHR region were shown to bind and interact, in particular, with the negatively charged phospholipids. Similar result was documented by Yu et al. (Citation1994) that, upon initial FP attachment to the target membrane, the NHR triplet coiled coil was sprayed apart on the membrane surface and inserted into the target membrane to promote fusion.
The above concept was disputed by Brunner and coworkers who employed the hydrophobic photolabeling technique to show that membrane-core insertion of the hemagglutinin fusion protein is mediated solely by FP (Harter et al. Citation1989, Durrer et al. Citation1996). Similarly, previous investigations pointed to the highly hydrophobic N-terminal segment generated by proteolysis as the membrane-inserting domain of the fusion protein (Brasseur et al. Citation1988). The FP domain also displayed the highest hemolytic activity among the peptide molecules representing the ectodomain of gp41 (Mobley et al. Citation2001). Moreover, membrane insertion of the peptide fragment derived from FP domain has been demonstrated in numerous investigations (Stegmann et al. Citation1991, Martin et al. Citation1994, Pereira et al. Citation1997, Chang et al. Citation1997, Bradshaw et al. Citation2000), and mutations of the residues within this region abolished fusion completely (Freed et al. Citation1990, 1992, Schaal et al. Citation1995). In a recent biophysical study, FP has been proposed to reduce the bending energy thereby promoting membrane curvature (Tristram-Nagle and Nagle Citation2007). It is noteworthy that, in many reports, short FP fragments were used which may not precisely reflect some FP functions in the context of full length gp41 protein.
Since membrane fusion involves disruption of the membrane architecture, it is possible that both FP and NHR segments perturb the membrane structure, i.e., membrane active. A recent report Korazim et al. (Citation2006) that a 70-residue gp41 N-terminal segment containing FP and NHR had 100-fold higher lipid mixing activity than FP fragment, attesting a synergism between the two segments of gp41. Since FP was shown to insert deeply into the membrane interior due to its high hydrophobicity, it is possible that the amphipathic NHR – downstream of FP – has an enhanced membrane affinity and embeds in the interface of the bilayer, thereby increasing the potency of N-70. To further elucidate the role of FP and NHR in the virus-mediated membrane fusion, we conducted fluorescence, infrared and surface plasmon resonance (SPR) experiments to probe the state of membrane insertion and perturbation for the two regions of gp41 by comparing the results obtained with the recombinant ectodomain proteins in the presence and absence of FP. To reinforce the results, the 23-mer peptide corresponding to gp41 FP was employed. Moreover, the intact gp41 ectodomain including FP and the loop region between the NHR and CHR was synthesized as an improvement to the one used in the previous study devoid of the loop region (Caffrey et al. Citation1999) to avert protein aggregation.
From these measurements on the full-length gp41 ectodomain we found that the FP, but not NHR, domain penetrated into the model membrane and destabilized the membrane to greater extent. Yet NHR was drawn deeper into the membrane by the N-terminal FP from fluorescence quenching experiments on the fusion proteins labeled in the NHR region. Our data underscored the notion that membrane insertion of gp41 is mediated by FP while NHR associates with the membrane peripherally. Notably, NHR was found to be drawn deeper into the membrane interior by FP, providing a rationale of functional synergism of the two gp41 N-terminal regions (Korazim et al. Citation2006). The hypothesis of different membrane interaction of the fusion protein, particularly NHR, during the prehairpin and postfusion phases of the fusion event affords a new insight into the mechanism of the virus-mediated fusion.
Materials and methods
Preparation of protein and peptide
The sequences of the peptides used in this work were shown in . Synthesis of the gp41 ectodomain with (gp41e-FP) and without FP (gp41e) followed the protocol described previously (Lin et al. Citation2008). Expression, purification and characterization of gp41e-FP are presented in Supplementary Figures S1–5 (online version only). The FP fragment, FP-23, was synthesized and purified as previously described (Chang et al. Citation1997). Fluorescent labeling with NBD (4-chloro-7-nitrobenz-2-oxa-1,3-diazole) or Rhodamine on residue 546 of S546C mutant of the proteins and on the N-terminus of FP-23 was conducted according to the method documented previously (Rapaport and Shai Citation1991, Kantchev et al. Citation2004).
Figure 1. The sequence of the ectodomain of HIV-1 gp41 a.a. 512-665, gp41e-FP, with underlined Trps. The black bar pointed out the FP region (FP-23, a.a. 512-534), *marked the Trp mutated residue on FP-23 (designated as FP-23-W2) and the arrows indicated the S598C/S604C double mutated positions. Gp41 ectodomain devoid of FP, including a.a. 535–665, was designated as gp41e.

Preparation of small unilamellar vesicles (SUV)
The SUV liposomes were prepared with DMPC, molar ratio 1:1 of DMPC:DMPG* (PC:PG) or 1:1:1 of DOPC/cholesterol/sphingomyelin (raft-liposome). The lipid mixture was solubilized in a 4:1 (v/v) chloroform: methanol solution. Aliquots of the liposomes were evaporated by a stream of nitrogen gas. The residual solvent was removed under vacuum for at least 1 h. The raft-liposome mixture was added with 2% CHS (cholesterylhemisuccinate/tris salt/10% DOM (n-dodecyl-s-D-maltopyranoside) / 10% Chaps and then dissolved in 1 ml solubilization buffer (Hepes buffer: 50 mM Hepes, 5 mM MgCl2, 1mM CaCl2 and 150 mM NaCl, pH 7.5). PC:PG liposomes were dissolved in PBS buffer pH 7, while DMPC was dissolved in H2O. The liposome suspensions were frozen, thawed and vortexed, followed by sonication and purification with ultracentrifugation at 14,000 rpm for 5 min (raft-liposome) or 20,000 rpm for 30 min (PC:PG). The raft-liposomes were diluted 10-fold in the solubilization buffer prior to use (Navratilova et al. Citation2006).
L1-SPR measurements
To evaluate the binding affinity of the proteins and peptide to the lipid bilayer, SPR experiments were performed on a BIAcore 3000 biosensor system using L1 biosensor chip (BIAcore AB, Uppsala, Sweden). The surface of an L1 sensor chip was cleaned with 40 mM octyl-D-glucoside and 50 mM NaCl followed by 30% ethanol. Raft-liposomes (0.5 mM lipid concentration) or DMPC (1 mM) were injected at a flow rate of 2 μl/min (200 μl) into the chamber. For raft binding experiments, each of the analytes, FP-23, gp41e-FP and gp41e, dissolved in distilled water, was injected at a flow rate of 10 μl/min and captured on the SUV coated surface. At the end of a binding measurement, the surface was cleaned by injection of 40 mM octyl-D-glucoside and 50 mM NaCl at a flow rate of 10 μl/min for 10 min. SPR data were analyzed with BIAevaluation 3.0 software (Biacore AB, Uppsala, Sweden). The kinetic data were fit by the software using a conformation change fitting model. The quality of fit was estimated by calculating χ2 values and inspecting residuals.
The DMPC-L1 system was employed for SPR shedding experiments. Rhodamine-labeled gp41 proteins were dissolved in 50 mM sodium formate (pH ∼ 3) which was also used as the running buffer. Collection of the eluent from the dissociation stage was controlled by the in-house automated recovery program and detected by fluorescence spectrophotometer using an extra-micro quartz cell with light path of 3 × 3 mm. Fluorescence spectra were recorded in the range of 550–650 nm with excitation wavelength at 530 nm and a cutoff filter at 540 nm.
Fluorescence spectrophotometry
All fluorescence experiments were performed on a Hitachi F-2500 Fluorescence Spectrophotometer at 37°C unless indicated otherwise. A scan rate of 300 nm/min with response of 0.4 s was used in the wavelength scan measurements.
Hydrophobic surface probed by bis-ANS (4,4′-dianilino-1,1′-binaphthyl- 5,5′-disulfonic acid, dipotassium salt)
Bis-ANS may bind to the hydrophobic patches of proteins to exhibit a significant enhancement of fluorescence. At ambient temperature, a hemi-micro cuvette with a magnet stirrer was used to measure the intensity changes as the peptide was added to bis-ANS aqueous solutions. This fluorescent probe was monitored by excitation and emission wavelengths of 390 nm and 490 nm, respectively, using a cut-off filter at 430 nm. Both slit bandwidths of excitation and emission were set at 10 nm. Wavelength scans in the range of 400–700 nm were performed after the intensity attained a steady value. The final concentrations of bis-ANS and peptides were 300 nM and 200 nM, respectively.
NBD fluorophore quenched by Co2+
NBD fluorescence can be quenched by divalent cobalt ions (Morris et al. Citation1985) via a collisional quenching mechanism. A large quenching constant by the aqueous cation signifies a closer proximity of the NBD tag to the membrane interface. In these experiments, the NBD fluorophore was labeled on residue 546 of S546C mutant of gp41e or gp41e-FP and the N-terminal of FP-23 (designated as NBD-S546C, NBD-S546C-FP and NBD-FP-23, respectively) according to the procedure of Rapaport and Shai (Citation1991) with modifications as described previously (Kantchev et al. Citation2004). For Co2+ quenching experiments, an aliquot of each of the NBD-labeled peptides (final concentration 0.3 μM) was incubated in 100 μM raft-liposome suspension in an ultra-micro cuvette until the signal reached a steady value. An incremental aliquot of CoCl2 stock solution (0.1 M) was then injected into the cuvette to give final Co2+ concentrations in the range of 0.5–2.5 mM. The observed fluorescence intensities (Fobs) were corrected for dilution (F'obs) and then adjusted for inner filter effects (Fcorr) using the equation (Lakowicz Citation1983):
where ODEX and ODEM are the optical densities of CoCl2 at the excitation wavelength (476 nm) and the emission wavelength (around 530 nm), respectively. The slit bandwidths of excitation and emission were set at 10 nm. A filter cut-off at 480 nm was used. The data were analyzed using the Stern-Volmer equation:
where F0 is the fluorescence intensity at the zero quencher concentration, F is the fluorescence intensity at any given quencher concentration [Q], whereas KSV represents the apparent Stern-Volmer quenching constant, obtained from the slope of the plot of F0/F versus [Q].
Acrylamide quenching experiments
The fluorescence quenching study monitors the accessibility of Trp to the acrylamide quencher. Similar to Co2+ quenching of NBD, a larger quenching constant of Trp by the aqueous phase quencher acrylamide indicates that the Trp is located closer to the membrane interface. 0.5 μM of gp41e-FP or gp41e was incubated in raft-liposomes of 100 μM or 5-fold diluted Hepes buffer (10 mM Hepes, 1 mM MgCl2, 0.2 mM CaCl2 and 30 mM NaCl, pH 7.5) for 2 h. Since there are 7 Trps in gp41e-FP or gp41e and no Trp in the FP region, the V513W variant of FP-23 (FP-23-W2) was synthesized to investigate the acrylamide quenching effect on FP and 2.5 μM of FP-23-W2 was used in 500 μM raft-liposome or the same diluted Hepes buffer. Fluorescence emission spectra in the 300-450 nm range were recorded by using an ultra-micro cuvette and a 280 nm excitation wavelength with a cut-off filter at 300 nm. The slit bandwidths of excitation and emission were set at 5 and 2.5 nm, respectively. An incremental amount of acrylamide stock solution (1 M) was then added to the peptide lipidic or buffered solution. Appropriate blanks were subtracted to obtain the corrected spectra and corrections for dilution were made to the observed fluorescence intensities. The data were analyzed using Stern-Volmer equation (cf. the last paragraph).
Lipid mixing assay by fluorescence resonance energy transfer (FRET)
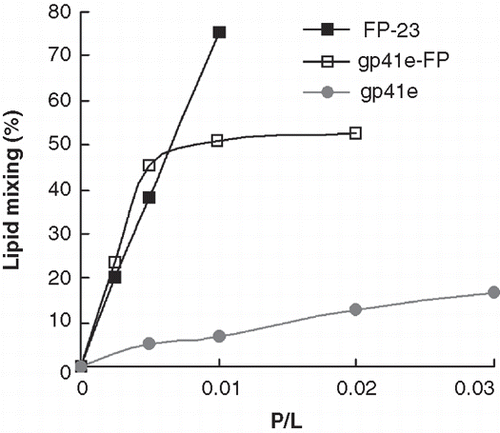
Membrane fusion assay is based on the measurement of FRET from NBD to rhodamine (Struck et al. Citation1981). Specifically, two lipid suspensions were prepared, one unlabeled (raft-liposomes 100 μM) and one labeled (raft-liposome: NBD-PE:Rho-PE 100:0.6:0.6 μM). A 9:1 molar ratio of unlabeled to labeled liposomes was mixed in the assay; hence the final molar ratio of raft-liposome to NBD-PE is 1666 after the lipid mixing. Varying aliquots of ∼ 50 μM peptide stock solution were injected into the liposome mixture to observe FRET effect. As the peptide is added to induce lipid mixing, the fluorescent probe is diluted by mixing of the unlabeled and labeled liposomes, resulting in reduced energy transfer efficiency and an increase in the fluorescence intensity of the energy donor, NBD-PE. To monitor the NBD probe, the excitation and the emission wavelengths were set at 467 and 530 nm, respectively. The fluorescence intensity after the addition of Triton X-100 (0.2% v/v) was referred to as 100%.
Tb3+/DPA (dipicolinic acid) leakage experiments
The method is based on the enhancement of the lanthanide metal ion Tb3+ fluorescence liganded to the aromatic chelator DPA. To encapsulate Tb3+ in the large unilamellar vesicles (LUVs) of raft-liposome, the dried lipid film of DOPC/cholesterol/sphingomyelin (prepared as described previously) was resuspended in the Hepes buffer mixed with 5 mM sodium citrate/0.5 mM terbium chloride. The lipid suspension was frozen and thawed 10 times and extruded 20 times through a polycarbonate membrane with a pore size of 0.1 μm. To remove the terbium citrate from the external liposome wall, it was dialyzed at 4°C against three 1-L changes of 50 mM Tris-HCl/150 mM NaCl, pH 7.5, containing EDTA (0.5 mM) for a total of 40 h and then exhaustively dialysed against 50 mM Tris-HCl/150 mM NaCl, pH 7.5, of three 1-L changes for 72 h in order to remove EDTA from the outside wall (Bacigalupo et al. Citation2003).
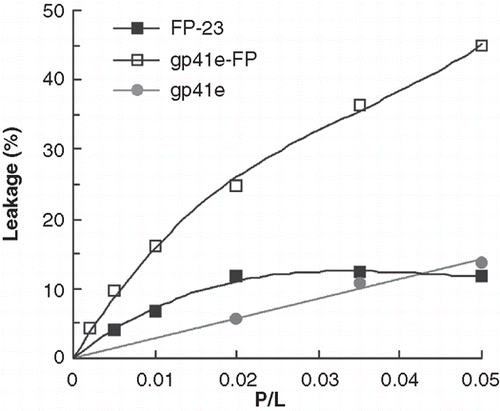
To quantitate the extent of leakage observed in the Tb3+/DPA assay, peptides of 0.2 ∼ 5 μM were added to a solution containing 100 μM raft-liposome/Tb3+, 50 μM DPA, 150 mM NaCl, 50 mM Tris at pH 7.5. The samples were shaken for 2 h at ambient temperature; the fluorescence was recorded with excitation and emission wavelengths of 270 and 545 nm, respectively, and 10 nm bandwidth for both excitation and emission. The percentage leakage of Tb3+ was calculated as follows:
where Fmax was defined as the fluorescence emitted after addition of TbCl3 (10 μM) and DPA (50 μM) to the suspension of lipids without EDTA and F0 was the fluorescence intensity of Tb3+ after addition of 0.2% Triton X-100 containing 1 mM EDTA (Bryl et al. Citation2000).
ATR-FTIR spectroscopy measurements
FP-23 dissolved in a small quantity of hexafluoro-2-propanol (HFIP) or gp41e-FP and gp41e dissolved in H2O was incubated in PC:PG SUV to make the final peptide-to-lipid molar ratio (L/P) 1:50. The sample was subsequently spread on a germanium surface to allow the solvent to evaporate. The ATR sample covered with a homemade box was kept in full D2O hydration with D2O/lipid ratio > 35 based on infrared absorbance ratio of D-O/C-H stretch peaks.
ATR-FTIR spectra were acquired on a BIO-RAD FTS-60A spectrometer equipped with a KBr beamsplitter and a liquid nitrogen-cooled MCT detector at 22°C. The incoming radiation was polarized with a germanium single diamond polarizer (Harrick, Ossining, NY). The 45° germanium ATR-plate (2 × 5 × 50 mm) was cleaned by a plasma cleaner (Harrick, Ossining, NY, USA) before depositing the sample. After 300 scans at a spectral resolution of 2 cm-1, the data were smoothed with triangular apodization and the absorption spectra were analyzed by Peakfit program to calculate the secondary structure components (Tamm and Tatulian Citation1997). The Peakfit program was also used for deconvolution of the carbonyl band at ∼ 1735 cm-1 into non-hydrogen bonded (dehydrated) and hydrogen bonded (hydrated) peaks.
Calculations of the tilt angles of lipid acyl chain (δ) and the insertion angles of α-helix (θ) or β-strands (Φ) peptides into membranes relative to the membrane director were described previously (Chang et al. Citation2008). θ and Φ were obtained by analyzing the amide-I α-helix and β-strand peaks at 1655 and 1628 cm-1, respectively; however, δ was related to the symmetric CH2 stretching vibration at 2850 cm-1. These angles were used to investigate the orientation and perturbation effect of peptide in lipidic medium.
Results
Fluorescence emission intensity experiments indicate FP is a major hydrophobic region of gp41 ectodomain
Synthesis and characterization of the recombinant ectodomain protein of gp41, gp41e, were reported in a previous communication (Lin et al. Citation2008). It oligomerizes into trimer and predominantly helical in aqueous solution.
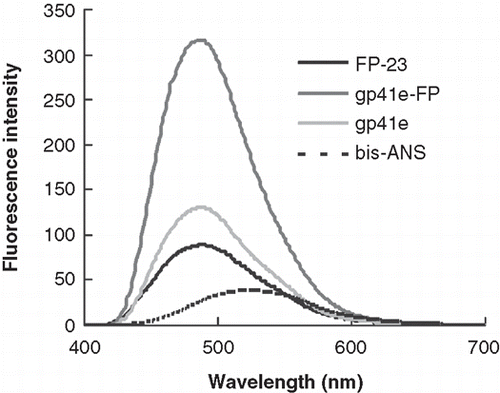
To probe the non-polar surface of the protein molecule, the polarity-sensitive bis-ANS fluorescence was utilized. In , the emission enhancement of gp41e-FP is highest among the three peptides tested, whilst FP-23 and gp41e caused a smaller increase in intensity. As the intensity of the probe mixed with gp41e is about 40% of that with gp41e-FP, the result indicates that the contribution of FP region to the hydrophobic surface of gp41 ectodomain is comparable to, if not more than, the remainder of the ectodomain. This is remarkable considering the size of FP-23 is much smaller than gp41e, in keeping with the notion of high hydrophobicity for the FP domain.
Figure 2. Bis-ANS emission spectra upon addition of gp41e-FP, gp41e or FP-23. Background fluorescence due to the medium has been subtracted. The intensities enhanced by the addition of the peptides were caused by exposure of the hydrophobic binding sites of the peptides and gp41e-FP has the strongest effect. This indicated the prominent contribution of FP region to the hydrophobic surface of gp41 ectodomain.
SPR experiments show membrane association region of gp41 ectodomain resides in the FP domain
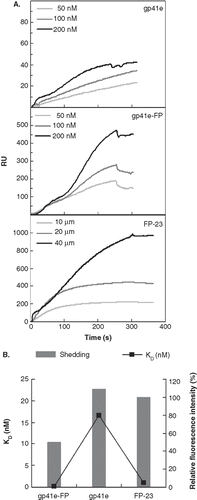
Since the FP region is highly hydrophobic, we surmised that it is a primary membrane binding segment in the gp41 ectodomain. To examine the membrane binding affinity, we employed SPR to measure the dissociation constant (Kd) of gp41e-FP, gp41e or FP-23 interaction with the raft-liposome SUV deposited on an L1 chip. Analysis was performed on a series of sensorgrams collected from several incremental concentrations of gp41 constructs (). The affinity constant was calculated by global fitting of the binding response to variation of the analyte concentration, evaluated with the conformation change fitting model of BiaEvaluation 3.0 software (BIAcore AB, Uppsala, Sweden). The derived dissociation constants (Kd) of gp41e-FP, gp41e and FP-23 are 0.120 ± 0.005, 16.6 ± 0.6 and 1.01 ± 0.03 nM, respectively. The data indicate that the FP region contributes to the membrane binding more significantly than other regions of gp41 ectodomain as gp41e devoid of FP has much weaker affinity for the membrane than the FP-containing gp41e-FP. Moreover, the much higher binding strength of gp41e-FP compared to its constituent constructs suggested that the two portions of the ectodomain act cooperatively in binding to the membrane. Remarkably, the sensorgrams of FP-23 and gp41e-FP display virtually little dissociation from the membrane. To quantitate the extent of dissociation, the relative amount of shed Rhodamine-labeled gp41 peptides collected in the dissociation stage of SPR measurements were compared as shown in . Gp41e-FP exhibited the least shedding among the three constructs whereas gp41e and FP-23 had essentially equal shedding from the liposome. The results provide evidence of synergism of FP and the remainder of gp41 in binding to the membrane. Moreover, it is deduced that the FP domain is more tightly bound to the liposome than gp41e in view of the size difference between FP-23 and gp41e.
Figure 3. Binding affinity and shedding of gp41e-FP, gp41e or FP-23 to the phospholipid membrane bilayer by SPR measurements. (A) Binding affinity of gp41e-FP, gp41e and FP-23 with raft-liposome. Despite the smaller size, FP-23 associated with the membranes better than gp41e, suggesting the high binding affinity of FP region for the membrane. (B) Dissociation constant (KD) analysed from (A) (black box) and relative shedding of gp41e-FP, gp41e and FP-23 from the DMPC liposomes (grey bar).
FP penetration into membrane can be deduced from differential cobalt quenching of NBD conjugated to FP of gp41, gp41e and gp41e-FP
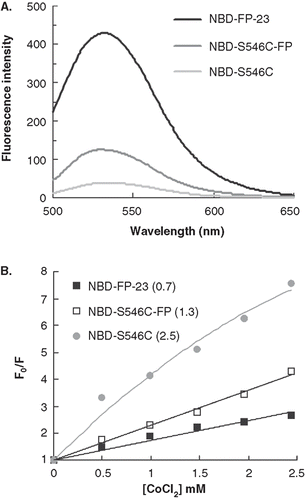
The vast discrepancy in binding affinity to the membrane bilayer between gp41e and gp41e-FP along with the paucity of dissociation from the membrane for the latter protein observed in the SPR experiments prompted us to investigate the membrane insertion of the proteins and peptide tested. To probe the insertion, we took advantage of the highly polarity-sensitive fluorescence of NBD. Thus, an increased fluorescence intensity of NBD attached to the N-terminus of the peptide signifies embedding of the peptide in the apolar milieu of membrane bilayer. As shown in , NBD-FP-23 has much higher intensity than NBD-S546C in the lipidic suspension indicating the hydrophobic medium encountered by the N-terminal of FP-23 in association with the membrane. To quantitatively evaluate the immersion depth of the peptides in the membrane, we measured the Stern-Volmer constant (KSV) obtained from cobalt quenching experiments. and , respectively, show NBD intensity in the gp41 constructs and KSV (indicated in the parentheses). A much smaller KSV for FP-23 than gp41e unequivocally signifies that the N-terminal of FP-23 inserts into the bilayer interior while gp41e associated with the membrane peripherally. Because of much larger size of gp41e than FP-23, KSV would have been larger for FP-23 if FP-23 were not located in the bilayer core. To further evaluate the membrane interaction of NHR under the influence of FP, we synthesized NBD-S546C-FP. As demonstrated in , the intensity and KSV for NBD-S546C-FP are intermediate between NBD-S546C and NBD-FP-23 implying that incorporating FP to gp41e pulls NHR of gp41e somewhat deeper into the membrane interior.
Figure 4. The insertion depth into the membrane measured from NBD labeled peptides quenched by Co2+. (A) The fluorescence emission spectra of NBD-FP-23, NBD-S546C and NBD-S546C-FP in raft-liposome show the higher intensity of the fusion peptide and indicate the strong hydrophobic interaction between membrane and FP region. The larger intensity of NBD-S546C-FP than NBD-S546C signifies that the NHR region is located deeper in the liposome due to the presence of FP, which is thought to penetrate into the membrane. (B) Stern-Volmer plot presents the small KSV (in the parentheses, unit of mM-1) of NBD-FP-23, demonstrating the deeper insertion of FP region into the membrane than gp41 ectodomain devoid of FP. The intermediate KSV value for S546C-FP between FP-23 and S546C concurs with the result displayed in (A). Both data consistently show that the NHR region penetrates deeper into the bilayer by the upstream FP sequence.
Insertion of FP-23 into membrane is also demonstrated by acrylamide quenching of tryptophan side chain
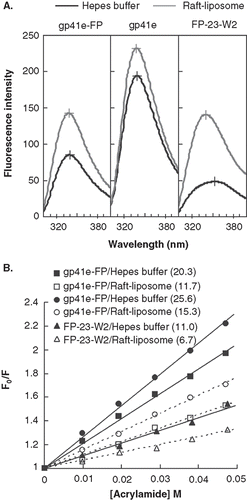
Another quantitative gauge on the locus of proteins in association with the membrane bilayer is afforded by Trp fluorescence variation with the aqueous quencher acrylamide. displays the Trp emission spectra of gp41e-FP, gp41e and FP-23-W2. The maximal fluorescence emission of FP-23-W2 in buffer was at 353 nm, indicating a highly polar environment for the Trp residue. However, a blue shift of the fluorescence maximum and distinct intensity increase for FP-23-W2 in the presence of liposomes compared to the aqueous medium indicate deep immersion of the Trp residue in the bilayer. Furthermore, in the small KSV demonstrated FP-23-W2 was more shielded against quenching by acrylamide in lipidic solutions, consistent with the idea that the Trp residue in FP-23-W2 was located in the lipidic interior of the membrane. Since there are seven Trps (underlined in ) in gp41e-FP or gp41e, the acrylamide quenching data represented an averaged effect of these 7 residues. Strikingly, for gp41e-FP and gp41e in buffer or in liposomes, the fluorescence emission maximum was at around 340 nm and the intensities were relatively low compared to that of FP-23-W2 which has only one Trp residue (). This could stem from aggregation of gp41e or gp41e-FP with Trp residues closely clustered, leading to fluorescence self-quenching. The significantly larger KSV values for these protein analogs than that for FP-23-W2 () in liposomes reflected a peripheral association with membranes for gp41e and the portion of gp41e-FP in which the Trp residues reside. For the three gp41 constructs, the KSV were larger in buffer solution than those in liposomes, again signifying their membrane interaction.
Figure 5. Tryptophan fluorescence emission spectra of gp41e-FP, gp41e and FP-23-W2 in raft-liposome and buffer solution (A) and Stern-Volmer plot and constants KSV (in the parentheses, unit of M-1) quenched by acrylamide (B). A typical maximal fluorescence emission for tryptophan in a polar environment was seen for FP-23-W2 in buffer solution (at 353 nm), and blue shifts were found for all other spectra, indicating the insertion of FP into the membranes and the aggregation of gp41e-FP and gp41e. A large KSV indicates the accessibility of the tryptophan residue for the quencher. Hence the smaller KSV for FP23-W2 than that for gp41e-FP or gp41e argues strongly for the deep immersion of FP in the model membrane.
ATR-FTIR measurements demonstrate the membrane perturbation of gp41 ectodomain is mainly mediated by FP
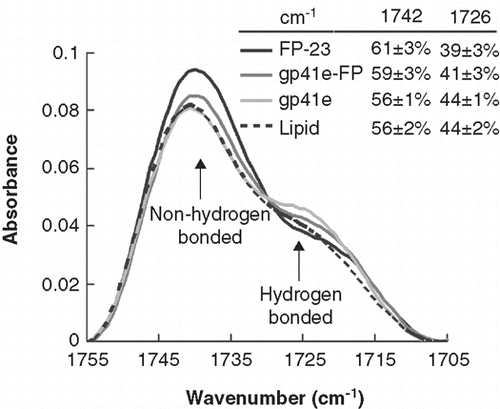
Because FP is highly hydrophobic and has been shown to insert into the membrane, we reasoned that FP would destabilize the membrane. To verify the membrane perturbation, ATR-FTIR experiments were conducted. displays the non-hydrogen bonded and hydrogen bonded carbonyl bands of the phospholipid in the presence and absence of the gp41 constructs. Note that the percentage of dehydration bands increases with the two FP-containing peptides. The spectra deconvoluted for the secondary structure analysis shows that FP-23 is dominantly β-sheet, while gp41e-FP and gp41e are mainly α-helix (Supplementary Figure S5, online version only).
Figure 6. Membrane dehydration as probed by the intensity of carbonyl stretching band of the gp41 fragments in association with DMPC/DMPG lipid bilayer at L/P = 50. The bands at 1742 and 1726 cm-1 arise from dehydrated and hydrated carbonyl stretching, respectively. Higher fraction of dehydrated band for FP-23 and gp41e-FP embedded lipid bilayer compared to the phospholipid alone signifies membrane perturbation by these fragments. In contrast, gp41e devoid of FP causes no change in the hydration state of the phopholipid.
Insertion angle and acyl chain angle deduced from the dichroic ratio
The angle of peptide insertion into membrane and the tilt angle of lipid acyl chains disturbed by the gp41 variants were listed in . The large angle observed with gp41e (66°) or gp41e-FP (63°) helix shows that the helical structure lies nearly parallel to the surface. On the other hand, the β-strand of FP-23 immerses in the membrane with an angle of 34° with respect to the membrane normal, close to that of gp41e-FP or gp41e β-strand structure.
Table I. Insertion angles and tilt angles of lipid acyl chain from analyzing ATR-FTIR spectra of lipid (DMPC:DMPG 1:1) and gp41e-FP, gp41e or FP-23 in the vesicular solution with L/P = 50 at 22°C and pH 7.0a.
A dramatic increase (from 27–50°) in the angle of lipid chain axis with respect to the normal of the membrane surface is observed for the two FP-containing gp41 analogs, reflecting the effect of FP insertion on the increase in the number of gauche conformations of the acyl backbone. At a high FP concentration (L/P = 50), the average tilt angle (50°) is close to the ‘magic angle’ which corresponds to a quasi-isotropic organization of the lipid chains or chain disorganization. In stark contrast, gp41e exerts little perturbation on the angle of the lipid acyl chain.
FRET evidence on the higher activity of gp41e-FP and FP-23 than gp41e in inducing lipid mixing
Because greater membrane perturbation was observed for gp41e-FP and FP-23, we further examined membrane mixing activity of the three gp41-derived molecules by FRET. displays the lipid mixing of raft-liposomes obtained from FRET measurements induced by the addition of each of the molecules investigated. Strikingly, both gp41e-FP and FP-23 exhibited strong lipid mixing activity, in contrast to that induced by gp41e. The pattern corroborates the membrane perturbation data from the FT-IR experiments, strongly suggesting that FP is the most fusogenic and memebrane-perturbing region in the gp41 ectodomain.
Fluorescence measurements on DPA-chelated terbium suggest strong membrane leakage activity of gp41e-FP as compared with gp41e and FP-23
Tb3+-encapsulated liposomes mixed with each of the tested peptides were used to monitor the leakage activity. The enhancement of terbium fluorescence by the external DPA is due to energy transfer from the aromatic ring of DPA. demonstrates that gp41e-FP has the strongest leakage activity in the neutral raft-liposomal suspension and gp41e caused little Tb3+ leakage. In consideration of its small size, it is remarkable that FP-23 has substantial leakage activity as compared to gp41e. The result leads to the conclusion that the FP region of gp41 is critical in disrupting the membrane.
Discussion
The study on the membrane interacting domain of gp41
It was demonstrated that the N-terminal FP domain inserts into and destabilizes the target membrane with extension of the NHR helical coiled coil that reorients the gp41 N-terminal region ([Durell et al. Citation1997] and references therein). The conformational plasticity of FP was deduced from its sensitivity to the composition of the membrane using solid state NMR technique (Zheng et al. Citation2006). Thus helical structure is dominant for the Leu7-Phe11 stretch in the absence of cholesterol, whereas β strand conformation prevails in the presence of 30% mole fraction of cholesterol. A recent electron-spin resonance study on the influenza hemagglutinin FP (Ge and Freed Citation2009) suggested that an increased order in the outer leaflet of the target membrane facilitates negative curvature of the bilayer to promote fusion.
The gp41 NHR domain, which constitutes the central coiled coil in SHB and was responsible for oligomerization of gp41 (Chen et al. Citation1998), was shown to be the target for the CHR-derived peptide fusion inhibitors such as T-20, T649 and CP32M (He et al. Citation2008); its membrane interaction underlying the mechanism of virus-mediated fusion event remains under debate. Using the electron-spin labeled 40-residue peptide encompassing the loop region and part of coiled coil region, Yu et al. (Citation1994) suggested that the coiled coil region are partitioned in the membrane interior. Biophysical investigation conducted by Lev and Shai using 54- and 36-residue peptides with and without acyl chain attached suggested that FP can be replaced by the palmitic chain for lipid mixing activity and the NHR of gp41 perturbed the membrane. Fluorescent experiments performed by Moreno et al. (Citation2007) using 15- and 23-residue peptides corresponding to the N-terminal portions of NHR demonstrated the membrane disruption and fusion activities of the peptides tested, implying permeation of the peptides in the membrane apolar milieu. These experiments, however, did not address the issue of membrane insertion, i.e., the locus in the membranous medium, of the NHR region. In the present study, comparison of membrane interaction among FP-23, gp41e and gp41e-FP and NBD labeling at 546 of the recombinant protein constructs allowed delineation of the role of FP in membrane perturbation, particularly in the context of the full length gp41 ectodomain
FP is the major hydrophobic stretch of gp41 ectodomain tightly bound to the membrane in the prefusion stage
Hydrophobicity is an essential property in identifying segments that partition to the membrane apolar milieu (Eisenberg Citation1984). As shown in , the polarity-sensitive bis-ANS exhibits high fluorescence intensity for FP-23 and gp41e-FP, indicating that FP is a primary hydrophobic site for gp41. In accord with this result, association with the model membrane examined by SPR displays a strong membrane affinity for the two FP-containing gp41 fragments (). Moreover, the small dissociated fraction of the two gp41 FP-containing derivatives observed in the dissociation phase of the SPR measurements reinforces the notion of the penetration of FP portion into the membrane core (). The data presented in the present work corroborate previous NMR evidence on FP-23 insertion into the micellar interior with the Ala15-Gly16 stretch located in the interface (Chang et al. Citation1997, Chang and Cheng Citation1998, Jaroniec et al. Citation2005). In contrast, gp41e exhibits the least affinity to the membrane among the three molecules tested. A recent photoactivated hydrophobic labeling experiment was consistent with our finding in that the NHR and the loop regions of gp41 ectodomain were not embedded in the apolar bilayer core (Viard et al. Citation2008). However, the FP region cannot be analyzed due to limited number of fragments that are generated with trypsin cleavage.
Having established that FP inserts into the membrane, we attempted to quantify the insertion depth by fluorescence quenching experiments. Thus by comparing the intensity of NBD and quenching by cobalt ion for the labeled FP-23, gp41e and gp41e-FP in association of the membrane (), it is deduced that the N-terminus of FP-23 resides much deeper in the membrane than gp41e and thereby pulls the NHR region deeper into the bilayer. The data corroborated that obtained in , in which the location of the peptides was monitored by the blue shift and acrylamide quenching of Trp fluorescence. Membrane-disruptive activity probed by lipid mixing assay () and leakage detection () also pointed to the FP domain as the principal determinant in destabilizing the membrane.
From the above several lines of fluorescence evidence and SPR binding measurements it is inferred that FP is the major membrane-inserting region of gp41 ectodomain and induces deeper penetration of the C-terminally adjoining amphiphilic NHR. To augment this assertion, other membrane interactions as probed by IR measurements, including membrane dehydration, acyl chain perturbation, are described below.
Dehydration effect of the HIV fusion peptide serves as further evidence of membrane perturbation
Lipid bilayers are stable, dynamic structures that do not fuse spontaneously. Hence membrane fusion is an energy costing event, requiring for instance the ATP hydrolysis in the cellular vesicle fusion (Sollner et al. Citation1993). In particular, a very high energy barrier must be surmounted before the merger can take place, which involves bilayer destabilization and dehydration of the interface regions of fusing membranes (Rand Citation1981). This concept is also compatible with the low efficiency of productive entry by the virus into the target cell (Marsh and Helenius Citation1989). The required energy presumably is in part supplied by the fusion protein (Chang et al. Citation1999, Bonnafous and Stegmann Citation2000), likely from the conformational change and interaction with the membrane. The latter may be effected predominantly from the FP domain insertion into the membrane apolar core. The hydrophobic interaction energy is likely to transduce to energy of membrane curvature, acyl chain order and removal of water of hydration at the membrane surface, which in turn facilitate the formation of fusion intermediate such as stalk (Ge and Freed Citation2009).
The membrane dehydration can be assayed by comparing the absorption peaks due to stretching of the phospholipid carbonyl bond with and without the hydrogen bond at 1726 and 1742 cm-1, respectively (; Tamm and Tatulian Citation1997). It is interesting to find that the fraction of non-hydrogen bonded peak increases substantially as FP (for FP-23 and gp41e-FP) binds to the lipid bilayer, indicating dehydration occurs upon FP insertion into the membrane whereas the superficial association of gp41e with the membrane induces little dehydration.
The orientation of viral FP inserted into the membrane has been suggested to correlate with its fusogenic activity (Martin et al. Citation1999), as mutations within the FP domain resulting in parallel or perpendicular membrane penetration abrogated fusion activity. It was deduced that the oblique insertion angle of viral fusion peptides increases the separation between lipid monolayer. Similar oblique angle was reported for many viral fusion peptides: 50° for HIV, 55° for simian immunodeficiency virus, and 30–55° for influenza virus (Luneberg et al. Citation1995, Han et al. Citation1999), in line with our data that FP β-sheet structure makes 35° angle with the membrane normal. The result strongly suggests that FP exerts its membrane-destabilizing action more effectively when the insertion angle falls in the 30–55° range.
Additional structural effect that can be gleaned from FT-IR measurements includes the hydrocarbon chain order. It has been demonstrated that the wild-type and several fusogenic mutants of the 23-residue influenza fusion peptide increased the acyl chain order parameter in fluid DMPC lipid bilayers (Han et al. Citation1999). In contrast, similar increase of the acyl chain order was not observed with several non-fusogenic constructs that had only one or two amino acids modified from the wild-type sequence. It is not yet known whether this increase in chain order is a primary effect imposed by the apolar portion of the peptide on the structure of hydrocarbon region of the lipid bilayer, or whether it is an indirect consequence of a specific peptide-induced dehydration at the membrane surface (Lis et al. Citation1982, Gawrisch et al. Citation1992). The latter hypothesis is attractive because the fusion peptide could then perform the tasks of removing water from the membrane surface at the site of closest contact, indenting the apposing membranes (Helm et al. Citation1989, Burgess et al. Citation1992).
Biological consequences of membrane insertion of fusion peptide: Organization, and effects on membrane topology, architecture and hydration
Oligomerization of the fusion protein has been suggested by a mutation within the FP region that blocked fusion activity and infectivity of HIV in a dominant negative manner (Freed et al. Citation1992), suggesting that FP exerted its function in a cooperative manner.
A mutation in the FP region has been shown to influence the coreceptor tropism (Huang et al. Citation2008) suggesting an interplay between structural alteration of gp41 FP and gp120 coreceptor binding site. The finding is compatible with our previous observation that the coreceptor-induced gp120 shedding from gp41 is a gradual process spanning 10 minutes after engagement of effector and target cells (Chien et al. Citation2008). As FP refolds at approximately 1 min after virus-receptor (CD4) engagement, it may trigger the conformational change of gp120 on its way to the target membrane, since complete gp120 dislodgment has not occurred. The altered gp120 structural change caused by a mutation in FP may in turn influence the coreceptor tropism (Huang et al. Citation2008).
The pre-transmembrane Trp-rich sequence (aa. 664-683) of gp41 has been shown to be membrane-active similar to the FP domain (Saez-Cirion et al. Citation2003) and embedded in the membrane interface. A previous EPR study implicated an interaction of the N-helix region with membrane (Yu et al. Citation1994). In the present study, we also deduced a peripheral association of the N-helix with the membrane bilayer. Hence it is hypothesized that, in the course of fusion, the domains immediately C-terminal to FP and N-terminal to TMD (i.e., the two membrane anchor domains) are immersed in and disrupt the interface regions of the fusing membranes to induce hemifusion.
Additionally, a loose association of FP with the transmembrane domain of influenza virus has been documented (Chang et al. Citation2008). This interaction is not strictly sequence-specific and was proposed to act at the late steps of fusion event, together with the membrane-perturbing lentivirus lytic peptide region in the gp41 cytopalsmic tail (Moreno et al. Citation2008), to promote the hemifusion-to-complete fusion transition. Thus FP may exert its function both at the early and late phases of the membrane fusion.
The oblique (30–50°) insertion of FP, possibly in conjunction with its conformational switch, disrupts the membrane architecture near the site of insertion. Alternatively, the deduced angle may be the resultant of the perpendicular orientation for initial FP insertion and the subsequent flattening of FP, possibly with the aid of conformationally plastic polar intervening segment C-terminal to FP. Conceivably, among the altered stability are those of lipid chain order, bilayer curvature and hydration of the interfacial region. Disordering of the lipid acyl chain facilitates migration of phosphotidylethanolamine – a molecule favouring formation of hexagonal phase – to the contacting site. In addition, negative curvature of the membrane may be induced by FP insertion, in preparation for fusion (Ge and Freed Citation2009). More importantly, as the fusing membranes approach within about 20–30 Å, they would experience the hydration repulsive force (Rand Citation1981). As observed in , the dehydration induced by FP insertion would contribute significantly to lowering this energy barrier. In conjunction with the membrane-disrupting Trp-rich pre-transmembrane domain (Vishwanathan and Hunter Citation2008), the conformationally flexible polar linker between FP and NHR renders viable the change in orientation of NHR and CHR relative to the membrane surface (Freed et al. Citation1990, Chang et al. Citation1999).
The finding that FP augments the membrane interaction of NHR () may shed light on the virus-mediated fusion mechanism. For example, the NHR sequence may be extended (becomes a rigid helical rod) as it is pulled by FP deeper into the bilayer to strengthen the membrane binding. The higher lipid mixing and leakage activity of gp41e-FP compared to gp41e and FP-23 can be accounted for by the cooperation of FP and NHR. Our finding provides a perspective to accommodate the discordant views of Lev and Shai (Citation2007), Yu et al. (Citation1994), Durrer et al. (Citation1996) and Harter et al. (Citation1989) in that the NHR can interact with the membrane effectively only in coordination with the adjoining FP.
Considering the enhanced fusogenic effect of NHR when pulled by FP observed herein (presumably in the prehairpin stage) and stabilization of (the fused) membrane by SHB in the postfusion stage, we hypothesized that NHR may have different roles during various steps of the fusion event. Acting alone, NHR has little membrane-disturbing effect but the activity is greatly raised with induction by membrane-anchored FP linked to NHR through the polar segment.
Lately, a full-length recombinant gp41 ectodomain similar to the one used herein was documented by Lev et al. (Citation2009). From comparison of lipid mixing activity with the core structure lacking FP, it was concluded that SHB acted to attenuate the membrane disruption by gp41 and to stabilize the fused membrane in the postfusion stage (Korazim et al. Citation2006). Future investigation is needed to test the proposal on the mode of action of gp41 ectodomain in the fusion mechanism.
Concluding remarks
A recent study on the function of the pre-transmembrane sequence (LWYIK; aa 679-683) indicated that this region of gp41 exerted its function posterior to the lipid mixing, probably at the pore opening, step(Chen et al. Citation2009). Consistent with this notion, the membrane proximal external sequence has been shown to exert its function at the pore-forming stage (Apellániz et al. Citation2009). In combination with the findings of the present work, it is concluded that the fusion peptide is the major segment of gp41 ectodomain that deeply embeds and perturbs the membrane and acts synergistically with NHR to drive the prefusion stage of fusion process.
Supplementary Material
Download PDF (254.3 KB)Acknowledgements
This work is supported by National Science Council (grant NSC 95-2113-M-001-011 to D.K. Chang) and Academia Sinica, Republic of China. Plasmid pSVE7'-puro was kindly provided by Dr S.S.L. Chen of the Institute of Biomedical Science of Academia Sinica. We are indebted to Mr Sean T.S. Wei for performing peptide shedding experiments of .
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Apellániz B, Nir S, Nieva JL. Distinct mechanisms of lipid bilayer perturbation induced by peptides derived from the membrane-proximal external region of HIV-1 gp41. Biochemistry. 2009;48:5320–5331.
- Bacigalupo MA, Ius A, Longhi R, Meroni G. Homogeneous immunoassay of atrazine in water by terbium-entrapping liposomes as fluorescent markers. Talanta. 2003;61:539–545.
- Bewley CA, Louis JM, Ghirlando R, Clore GM. Design of a novel peptide inhibitor of HIV fusion that disrupts the internal trimeric coiled-coil of gp41. J Biol Chem. 2002;277:14238–14245.
- Bonnafous P, Stegmann T. Membrane perturbation and fusion pore formation in influenza hemagglutinin-mediated membrane fusion. A new model for fusion. J Biol Chem. 2000;275:6160–6166.
- Bradshaw JP, Darkes MJ, Harroun TA, Katsaras J, Epand RM. Oblique membrane insertion of viral fusion peptide probed by neutron diffraction. Biochemistry. 2000;39:6581–6585.
- Brasseur R, De Loof H, Ruysschaert JM, Rosseneu M. Conformational analysis of lipid-associating proteins in a lipid environment. Biochim Biophys Acta. 1988;943:95–102.
- Bryl K, Kedzierska S, Laskowska M, Taylor A. Membrane fusion by proline-rich Rz1 lipoprotein, the bacteriophage lambda Rz1 gene product. Eur J Biochem. 2000;267:794–799.
- Burgess SW, McIntosh TJ, Lentz BR. Modulation of poly(ethylene glycol)-induced fusion by membrane hydration: Importance of interbilayer separation. Biochemistry. 1992;31:2653–2661.
- Caffrey M, Kaufman J, Stahl S, Wingfield P, Gronenborn AM, Clore GM. Monomer-trimer equilibrium of the ectodomain of SIV gp41: Insight into the mechanism of peptide inhibition of HIV infection. Protein Sci. 1999;8:1904–1907.
- Cao J, Bergeron L, Helseth E, Thali M, Repke H, Sodroski J. Effects of amino acid changes in the extracellular domain of the human immunodeficiency virus type 1 gp41 envelope glycoprotein. J Virol. 1993;67:2747–2755.
- Chang DK, Cheng SF. Determination of the equilibrium micelle-inserting position of the fusion peptide of gp41 of human immunodeficiency virus type 1 at amino acid resolution by exchange broadening of amide proton resonances. J Biomol NMR. 1998;12:549–552.
- Chang DK, Cheng SF, Chien WJ. The amino-terminal fusion domain peptide of human immunodeficiency virus type 1 gp41 inserts into the sodium dodecyl sulfate micelle primarily as a helix with a conserved glycine at the micelle-water interface. J Virol. 1997;71:6593–6602.
- Chang DK, Cheng SF, Kantchev EA, Lin CH, Liu YT. Membrane interaction and structure of the transmembrane domain of influenza hemagglutinin and its fusion peptide complex. BMC Biol. 2008;6:2.
- Chang DK, Cheng SF, Trivedi VD. Biophysical characterization of the structure of the amino-terminal region of gp41 of HIV-1. Implications on viral fusion mechanism. J Biol Chem. 1999;274:5299–5309.
- Chen SS, Lee SF, Hao HJ, Chuang CK. Mutations in the leucine zipper-like heptad repeat sequence of human immunodeficiency virus type 1 gp41 dominantly interfere with wild-type virus infectivity. J Virol. 1998;72:4765–4774.
- Chen SSL, Yang P, Ke PY, Li HF, Chan WE, Chang DK, Chuang CK, Tsai Y, Huang SC. Identification of the LWYIK motif located in the human immunodeficiency virus type 1 transmembrane gp41 protein as a distinct determinant for viral infection. J Virol. 2009;83:870-883.
- Chien MP, Jiang S, Chang DK. The function of coreceptor as a basis for the kinetic dissection of HIV type 1 envelope protein-mediated cell fusion. Faseb J. 2008;22:1179–1192.
- Durell SR, Martin I, Ruysschaert JM, Shai Y, Blumenthal R. What studies of fusion peptides tell us about viral envelope glycoprotein-mediated membrane fusion (review). Mol Membr Biol. 1997;14:97–112.
- Durrer P, Galli C, Hoenke S, Corti C, Gluck R, Vorherr T, Brunner J. H+-induced membrane insertion of influenza virus hemagglutinin involves the HA2 amino-terminal fusion peptide but not the coiled coil region. J Biol Chem. 1996;271:13417–13421.
- Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623.
- Freed EO, Delwart EL, Buchschacher GL Jr, Panganiban AT. A mutation in the human immunodeficiency virus type 1 transmembrane glycoprotein gp41 dominantly interferes with fusion and infectivity. Proc Natl Acad Sci USA. 1992;89:70–74.
- Freed EO, Myers DJ, Risser R. Characterization of the fusion domain of the human immunodeficiency virus type 1 envelope glycoprotein gp41. Proc Natl Acad Sci USA. 1990;87:4650–4654.
- Furuta RA, Wild CT, Weng Y, Weiss CD. Capture of an early fusion-active conformation of HIV-1 gp41. Nat Struct Biol. 1998;5:276–279.
- Gawrisch K, Ruston D, Zimmerberg J, Parsegian VA, Rand RP, Fuller N. Membrane dipole potentials, hydration forces, and the ordering of water at membrane surfaces. Biophys J. 1992;61:1213–1223.
- Ge M, Freed JH. Fusion peptide from influenza hemagglutinin increases membrane surface order: An electron-spin resonance study. Biophys J. 2009;96:4925–4934.
- Han X, Steinhauer DA, Wharton SA, Tamm LK. Interaction of mutant influenza virus hemagglutinin fusion peptides with lipid bilayers: Probing the role of hydrophobic residue size in the central region of the fusion peptide. Biochemistry. 1999;38:15052–15059.
- Harter C, James P, Bachi T, Semenza G, Brunner J. Hydrophobic binding of the ectodomain of influenza hemagglutinin to membranes occurs through the ‘fusion peptide’. J Biol Chem. 1989;264:6459–6464.
- He Y, Cheng J, Lu H, Li J, Hu J, Qi Z, Liu Z, Jiang S, Dai Q. Potent HIV fusion inhibitors against Enfuvirtide-resistant HIV-1 strains. Proc Natl Acad Sci USA. 2008;105:16332–16337.
- Helm CA, Israelachvili JN, McGuiggan PM. Molecular mechanisms and forces involved in the adhesion and fusion of amphiphilic bilayers. Science. 1989;246:919–922.
- Hernandez LD, Hoffman LR, Wolfsberg TG, White JM. Virus-cell and cell-cell fusion. Annu Rev Cell Dev Biol. 1996;12:627–661.
- Huang W, Toma J, Fransen S, Stawiski E, Reeves JD, Whitcomb JM, Parkin N, Petropoulos CJ. Coreceptor tropism can be influenced by amino acid substitutions in the gp41 transmembrane subunit of human immunodeficiency virus type 1 envelope protein. J Virol. 2008;82:5584–5593.
- Jaroniec CP, Kaufman JD, Stahl SJ, Viard M, Blumenthal R, Wingfield PT, Bax A. Structure and dynamics of micelle-associated human immunodeficiency virus gp41 fusion domain. Biochemistry. 2005;44:16167–16180.
- Kantchev EA, Cheng SF, Wu CW, Huang HJ, Chang DK. Secondary structure, phospholipid membrane interactions, and fusion activity of two glutamate-rich analogs of influenza hemagglutinin fusion peptide. Arch Biochem Biophys. 2004;425:173–183.
- Korazim O, Sackett K, Shai Y. Functional and structural characterization of HIV-1 gp41 ectodomain regions in phospholipid membranes suggests that the fusion-active conformation is extended. J Mol Biol. 2006;364:1103–1117.
- Lakowicz JR. 1983. Principles of fluorescence spectrosopy. New York: Plenum Press.
- Lev N, Fridmann-Sirkis Y, Blank L, Bitler A, Epand RF, Epand RM, Shai Y. Conformational stability and membrane interaction of the full-length ectodomain of HIV-1 gp41: Implication for mode of action. Biochemistry. 2009;48:3166–3175.
- Lev N, Shai Y. Fatty acids can substitute the HIV fusion peptide in lipid merging and fusion: An analogy between viral and palmitoylated eukaryotic fusion proteins. J Mol Biol. 2007;374:20–230.
- Lin CH, Chang CC, Cheng SF, Chang DK. The application of perfluorooctanoate to investigate trimerization of the human immunodeficiency virus-1 gp41 ectodomain by electrophoresis. Electrophoresis. 2008;29:3175–3182.
- Lis LJ, McAlister M, Fuller N, Rand RP, Parsegian VA. Interactions between neutral phospholipid bilayer membranes. Biophys J. 1982;37:657–665.
- Luneberg J, Martin I, Nussler F, Ruysschaert JM, Herrmann A. Structure and topology of the influenza virus fusion peptide in lipid bilayers. J Biol Chem. 1995;270:27606–27614.
- Maddon PJ, Dalgleish AG, McDougal JS, Clapham PR, Weiss RA, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348.
- Marsh M, Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–1051.
- Martin I, Dubois MC, Defrise-Quertain F, Saermark T, Burny A, Brasseur R, Ruysschaert JM. Correlation between fusogenicity of synthetic modified peptides corresponding to the NH2-terminal extremity of simian immunodeficiency virus gp32 and their mode of insertion into the lipid bilayer: An infrared spectroscopy study. J Virol. 1994;68:1139–1148.
- Martin II, Ruysschaert J, Epand RM. Role of the N-terminal peptides of viral envelope proteins in membrane fusion. Adv Drug Deliv Rev. 1999;38:233–255.
- Mobley PW, Pilpa R, Brown C, Waring AJ, Gordon LM. Membrane-perturbing domains of HIV type 1 glycoprotein 41. AIDS Res Hum Retroviruses. 2001;17:311–327.
- Moreno MR, Guillen J, Perez-Berna AJ, Amoros D, Gomez AI, Bernabeu A, Villalain J. Characterization of the interaction of two peptides from the N terminus of the NHR domain of HIV-1 gp41 with phospholipid membranes. Biochemistry. 2007;46:10572–10584.
- Moreno MR, Perez-Berna AJ, Guillen J, Villalain J. Biophysical characterization and membrane interaction of the most membranotropic region of the HIV-1 gp41 endodomain. Biochim Biophys Acta. 2008;1778:1298–1307.
- Morris SJ, Bradley D, Blumenthal R. The use of cobalt ions as a collisional quencher to probe surface charge and stability of fluorescently labeled bilayer vesicles. Biochim Biophys Acta. 1985;818:365–372.
- Navratilova I, Pancera M, Wyatt RT, Myszka DG. A biosensor-based approach toward purification and crystallization of G protein-coupled receptors. Anal Biochem. 2006;353:278–283.
- Pereira FB, Goni FM, Muga A, Nieva JL. Permeabilization and fusion of uncharged lipid vesicles induced by the HIV-1 fusion peptide adopting an extended conformation: Dose and sequence effects. Biophys J. 1997;73:1977–1986.
- Rand RP. Interacting phospholipid bilayers: Measured forces and induced structural changes. Annu Rev Biophys Bioeng. 1981;10:277–314.
- Rapaport D, Shai Y. Interaction of fluorescently labeled pardaxin and its analogues with lipid bilayers. J Biol Chem. 1991;266:23769–23775.
- Rizzuto CD, Wyatt R, Hernandez-Ramos N, Sun Y, Kwong PD, Hendrickson WA, Sodroski J. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280:1949–1953.
- Saez-Cirion A, Arrondo JL, Gomara MJ, Lorizate M, Iloro I, Melikyan G, Nieva JL. Structural and functional roles of HIV-1 gp41 pretransmembrane sequence segmentation. Biophys J. 2003;85:3769–3780.
- Sattentau QJ, Moore JP. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med. 1991;174:407–415.
- Schaal H, Klein M, Gehrmann P, Adams O, Scheid A. Requirement of N-terminal amino acid residues of gp41 for human immunodeficiency virus type 1-mediated cell fusion. J Virol. 1995;69:3308–3314.
- Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–418.
- Stegmann T, Delfino JM, Richards FM, Helenius A. The HA2 subunit of influenza hemagglutinin inserts into the target membrane prior to fusion. J Biol Chem. 1991;266:18404–18410.
- Struck DK, Hoekstra D, Pagano RE. Use of resonance energy transfer to monitor membrane fusion. Biochemistry. 1981;20:4093–4099.
- Sullivan N, Sun Y, Binley J, Lee J, Barbas CF 3rd, Parren PW, Burton DR, Sodroski J. Determinants of human immunodeficiency virus type 1 envelope glycoprotein activation by soluble CD4 and monoclonal antibodies. J Virol. 1998;72:6332–6338.
- Tamm LK, Tatulian SA. Infrared spectroscopy of proteins and peptides in lipid bilayers. Q Rev Biophys. 1997;30:365–429.
- Tristram-Nagle S, Nagle JF. HIV-1 fusion peptide decreases bending energy and promotes curved fusion intermediates. Biophys J. 2007;93:2048–2055.
- Viard M, Ablan SD, Zhou M, Veenstra TD, Freed EO, Raviv Y, Blumenthal R. Photoinduced reactivity of the HIV-1 envelope glycoprotein with a membrane-embedded probe reveals insertion of portions of the HIV-1 Gp41 cytoplasmic tail into the viral membrane. Biochemistry. 2008;47:1977–1983.
- Vishwanathan SA, Hunter E. Importance of the membrane-perturbing properties of the membrane-proximal external region of human immunodeficiency virus type 1 gp41 to viral fusion. J Virol. 2008;82:5118–51126.
- White JM, Delos SE, Brecher M, Schornberg K. Structures and mechanisms of viral membrane fusion proteins: Multiple variations on a common theme. Crit Rev Biochem Mol Biol. 2008;43:189–219.
- Yu YG, King DS, Shin YK. Insertion of a coiled-coil peptide from influenza virus hemagglutinin into membranes. Science. 1994;266:274–276.
- Zheng Z, Yang R, Bodner ML, Weliky DP. Conformational flexibility and strand arrangements of the membrane-associated HIV fusion peptide trimer probed by solid-state NMR spectroscopy. Biochemistry. 2006;45:12960–12975.
- Lev N, Fridmann-Sirkis Y, Blank L, Bitler A, Epand RF, Epand RM, Shai Y. Conformational stability and membrane interaction of the full-length ectodomain of HIV-1 gp41: Implication for mode of action. Biochemistry. 2009;48:3166–3175.
- Lin CH, Chang CC, Cheng SF, Chang DK. The application of perfluorooctanoate to investigate trimerization of the human immunodeficiency virus-1 gp41 ectodomain by electrophoresis. Electrophoresis. 2008;29:3175–3182.