Abstract
Golgi-Associated Plant Pathogenesis-Related protein 1 (GAPR-1) is a mammalian protein that belongs to the superfamily of plant pathogenesis-related proteins group 1 (PR-1). GAPR-1 strongly associates with lipid rafts at the cytosolic leaflet of the Golgi membrane. The myristoyl moiety at the N-terminus of GAPR-1 contributes to membrane binding but is not sufficient for stable membrane anchorage. GAPR-1 is positively charged at physiological pH, which allows for additional membrane interactions with proteins or lipids. To determine the potential contribution of lipids to membrane binding of GAPR-1, we used a liposome binding assay. Here we report that non-myristoylated GAPR-1 stably binds liposomes that contain the negatively charged lipids phosphatidylserine, phosphatidylglycerol, phosphatidylinositol, or phosphatidic acid. GAPR-1 displays the highest preference for phosphatidic acid-containing liposomes. In contrast, lysozyme, which contains a similar surface charge, did not bind to these liposomes, except for a weak membrane association with PA-containing liposomes. Interestingly, GAPR-1 binds to phosphatidylinositol with unusual characteristics. Denaturation or organic extraction of GAPR-1 does not result in dissociation of phosphatidylinositol from GAPR-1. The association of phosphatidylinositol with GAPR-1 results in a diffuse gel-shift in SDS-PAGE. Mass spectrometric analysis of gel-shifted GAPR-1 showed the association of up to 3 molecules of phosphatidylinositol with GAPR-1. These results suggest that the lipid composition contributes to the GAPR-1 binding to biological membranes.
| Abbreviations: | ||
| CAP | = | proteins, cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins |
| GAPR-1, | = | Golgi Associated plant Pathogenesis Related protein 1; GPI, glycosylphosphatidylinositol |
| MALDI-TOF, | = | Matrix-assisted LASER desorption/ionization time-of-flight |
| PA, | = | Phosphatidic acid; PC, Phosphatidylcholine |
| PE, | = | Phosphatidylethanolamine |
| PI, | = | Phosphatidylinositol |
| PR-1, | = | plant pathogenesis-related proteins group 1 |
| PS, | = | Phosphatidylserine; SCP, Sperm-coating protein |
| SDS-PAGE, | = | Sodium dodecyl sulphate polyacrylamide gelelectrophoresis |
| SM, | = | sphingomyelin |
| TX114, | = | Triton X-114 |
Introduction
Golgi-Associated Plant Pathogenesis-Related protein 1 (GAPR-1) is a mammalian protein, which is well conserved among vertebrates and is known under different aliases such as GLIPR-2 and C9orf19 [Citation1–3]. In mice, GAPR-1 is predominantly expressed in the lung, spleen, monocytes, uterus and embryonic tissue [Citation3]. In humans, GAPR-1 expression has thus far been described in peripheral leukocytes and lung [Citation2]. Its expression in immunocompetent cells and tissues suggests a role in immunity. Furthermore, it was speculated that GAPR-1 may be involved in the differentiation of epithelial cells into mesenchymal cells as GAPR-1 was found to be up-regulated in fibrotic human kidneys [Citation1]. The biological function of GAPR-1 remains, however, unclear.
Structural analysis in silico has shown that GAPR-1 contains a sperm-coating protein (SCP) domain (13 kDa), which encompasses almost the entire protein (17 kDa) [Citation4]. This domain is also referred to as the CAP domain (reviewed in [Citation5]). So far, no specific function has been assigned to the SCP/CAP domain. Proteins containing the SCP/CAP domain display heterogeneous functions [Citation5–10]. In humans, GAPR-1 shows significant homology to GLIPR-1 (RTVP-1) and CRISP proteins [Citation11,Citation12]. Overall, GAPR-1 shares the highest homology with plant Pathogenesis Related-1 (PR-1) proteins, which have been implicated in acquisition of resistance by plants against pathogens (reviewed in [Citation13]). Members of this protein family are comparable to GAPR-1 in size, isoelectric point and 3D structure [Citation14]. In contrast to plant PR-1 proteins and other mammalian SCP/CAP domain containing proteins, GAPR-1 is not secreted as it lacks a signal peptide. Instead, it localizes to lipid rafts at the cytosolic leaflet of the Golgi complex [Citation15].
Its membrane binding is very strong, as salt-stripping of membranes or treatment of cells with Brefeldin A, which causes a redistribution of GAPR-1 in cells, do not release GAPR-1 from membranes. The strong binding to Golgi membranes is unique as most of the proteins containing an SCP/CAP domain are localized to the extracellular leaflet of the plasma membrane or remain soluble upon secretion [Citation6,Citation7,Citation13,Citation16]. GAPR-1 is myristoylated and this fatty acid modification could provide a mechanism to anchor this protein to the membrane. The binding energy of myristate is however not sufficient for stable membrane anchorage [Citation17]. In agreement with this, several myristoylated proteins do not show exclusive membrane localization, and a second interaction is required for efficient membrane-binding (reviewed in [Citation18,Citation19]). GAPR-1 has been shown to interact with caveolin-1, a well known membrane resident protein [Citation3]. In addition, GAPR-1 has a pI of 9.4, resulting in a net positive charge at physiological pH. According to the crystal structure of GAPR-1, most of the positive charged residues are localized to one area of the protein surface [Citation4], creating an opportunity for efficient electrostatic interactions with negatively charged lipids in the membrane. Electrostatic lipid-protein interactions combined with myristoyl anchorage can be sufficient for stable membrane binding [Citation18].
To gain more insight into the membrane-binding mechanism of GAPR-1, we investigated how GAPR-1 interacts with lipids. By use of a liposome binding assay, we show that GAPR-1 binds to negatively charged lipids. During the screening of these lipids, we observed unusual binding characteristics of GAPR-1 to phosphatidylinositol (PI)-containing liposomes.
Materials and methods
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine, 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphate, L-a-phosphatidylinositol (Bovine liver), sphingomyelin (egg), cholesterol, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine and 1-palmitoyl-2-oleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] were purchased from Avanti Polar Lipids (Alabaster, USA). Lysozyme (chicken egg) was purchased from Sigma (St. Louis, USA). L-3-Phosphatidyl [U-14C]Inositol (CFA 641) was from Amersham Biosciences (Piscataway, USA).
Liposome binding assay
Stock solutions of lipids were made in chloroform/methanol (1:2 v/v) and stored in the freezer at −20°C under N2. Liposomes were made freshly for each liposome binding assay. To generate liposomes, phospholipids and cholesterol were mixed from stock solutions in a molar ratio of 2.28:1. The solvent was evaporated using a flow of N2 with subsequent drying at room temperature in a speedvac (Savant SVC100H, Farmingdale, USA) for at least 90 min. Subsequently 50-NT buffer (50 mM NaCl, 25 mM tris, pH 7.4) was added on the dried lipid film to a final concentration of 6.8 μmol/ml phospholipids. The tube was vortexed at least 3 times for 15 sec until all lipids had been resuspended. Liposomes were generated by sonicating the lipid suspension 4 times for 15 sec on ice using an ultrasonic probe (MSE Soniprep 150, London, UK).
In a typical experiment, 50 µl of the liposome suspension was incubated with 20 µg of recombinant GAPR-1 [Citation4,Citation20] and lysozyme (100 μg) in 50-NT buffer (75 µl total volume). The samples were incubated for 90 min at 37°C or using indicated conditions. The incubation was stopped by cooling the samples on ice. Sucrose (60% (w/v) in 50-NT) was mixed with the sample to a final concentration of 36.5%. The samples were overlayed with 500 µl 25% (w/v) sucrose in 50-NT buffer and subsequently with 100 µl 50-NT buffer.
The samples were centrifuged in a TLA-55 rotor (Beckman, Fullerton, USA) for 90 min at 136,000 g (4°C). After centrifugation, liposome-bound protein was collected in 300 μl from the top of the gradient. To ensure that liposomes floated to the top of the gradient, floated lipids were quantified by a phosphate determination according to Rouser with minor modifications [Citation21]. Briefly, 37.5 µl of the floated liposome fraction was taken and 150 µl perchloric acid (70–72%) was added, after which samples were heated at 180°C for at least 1 h until the sample had become clear. After cooling to room temperature, 625 µl water, 125 µl 2.5% (w/v) heptamolybdate and 125 µl 10% (w/v) ascorbic acid were added. Subsequently, samples were warmed to 50–60°C for 20 min, after which absorbance was measured at 820 nm wave length. The recovery efficiency of all liposomes tested was 81 ± 5%. Prior to analysis of the sample by SDS-PAGE and subsequent coomassie staining, proteins were precipitated by chloroform/methanol to concentrate the proteins and to remove liposomal lipids. Briefly, 775 μl chloroform/methanol (1:2) was added to 225 μl of the floated fraction. The sample was mixed and centrifuged for 30 min at 13,000 g. The supernatant containing liposomal lipids was removed and the pellet containing precipitated protein was air-dried.
Triton X-114 phase separation assay
Triton X-114 (TX114) phase separation was performed as described [Citation22]. Briefly, TX114 (Sigma, St. Louis, US) was dissolved in 25 mM HEPES pH 7.4, 50 mM NaCl at 4°C. To remove impurities, phase separation was induced by shifting the solution to 37°C in a water bath O/N. The upper phase was discarded and an equal volume of fresh buffer was added. This step was repeated twice. The final TX114 concentration (16%) was determined by measuring the O.D. at 268 nm. PI-bound GAPR-1 was made by binding GAPR-1 to PI liposomes and subsequent flotation as described above. For each TX114 assay, 100 µl of a top fraction of a liposome binding assay PI or PA liposomes was used. As a control, non-bound GAPR-1 was diluted in 100 µl 50-NT buffer. An equal volume of 1% TX114 (v/v) in 50-NT buffer was added to the samples and the mixture was incubated on ice for 40 min. Phase separation was induced by incubation of the samples for 5 min at 37°C in a water bath followed by centrifugation for 2 min at 13,000 g in a microtube centrifuge, allowing the separation of the detergent phase from the aqueous phase. Both phases were washed once by adding 100 µl 50-NT buffer to the detergent phase and 100 µl 1% TX114 in 50-NT buffer to the aqueous phase, and centrifugation as above. Both aqueous phases and both detergent phases were pooled. To all samples 50-NT buffer was added to a total volume of 300 µl.
Binding of GAPR-1 to radio-labeled PI
Liposomes were made as described above containing 250 nCi L-3-phosphatidyl[U-14C]Inositol with non-labeled PI, PC and cholesterol (1:9:3 mol ratio). 50 µl liposomes, 20 µg GAPR-1 and 100 µg lysozyme in 50-NT buffer with 10 mM β-mercaptoethanol were incubated in a total volume of 75 µl for 100 min at 37°C. Liposomes were pelleted for 1 h at 112,000 g in a TLA-55 rotor (Beckman, Fullerton, USA). Proteins in the pellet were resolved on a Novex precast 4–12% gradient gel (Invitrogen, Carlsbad, USA) and visualized by staining with coomassie blue R-250 (Serva Electrophoresis, Heidelberg, Germany). To enhance the signal, the gel was soaked in N-AMP (Amersham Biosciences, Piscataway, USA) and analyzed by autoradiography using Biomax MR films (Kodak, Rochester, USA).
SDS-PAGE and Western blot
Unless indicated otherwise, 14% (w/v) polyacrylamide gels were used. Gels were analyzed by staining with coomassie blue R-250 (Serva Electrophoresis, Heidelberg, Germany), or by Western blotting. For Western blotting an affinity purified polyclonal antibody to GAPR-1 was used as described [Citation3]. Peroxidase labeled goat anti rabbit (Nordic Immunology, Tilburg, The Netherlands) was used as secondary antibody. Supersignal West Pico Chemiluminescent was purchased from Pierce (Rockford, USA). Molecular masses were estimated by comparison with SeeBlue® Plus2 Pre-Stained Standard molecular mass marker proteins (Invitrogen, Carlsbad, USA).
MALDI-TOF analysis
PI-bound GAPR-1 was made using the liposome binding assay as described above. Briefly, GAPR-1 was incubated with liposomes containing PI, PC and cholesterol (1:4:2.19 mol ratio) for 90 min at 37°C and subsequently liposomes were floated on a sucrose gradient. PI-bound GAPR-1 was collected from the upper fraction of the gradient. GAPR-1 (from a stock solution) and PI-bound GAPR-1 were precipitated using HPLC grade chloroform/methanol (1:2 v/v). The pellet was washed 2 times with HPLC grade chloroform/methanol (1:2 v/v) and resuspended in 10 μl MALDI matrix suspension (5 mg/ml sinapinic acid in 50% acetonitrile/0.1% trifluoroacetic acid). One μl of this suspension was spotted on a MALDI target plate and analyzed using an Applied Biosystems 4700 MALDI Proteomics analyzer (Applied Biosystems, Foster City, USA) in a positive linear mode, using an m/z 4,000–40,000 mass range with a focus m/z of 17,000. Data were acquired at a 200 Hz laser repetition rate, a laser intensity of 3500, an acceleration voltage of 20 kV and a digitizer bin size of 2.0 ns. In total, data from 9000 shots were used for the final spectrum.
Results
GAPR-1 binds negatively charged lipids
To determine the potential contribution of lipids to the membrane binding of GAPR-1, a liposome binding assay was used. Liposomes were made using phosphatidylcholine (PC) and cholesterol in the presence of various other phospholipids. As the myristoyl-moiety of GAPR-1 increases the affinity of GAPR-1 for lipid membranes and thus interferes with the analysis of the phospholipid headgroup specificity for binding, non-myristoylated GAPR-1 [Citation4] was used for the binding experiments. The GAPR-1 binding assay was performed in the presence of an excess of lysozyme, which is similar to GAPR-1 in terms of size (14.3 kDa) and charge (pI = 9.3). Lysozyme may therefore allow the discrimination between specific and non-specific interactions of GAPR-1 with negatively charged lipids. After incubation, the amount of liposome-bound GAPR-1 was assessed by flotation of the liposomes on a sucrose gradient. The proteins in the top fraction containing the floated liposomes were resolved by SDS-PAGE and visualized using coomassie blue (). GAPR-1 binding to liposomes composed of only PC and cholesterol is minimal. In the presence of negatively charged lipids, increased binding of GAPR-1 to liposomes is observed with the highest affinity for phosphatidic acid (PA). Lysozyme, however, was not recovered in the liposome fractions. Only in the sample with PA-containing liposomes small amounts of lysozyme can be detected in the liposome fraction, indicating some potential for non-specific interactions under these conditions. GAPR-1 binding to phosphatidylinositol (PI)-containing liposomes showed some unexpected behaviour. After separation of proteins by SDS-PAGE, GAPR-1 did not migrate as a defined sharp band but as a diffuse protein band caused by reduced migration of some GAPR-1 in the polyacrylamide gel. Western blot analysis confirmed that the diffuse material contained GAPR-1 (see below).
Figure 1. GAPR-1 binds negatively charged lipids. (A) Affinity of GAPR-1 for liposomes containing different membrane lipids. GAPR-1 and lysozyme were incubated with cholesterol/phospholipid liposomes containing PC as the only phospholipid (-), or 85 (mol) % PC and in addition one of the following lipids (15%): sphingomyelin (SM), phosphatidylethanolamine (PE), phosphatidic acid (PA), phosohatidylserine (PS), phosphatidylinositol (PI) or phosphatidylglycerol (PG). After incubation at 37°C for 90 min, liposomes were floated on a sucrose gradient. Proteins in the top fraction of the gradient were precipitated with chloroform/methanol (1:2) and subsequently resolved on an SDS-PAGE gel and visualized using coomassie blue. The 17 kDa bands represent GAPR-1. Input of GAPR-1 (50%) and lysozyme (50%) are shown on the right (B) GAPR-1 was incubated with liposomes containing PC alone or 90% PC with 10% PA or PI. After the incubation the liposomes were floated on a sucrose gradient. Fractions of the gradient were collected from top (fraction 1, low sucrose) to bottom (fraction 4, high sucrose). Proteins in each fraction were resolved by SDS PAGE and analyzed by Western blotting with an antibody to GAPR-1.
The characteristics of GAPR-1 binding to PA and PI were further investigated. After incubation with PA- or PI-containing liposomes, the liposomes were floated on a sucrose step gradient and each fraction was subsequently analyzed by Western blotting using anti-GAPR-1 antibodies (). In agreement with the results shown in , we observed increased binding of GAPR-1 to floated PA or PI-containing liposomes as compared to floated liposomes that do not contain negatively charged lipids. When GAPR-1 is bound to PI-containing liposomes, only GAPR-1 in the top fraction has an increased apparent molecular mass, whereas GAPR-1 in the bottom fractions is not affected by the incubation with PI-containing liposomes. This indicates that only the pool of GAPR-1 that interacts with PI-containing liposomes has changed its physical properties. This is in marked contrast with the binding of GAPR-1 to PA liposomes, which did not lead to a gel-shift of GAPR-1.
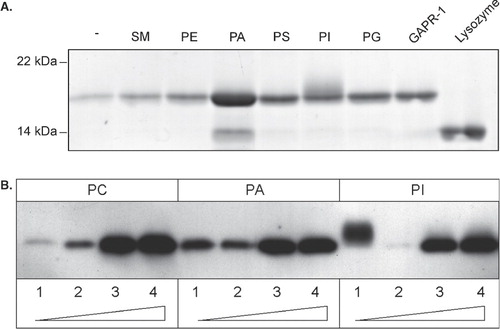
To further characterize the interaction of GAPR-1 with PI-containing liposomes, the temperature dependency of liposome binding of GAPR-1 was investigated. GAPR-1 was incubated with PA- or PI-containing liposomes at various temperatures (4°C, 20°C, and 37°C) (). When GAPR-1 is incubated with PA-containing liposomes, the temperature has little effect on the binding characteristics. However, binding of GAPR-1 to PI-containing liposomes is temperature dependent. Upon incubation at 37°C GAPR-1 binding to PI-containing liposomes is strongly increased. In addition, the molecular weight of GAPR-1 appears higher after incubation at 37°C as compared to incubations at the two lower temperatures. These data suggest that GAPR-1 binds to PA- and PI-containing liposomes via different mechanisms.
Figure 2. Binding to PI is time, temperature and concentration dependent. (A) GAPR-1 was incubated with cholesterol/phospholipid liposomes containing 85 (mol)% PC and 15% PA or PI at 4°C, 20°C or 37°C for 90 min, after which the liposomes were floated on a sucrose gradient. The top fractions from the gradient were resolved by SDS–PAGE and analyzed by Western blotting with an antibody to GAPR-1. GAPR-1 from stock is shown in the input lane. (B) GAPR-1 and lysozyme were incubated with liposomes (described in panel A) containing PI (upper panel) or PA (lower panel) at the indicated times at 37°C. After flotation of the liposomes on a sucrose gradient, proteins were precipitated with chloroform/methanol. The proteins were visualized using SDS-PAGE and coomassie blue. Input of GAPR-1 (25%) and lysozyme (25%) are shown on the right. (C) GAPR-1 and lysozyme were incubated with PC/cholesterol liposomes containing the indicated percentages (mol % phospholipid) of PI or PA as indicated at 37°C for 90 min. After incubation, the liposomes were floated on a sucrose gradient and bound proteins were visualized using SDS-PAGE and coomassie blue. Input of GAPR-1 (50%) and lysozyme (50%) are shown on the right.
The temperature dependency of the GAPR-1 binding to PI-containing liposomes indicates that this interaction is not solely electrostatic. Additional evidence for this was obtained by determining the time dependency of GAPR-1 binding to liposomes. GAPR-1 was incubated with PC liposomes in the absence or presence of PA or PI for different time periods at 37°C (0–240 min). After incubation the liposomes were floated on a sucrose gradient and analyzed for the presence of GAPR-1 using coomassie blue staining (). In the presence of PI, a time-dependent increase of GAPR-1 binding was observed. Under these conditions, lysozyme only showed a minimal time-dependent increase, except for a small increase at the latest timepoint. In the presence of PA, maximal binding is already observed at the earliest possible timepoint, without incubation at 37°C. In the absence of PI or PA, GAPR-1 association with liposomes remained minimal. In addition to a gradual increase of GAPR-1 association with PI-containing liposomes, the gel-shift of GAPR-1 can also be observed at early time points, indicating no significant delay of this GAPR-1 modification upon binding to PI-containing liposomes. Lysozyme, however, does not show any gel-shift.
The affinity of GAPR-1 for negatively charged lipids was determined by titrating increasing amounts of PI or PA into PC-containing liposomes in the presence of lysozyme (). Both in the presence of PI and PA, GAPR-1 association with liposomes increased with increasing concentrations of negatively charged lipids. The GAPR-1 gel-shift appears independent of the PI concentration since it can be observed at all tested PI concentrations.
Unusual strong GAPR-1 binding to PI
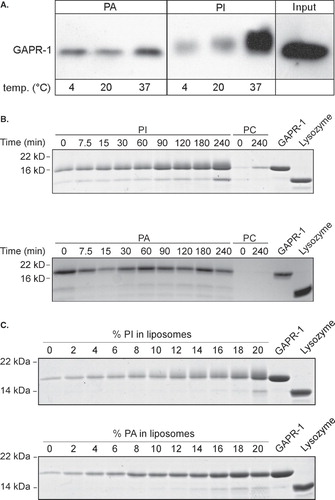
A straightforward explanation for the observed gel-shift of GAPR-1 upon binding to PI-containing liposomes is binding of GAPR-1 to PI. Therefore, it was investigated whether GAPR-1 becomes modified by PI under experimental conditions that result in a gel-shift of GAPR-1. GAPR-1 is a hydrophilic molecule as it is highly charged at neutral pH (pI 9.4). When modified by a lipid, GAPR-1 is expected to acquire hydrophobic properties, similar to that observed for, e.g., glycosylphosphatidylinositol(GPI)-anchored proteins. In phase-partitioning experiments, GPI-anchored proteins partition in the hydrophobic (TX114 detergent) phase, but after removal of the GPI-anchor by phospholipase C, they partition in the hydrophilic (aqueous) phase [Citation22]. A similar TX114 partitioning assay was therefore performed using GAPR-1, both before and after binding to PI-containing liposomes. As expected, before binding to PI-containing liposomes GAPR-1 partitioned in the aqueous phase (). After binding to PI-containing liposomes, however, a large fraction of GAPR-1 partitioned in the detergent phase, indicating a hydrophobic modification of the protein. The observed gel-shift together with the increase in hydrophobicity suggests stable binding of GAPR-1 to a lipid, most likely PI. In contrast, after binding to PA-containing liposomes, GAPR-1 does not partition in the detergent phase ().
Figure 3. GAPR-1 is modified by PI. (A) Non-bound GAPR-1 (lanes 7–9), GAPR-1 that was bound to liposomes containing PI for 100 min at 37°C (lanes 1–3), or GAPR-1 that was bound to liposomes containing PA for 100 min at 37°C (lanes 4–6), were extracted with TX114. GAPR-1 in the aqueous phase (A), detergent phase (D) and total (T) was analyzed using Western blot. (B) GAPR-1 and lysozyme were incubated with liposomes containing radio-labeled PI. After pelleting the liposomes, proteins were resolved on SDS-PAGE and were visualized using coomassie blue (C.B.). Radioactive material in the polyacrylamide gel was visualized by autoradiography (R.A.).
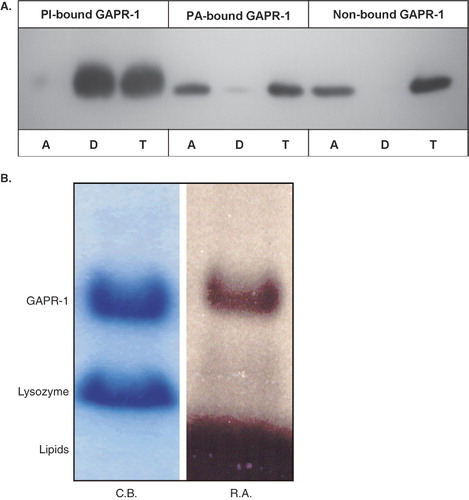
To directly confirm the association of GAPR-1 with PI, GAPR-1, together with lysozyme, was incubated with liposomes containing radiolabeled phosphatidyl[14C]inositol. After incubation, the liposomes were pelleted and GAPR-1 and lysozyme in the pellet were separated by SDS-PAGE and analyzed by coomassie blue staining and autoradiography. Due to experimental conditions (pelleting instead of flotation of liposomes), more lysozyme was recovered in this fraction. Neither the pelleted lysozymal fraction () nor the lysozymal fraction in the supernatant (data not shown) became radioactively labeled. GAPR-1, however, is radioactively labeled, demonstrating that GAPR-1 directly binds to PI. These results do not exclude the possibility that the presence of PI also stimulates GAPR-1 binding to PC. Therefore, GAPR-1 was incubated under similar conditions with PI-containing liposomes with radiolabeled phosphatidyl[14C]choline. Under these conditions, no radioactivity co-migrated with GAPR-1 (data not shown).
The GAPR-1 binding to PI is resistant to SDS-PAGE separation and to denaturing conditions (boiling in SDS-PAGE sample buffer prior to SDS-PAGE separation). In addition, before separation by SDS-PAGE, the samples were routinely subjected to chloroform/methanol extraction to remove excess liposomal lipids that interfere with SDS-PAGE protein separation (Materials and Methods). Thus, the binding of GAPR-1 to PI also resists organic extraction procedures, implying strong binding characteristics.
GAPR-1 can bind multiple PI molecules
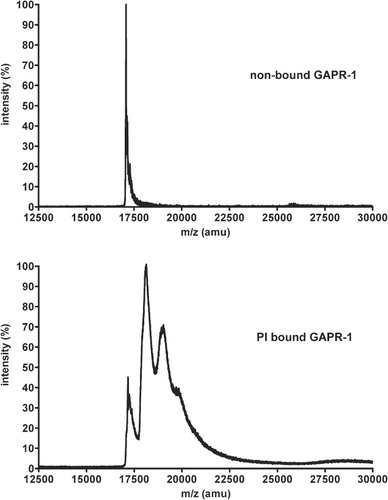
The TX114 analysis (increase in hydrophobicity of GAPR-1) together with the autoradiography experiment (labeling of GAPR-1 with the [14C]inositol-headgroup of PI) suggest binding of the entire PI-molecule to GAPR-1. To show this, GAPR-1 was bound to PI-containing liposomes and was analyzed by MALDI-TOF mass spectroscopy. GAPR-1 that has not been incubated with PI-containing liposomes has the expected m/z value of 17.1 kDa (, top panel). After binding to PI-containing liposomes and subsequent MALDI-TOF analysis of GAPR-1, multiple additional peaks were observed with repeating distances of ∼0.9 kDa corresponding to the molecular weight of a complete phospholipid with two fatty acids and a headgroup (, bottom panel). This indicates that a GAPR-1 molecule can bind up to 3 molecules of PI. In contrast to the well defined GAPR-1 peak (, upper panel), the peaks of GAPR-1 with bound PI-molecules showed peak broadening. This may be explained by the use of PI containing various acyl chains, which add different masses to GAPR-1. It is of note, however, that others have observed broadening of peaks in MALDI-TOF when a lipid with defined acyl chains was coupled to a protein [Citation23]. Probably, the combination of hydrophilic (protein) and hydrophobic (lipid) properties causes interaction with the MALDI matrix resulting in peak diffuseness. The heterogeneous PI binding pattern of GAPR-1 may help to explain why PI-bound GAPR-1 runs as a diffuse band during SDS-PAGE rather than as a sharply defined protein band.
Figure 4. GAPR-1 binds multiple PI molecules. GAPR-1 was incubated with liposomes containing PI, PC and cholesterol (1:9:3) for 90 min at 37°C, after which liposomes were floated on a sucrose gradient. Subsequently, proteins were precipitated using chloroform and methanol. Non-bound GAPR-1 was directly precipitated from stock. After this precipitation, the proteins were resuspended in MALDI matrix suspension and loaded onto the mass spectrometer. Top panel: analysis of purified GAPR-1; bottom panel, GAPR-1 bound to PI-containing liposomes.
Discussion
GAPR-1 interacts with membranes containing negatively charged lipids
We have presented the first evidence that a member of the superfamily of PR-1 proteins can interact with lipids in biological membranes. PR-1 proteins, including the plant PR-1 proteins and the CRISP proteins in humans, contain a conserved SCP-domain and are secreted. It remains to be established whether the secreted homologues also can interact with lipids. There are indications that the PR-1/SCP family also contains a subset of intracellular proteins. However, their subcellular localization remains to be determined [Citation24].
Using a liposome binding assay, we show that non-myristoylated GAPR-1 binds to negatively charged lipids. At neutral pH GAPR-1 is positively charged as it has a calculated pI of 9.4 [Citation3]. The charge is not equally distributed but is concentrated at one side of the protein molecule, resulting in a positively charged surface [Citation4], which may increase its affinity for negatively charged lipids. This may explain the observed differences in lipid binding between GAPR-1 and lysozyme. Lysozyme does not bind to the liposomes, although it has a similar pI as compared to GAPR-1 and it is added to the reaction at 5-fold higher concentrations than GAPR-1. Whereas PI, PS and PG have a relative charge at pH 7.4 of −1.0, PA has a relative charge of −1.3 [Citation25]. This charge difference may also explain the observed binding of lysozyme to PA liposomes. Based on these observations, we propose that the affinity of GAPR-1 for lipids may be determined by both charge and structure (PI) or by charge alone (negatively charged lipids) of the phospholipid head group.
GAPR-1 in vivo is strongly bound to membranes [Citation3], whereas in the binding assay in vitro only a fraction of the total amount of GAPR-1 binds. This suggests that negative membrane lipids are not sufficient for membrane binding and that GAPR-1 requires one or more additional binding signal(s). GAPR-1 in vivo is myristoylated and this modification, together with the lipid interactions described above, may be sufficient for membrane binding according to the two-signal model of Resh [Citation18]. A similar mechanism has been proposed for the MARCKS proteins that are stably anchored to membranes by a myristoyl group and positively charged residues at the surface of the protein [Citation26].
GAPR-1 binding to PI
During screening of the affinity for phospholipid classes, it appeared that GAPR-1 binds strongly to PI. Other proteins known to bind PI include the phosphatidylinositol transfer protein that binds PI to facilitate the intracellular distribution of PI [Citation27,Citation28], and the cytochrome bc1 complex that is stabilized by PI [Citation29]. In these cases, the interaction between PI and the protein are mainly based on hydrophobic and electrostatic interactions, and the lipid can be recovered from the protein only under native conditions. There are also few examples of protein-phospholipid interactions that are partially resistant to strong denaturing conditions. In erythrocytes, Band 3 (AE1) [Citation30] and glycophorin [Citation31] bind multiple phospholipids in a non-covalent but very stable manner, as extraction with chloroform and methanol does not release lipids from glycophorin. Since these proteins contain membrane-spanning domains, interaction with both the lipid head group and the acyl chain is possible.
In contrast to the above described examples, GAPR-1 does not have a transmembrane domain that stabilizes lipid binding via the acyl chain. Nevertheless, PI binding of GAPR-1 is resistant to chloroform/methanol treatment, trichloroacetic Acid (data not shown), TX114 phase separation, SDS-PAGE under reducing conditions and remains intact during mass-spectrometric analysis. Reminiscent of this behaviour are GPI-anchored proteins. A GPI-anchor is a posttranslational modification of proteins with a glycosylphosphatidylinositol. This requires a C-terminal signal sequence, which is cleaved off and replaced by an GPI-anchor upon membrane translocation of the protein (reviewed in [Citation32]). GAPR-1, however, is not translocated to the lumen of the endoplasmic reticulum and does not contain the signal sequence for GPI-anchorage. Another characteristic of GPI-anchored proteins is their sensitivity to phospholipase C. Attempts to release PI from GAPR-1 by phospholipase C were unsuccessful (data not shown). To our knowledge, there are only a few examples of cytosolic proteins that are lipidated by a phospholipid. Ubiquitin becomes attached to membranes after baculovirus infection by a novel type of phospholipid anchor [Citation33,Citation34]. During formation of autophagosomes, the protein Atg3 couples phosphatidylethanolamine (PE) to Atg8 at the C-terminal glycine via an amide bond [Citation35]. In contrast to PE, PI does not have an amine, which makes formation of amide bonds with the protein less likely. In addition, this coupling requires enzymatic activity (Atg3 in the case of PE coupling to Atg8 [Citation35]), which in the case of the liposome binding assay could only be provided by GAPR-1 itself. Therefore, it remains a formal possibility that GAPR-1 itself may have a catalytic activity. Few examples of such a principle are known. The Hedgehog protein autocatalyzes its binding to cholesterol [Citation36]. In this process cysteine-residues are involved, which become irreversibly modified. GAPR-1 contains 2 cysteines, whereas, based on the results from mass spectrometry, GAPR-1 can bind three PI molecules.
All attempts by mass spectrometry, including performing MALDI-MS/MS in the negative mode, to identify a GAPR-1 peptide with lipid bound to it have been unsuccessful so far. The most likely explanation is that upon digestion of the GAPR-1 protein by trypsin prior to MALDI-TOF analysis, the interaction with PI is disrupted. However, we cannot exclude the possibility that PI is covalently linked to GAPR-1. Upon digestion of GAPR-1 into peptides, the potential hydrophobic modification of peptides may hamper the analysis. Others have reported that this modification results in aberrant behaviour of the modified peptide in HPLC or mass spectrometric analysis [Citation37]. Application of MS analyses that allows for the identification of lipid-modified peptides [Citation37] did also not result in the identification of PI-modified GAPR-1 peptides.
It is tempting to speculate on the mechanism of a potential chemical bond formation between GAPR-1 and PI. In case the hydroxyl groups in the inositol ring of PI are involved in the attachment to GAPR-1, the most likely reaction would be the formation of an ester bond after reacting with carboxyl groups from an aspartate or glutamate of GAPR-1. This is an equilibrium reaction, which can be shifted to the product side by excluding water from the reaction. The capacity of GAPR-1 to form dimers may play a role in the formation of a hydrophobic environment, which may exclude water from the reaction and drives the ester formation to the product side. An alternative possibility is that GAPR-1 initially binds to the head group of PI, but that the actual ‘cross-linking’ is to a fatty acid moiety in the membrane. A Computer simulation program that models the interaction of proteins with lipid membranes (MAPAS: Membrane Associated Proteins Assessment (http://cancer-tools.sdsc.edu/MAPAS/pro2.html)) predicts a partial insertion of the protein into the membrane. This raises the possibility that GAPR-1 residues, after binding to the membrane, can interact with the fatty-acid moiety of PI, and reacts with its double bonds. In line with this possibility we observe that PI-binding to GAPR-1 is temperature-dependent. This dependency may suggest that membrane fluidity plays a role. These suggestions are however speculative and we do not (yet) see this modification in vivo. This strongly implies that there are others mechanisms active that regulate/control this modification.
Possible functions of GAPR-1 lipid modification
The most straightforward function for lipid binding to GAPR-1 is the anchoring to biological membranes, much like the GPI moiety of GPI-anchored proteins. The anchoring by PI may also facilitate the localization of GAPR-1 to lipid rafts, similar to GPI-anchored proteins that are also known to partition into lipid rafts [Citation32,Citation38]. Alternatively, PI binding to GAPR-1 may serve the same purpose as PE binding to Atg8, despite mechanistically different binding properties. When PE is coupled to Atg8, the protein becomes membrane-bound, clusters, and changes its conformation [Citation35,Citation39,Citation40]. In an analogous fashion, GAPR-1 may change its conformation by binding to PI and enter an active or inactive state. In this way PI binding of GAPR-1 could act as a molecular switch.
Judged from Western blotting experiments, GAPR-1 isolated from biological material does not show the gel-shift that was observed in the present study [Citation3]. At the Golgi membrane, sufficient PI is available to bind GAPR-1. GAPR-1 shows a slight shift already when bound to liposomes containing 4% PI, whereas Golgi membranes contain 6.5% PI [Citation41]. This may indicate that GAPR-1 in vivo has bound only a small and fixed number of PI molecules or that PI binding is limited to a small pool of GAPR-1. In addition, it is possible that PI modification is a dynamic and reversible event, allowing cells to uncouple GAPR-1 from PI. Finally, as all experiments have been performed with non-myristoylated GAPR-1, we cannot exclude the possibility that myristoylation of GAPR-1 prevents this interaction in vivo.
In conclusion, we have shown that GAPR-1 can bind negatively charged membranes. This behaviour is not only based on electrostatic interactions, as lysozyme does not bind to these membranes. The binding to phosphatidylinositol bears unusual characteristics.
These results suggest that the lipid composition contributes to the GAPR-1 binding to biological membranes. The specificity of these interactions may also be involved in the regulation of the so far unknown function of GAPR-1.
Acknowledgements
We thank Jos Brouwers for help with initial mass spectroscopic analyses of lipids bound to GAPR-1, and Coos Batenburg for critically reviewing our manuscript.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Baxter RM, Crowell TP, George JA, Getman ME, Gardner H. 2007. The plant pathogenesis related protein GLIPR-2 is highly expressed in fibrotic kidney and promotes epithelial to mesenchymal transition in vitro. Matrix Biol 26:20–29.
- Eisenberg I, Barash M, Kahan T, Mitrani-Rosenbaum S. 2002. Cloning and characterization of a human novel gene C9orf19 encoding a conserved putative protein with an SCP-like extracellular protein domain. Gene 293:141–148.
- Eberle HB, Serrano RL, Füllekrug J, Schlosser A, Lehmann WD, Lottspeich F, Kaloyanova D, Wieland FT, Helms JB. 2002. Identification and characterization of a novel human plant pathogenesis-related protein that localizes to lipid-enriched microdomains in the Golgi complex. J Cell Sci 115:827–838.
- Serrano RL, Kuhn A, Hendricks A, Helms JB, Sinning I, Groves MR. 2004. Structural analysis of the human Golgi-associated plant pathogenesis related protein GAPR-1 implicates dimerization as a regulatory mechanism. J Mol Biol 339:173–183.
- Gibbs GM, Roelants K, O'Bryan MK. 2008. The CAP superfamily: cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins--roles in reproduction, cancer, and immune defense. Endocr Rev 29:865–897.
- Busso D, Goldweic NM, Hayashi M, Kasahara M, Cuasnicú PS. 2007. Evidence for the involvement of testicular protein CRISP2 in mouse sperm-egg fusion. Biol Reprod 76:701–708.
- Milne TJ, Abbenante G, Tyndall JDA, Halliday J, Lewis RJ. 2003. Isolation and characterization of a cone snail protease with homology to CRISP proteins of the pathogenesis-related protein superfamily. J Biol Chem 278:31105–31110.
- Wang F, Li H, Liu M, Song H, Han H, Wang Q, Yin C, Zhou Y, Qi Z, Shu Y, Lin Z, Jiang T. 2006. Structural and functional analysis of natrin, a venom protein that targets various ion channels. Biochem Biophys Res Commun 351:443–448.
- Wang J, Shen B, Guo M, Lou X, Duan Y, Cheng XP, Teng M, Niu L, Liu Q, Huang Q, Hao Q. 2005. Blocking effect and crystal structure of natrin toxin, a cysteine-rich secretory protein from Naja atra venom that targets the BKCa channel. Biochemistry 44:10145–10152.
- Yamazaki Y, Koike H, Sugiyama Y, Motoyoshi K, Wada T, Hishinuma S, Mita M, Morita T. 2002. Cloning and characterization of novel snake venom proteins that block smooth muscle contraction. Eur J Biochem 269:2708–2715.
- Rosenzweig T, Ziv-Av A, Xiang C, Lu W, Cazacu S, Taler D, Miller CG, Reich R, Shoshan Y, Anikster Y, Kazimirsky G, Sarid R, Brodie C. 2006. Related to testes-specific, vespid, and pathogenesis protein-1 (RTVP-1) is overexpressed in gliomas and regulates the growth, survival, and invasion of glioma cells. Cancer Res 66:4139–4148.
- Cohen DJ, Busso D, Da Ros V, Ellerman DA, Maldera JA, Goldweic N, Cuasnicu PS. 2008. Participation of cysteine-rich secretory proteins (CRISP) in mammalian sperm-egg interaction. Int J Dev Biol 52:737–742.
- van Loon LC, van Strien EA. 1999. The families of plant pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol 55:85–97.
- Szyperski T, Fernández C, Mumenthaler C, Wüthrich K. 1998. Structure comparison of human glioma pathogenesis-related protein GliPR and the plant pathogenesis-related protein P14a indicates a functional link between the human immune system and a plant defense system. Proc Natl Acad Sci USA 95:2262–2266.
- Gkantiragas I, Brügger B, Stüven E, Kaloyanova D, Li X, Löhr K, Lottspeich F, Wieland FT, Helms JB. 2001. Sphingomyelin-enriched microdomains at the Golgi complex. Mol Biol Cell 12:1819–1833.
- Li S, Shin Y, Cho KW, Merzdorf CS. 2006. The Xfeb gene is directly upregulated by Zic1 during early neural development. Dev Dyn 235:2817–2827.
- Peitzsch RM, McLaughlin S. 1993. Binding of acylated peptides and fatty acids to phospholipid vesicles: Pertinence to myristoylated proteins. Biochemistry 32:10436–10443.
- Resh MD. 1999. Fatty acylation of proteins: New insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta 1451:1–16.
- Taniguchi H. 1999. Protein myristoylation in protein-lipid and protein-protein interactions. Biophys Chem 82:129–137.
- Groves MR, Kuhn A, Hendricks A, Radke S, Serrano RL, Helms JB, Sinning I. 2004. Crystallization of a Golgi-associated PR-1-related protein (GAPR-1) that localizes to lipid-enriched microdomains. Acta Crystallogr D Biol Crystallogr 60:730–732.
- Rouser G, Fkeischer S, Yamamoto A. 1970. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 5:494–496.
- Bordier C. 1981. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem 256:1604–1607.
- Gubbens J, Vader P, Damen JMA, O'Flaherty MC, Slijper M, de Kruijff B, de Kroon AIPM. 2007. Probing the membrane interface-interacting proteome using photoactivatable lipid cross-linkers. J Proteome Res 6:1951–1962.
- Kovalick GE, Griffin DL. 2005. Characterization of the SCP/TAPS gene family in Drosophila melanogaster. Insect Biochem Mol Biol 35:825–835.
- Marsh D. CRC Handbook of lipid bilayers. 1st edn. Boca Raton, FL: CRC Press; 1990.
- McLaughlin S, Aderem A. 1995. The myristoyl-electrostatic switch: A modulator of reversible protein-membrane interactions. Trends Biochem Sci 20:272–276.
- Cockcroft S, Carvou N. 2007. Biochemical and biological functions of class I phosphatidylinositol transfer proteins. Biochim Biophys Acta 1771:677–691.
- Wirtz KWA. 1991. Phospholipid transfer proteins. Annu Rev Biochem 60:73–99.
- Lange C, Nett JH, Trumpower BL, Hunte C. 2001. Specific roles of protein-phospholipid interactions in the yeast cytochrome bc1 complex structure. Embo J 20:6591–6600.
- Maneri LR, Low PS. 1989. Fatty acid composition of lipids which copurify with band 3. Biochem Biophys Res Commun 159:1012–1019.
- van Zoelen EJJ, Verkleij AJ, Zwaal RFA, van Deenen LLM. 1978. Incorporation and asymmetric orientation of glycophorin in reconstituted protein-containing vesicles. Eur J Biochem 86:539–546.
- Mayor S, Riezman H. 2004. Sorting GPI-anchored proteins. Nat Rev Mol Cell Biol 5:110–120.
- Webb JH, Mayer RJ, Dixon LK. 1999. A lipid modified ubiquitin is packaged into particles of several enveloped viruses. FEBS Lett 444:136–139.
- Guarino LA, Smith G, Dong W. 1995. Ubiquitin is attached to membranes of baculovirus particles by a novel type of phospholipid anchor. Cell 80:301–309.
- Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, Noda T, Ohsumi Y. 2000. A ubiquitin-like system mediates protein lipidation. Nature 408:488–492.
- Porter JA, Young KE, Beachy PA. 1996. Cholesterol modification of hedgehog signaling proteins in animal development. Science 274:255–259.
- Ujihara T, Sakurai I, Mizusawa N, Wada H. 2008. A method for analyzing lipid-modified proteins with mass spectrometry. Anal Biochem 374:429–431.
- Simons K, Ikonen E. 1997. Functional rafts in cell membranes. Nature 387:569–572.
- Kirisako T, Ichimura Y, Okada H, Kabeya Y, Mizushima N, Yoshimori T, Ohsumi M, Takao T, Noda T, Ohsumi Y. 2000. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J Cell Biol 151:263–276.
- Ichimura Y, Imamura Y, Emoto K, Umeda M, Noda T, Ohsumi Y. 2004. In vivo and in vitro reconstitution of Atg8 conjugation essential for autophagy. J Biol Chem 279:40584–40592.
- Higgins JA, Hitchin BW, Low MG. 1989. Phosphatidylinositol-specific phospholipase C of Bacillus thuringiensis as a probe for the distribution of phosphatidylinositol in hepatocyte membranes. Biochem J 259:913–916.