Abstract
Novel compound heterozygous mutations, G701D, a recessive mutation, and A858D, a mild dominant mutation, of human solute carrier family 4, anion exchanger, member 1 (SLC4A1) were identified in two pediatric patients with distal renal tubular acidosis (dRTA). To examine the interaction, trafficking, and cellular localization of the wild-type and two mutant kidney AE1 (kAE1) proteins, we expressed the proteins alone or together in human embryonic kidney (HEK) 293T and Madin-Darby canine kidney (MDCK) epithelial cells. In individual expressions, wild-type kAE1 was localized at the cell surface of HEK 293T and the basolateral membrane of MDCK cells. In contrast, kAE1 G701D was mainly retained intracellularly, while kAE1 A858D was observed intracellularly and at the cell surface. In co-expression experiments, wild-type kAE1 formed heterodimers with kAE1 G701D and kAE1 A858D, and promoted the cell surface expression of the mutant proteins. The co-expressed kAE1 G701D and A858D could also form heterodimers but showed predominant intracellular retention in HEK 293T and MDCK cells. Thus impaired trafficking of the kAE1 G701D and A858D mutants would lead to a profound decrease in functional kAE1 at the basolateral membrane of α-intercalated cells in the distal nephron of the patients with dRTA.
Introduction
Solute carrier family 4, anion exchanger, member 1 (SLC4A1) or anion exchanger 1 (AE1) encodes both erythroid AE1 (eAE1) and kidney AE1 (kAE1), which mediates chloride/bicarbonate (Cl−/HCO−3) exchange in red cells and acid-secreting ∝-intercalated cells of renal collecting ducts, respectively. Erythroid AE1 also regulates red cell morphology through interactions with the underlying membrane cytoskeleton [Citation1,Citation2]. Thus SLC4A1 mutations can result in morphological abnormalities of red cells, anion transport defects in red cells, and/or impaired acid secretion in the kidney causing distal renal tubular acidosis (dRTA) [Citation3,Citation4]. The SLC4A1 mutations have been found to be associated with either autosomal dominant (AD) or autosomal recessive (AR) dRTA, determined by mutational sites and trafficking behaviors of the mutant proteins [Citation5,Citation6]. AR dRTA may result from either homozygosity of a single SLC4A1 mutation or compound heterozygosity of two different SLC4A1 mutations. Several reported genotypes include: G701D/G701D, SAO/G701D, SAO/delV850, SAO/A858D, delV850/delV850, V488M/V488M, G701D/S773P and SAO/Q759H [Citation7–13]. The SLC4A1 G701D recessive mutation (band 3 Bangkok I), first described in a Thai family [Citation11], was frequently observed in Southeast Asia in both homozygote and compound heterozygote with Southeast Asian ovalocytosis (SAO) mutation [Citation5,Citation7,Citation8,Citation12,Citation14]. Heterozygous individual expressing the A858D mutation were symptomless but unable to acidify their urine when challenged with frusemide/fludrocortisone and were classified as incomplete dRTA, although this test is not the ‘gold standard’ as oral ammonium chloride administration test for dRTA. The mild dominant phenotype for the A858D mutation is in contrast to the complete dRTA induced by other dominant dRTA mutations such as R589H. Recently, we have identified two pediatric patients with AR dRTA caused by novel compound heterozygous SLC4A1 G701D/A858D mutations in a Thai family [Citation14,Citation15]. To better understand the molecular mechanism of the disease caused by this compound heterozygous SLC4A1 mutations, we investigated the interaction, trafficking and cellular localization of mutant kidney AE1 (kAE1) proteins (G701D and A858D) expressed alone or together in human embryonic kidney (HEK) 293T and Madin-Darby canine kidney (MDCK) epithelial cells.
Materials and methods
Plasmid constructs and mutagenesis
Plasmid constructs and mutagenesis for experiments in HEK 293T cells
Recombinant plasmids, pcDNA3-kAE1 WT-His, pcDNA3-kAE1 WT-HA, pcDNA3-kAE1 WT-Myc, pcDNA3-kAE1 G701D-His, pcDNA3-kAE1 G701D-HA, and pcDNA3-kAE1 G701D-Myc were constructed as previously described [Citation16]. pcDNA3-kAE1 A858D-His, pcDNA3-kAE1 A858D-HA and pcDNA3 kAE1 A858D-Myc were generated by site-directed mutagenesis.
Plasmid constructs and mutagenesis for experiments in MDCK epithelial cells
Retroviral expression plasmid pFBneo-kAE1HA557 was constructed by shuttling the cDNA encoding the entire human kidney anion exchanger carrying an external hemagglutinin (HA) tag inserted into position 557 [Citation17] into the Xho I site of the retroviral expression vector pFBneo (Stratagene, La Jolla, CA). The pFBneo-kAE1HA557 mutants were constructed using Stratagene QuickChange site-directed mutagenesis kit and confirmed by automated sequencing (ACGT, Toronto, Canada).
Expression studies in HEK 293T cells
Cell culture and transfection
Cultured HEK 293T cells were transfected with 1 μg of recombinant plasmid DNA per 3.5 cm well either by using Lipofectamine 2000 (Invitrogen, USA) or the DEAE-dextran method as previously described [Citation16]. The HEK 293T cells were individually transfected with each of the recombinant constructs, and were also co-transfected with pcDNA-kAE1 WT-His and pcDNA-kAE1 G701D-HA (or pcDNA-kAE1 A858D-HA), pcDNA-kAE1 G701D-His and pcDNA-kAE1 A858D-HA, pcDNA-kAE1 G701D-HA and pcDNA-kAE1 A858D-His.
SDS-PAGE and Western blot analysis
The kAE1 and mutant proteins were analyzed by 10% SDS-PAGE and Western blot analysis as previously described [Citation16]. Briefly, the transfected HEK 293T cells were solubilized with lysis buffer [1mM EDTA, 0.5% (v/v) Igepal (Nonidet P-40 detergent), 150 mM NaCl, 0.2% (v/v) bovine serum albumin, 10 mM Tris-HCl pH 7.5 and protease inhibitor cocktail]. After centrifugation to remove insoluble material, whole cell extracts were subjected to SDS gel electrophoresis and electroblotted to nitrocellulose membranes. The proteins were detected by incubating the membranes with primary antibodies (anti-His, anti-HA or anti-Myc), then with the secondary antibody conjugated to horseradish peroxidate (HRP), and detected with SuperSignal West Pico Chemiluminescent Substrate (Pierce, USA) plus Western Blotting Detection System. The chemiluminescence signal was captured by exposing the membrane to an X-ray film, which was then developed to reveal the immunoreactive bands.
Affinity co-purification
HEK 293T cells were individually transfected with the plasmid constructs or were co-transfected with four different pairs of the constructs described above. Two days after transfection, the cells were washed, lysed, and the lysate incubated with 40 μl of washed Co2+ chelate resins (BD Bioscience, USA) at 4°C for 12–16 h as previously described [Citation16]. After incubation, the resin was collected and washed thoroughly. 6×Histidine-tagged-kAE1 proteins that bound to Co2+ chelate resins were eluted with 2× SDS-PAGE sample loading buffer containing 2% (v/v) 2-mercaptoethanol and heated at 65°C for 5 min. The samples were subjected to electrophoresis on 10% SDS-PAGE and the co-purified kAE1-HA proteins were detected by Western blot analysis using anti-HA antibody and HRP-conjugated secondary antibody.
Co-immunoprecipitation
The transfected and co-transfected HEK 293T cells were prepared as mentioned in the previous section and processed as previously described [Citation16]. The non-specific binding proteins in the cell lysate were removed by Protein G-Sepharose beads and recovered by centrifugation. The supernatant was then used for immunoprecipitation by adding anti-6×His antibody and then Protein G-Sepharose beads. The mixture was incubated at 4°C with shaking for 12–16 h and then the beads were collected and washed thoroughly. Protein complexes were eluted with 2× SDS-PAGE sample loading buffer and heated at 65°C for 5 min. The protein complexes were analyzed by 10% SDS-PAGE and Western blot method using anti-HA antibody and HRP conjugated secondary antibody.
Immunofluorescence and confocal microscopy
HEK 293T cells were grown on cover glasses for transfection and co-transfection with the recombinant plasmid constructs. To examine cellular localization of HA-tagged or Myc-tagged kAE1, 48 h after transfection, the transfected HEK 293T cells were processed as previously described [Citation16]. Briefly, the transfected HEK 293T cells were washed with PBS and fixed with 4% paraformaldehyde in PBS. After washing with 100 mM glycine in PBS, the cells were permeabilized with 0.2% Triton X-100. The cells were then incubated with primary antibody followed by secondary antibody conjugated with a fluorescein dye. Localization of the expressed proteins was examined by a confocal microscope (Zeiss LSM510 META, Germany).
Flow cytometry
Myc or HA epitopes were inserted into the third extracellular loop of kAE1, known to have no effect on kAE1 conformation or transport function, to allow detection of the recombinant protein on the surface of intact cells. Thus, expression of kAE1-Myc or kAE1-HA on the cell surface could be determined by fluorescence staining and flow cytometry as previously described [Citation16]. Briefly, 2 days after transfection, the cells were collected and re-suspended in chilled DMEM containing 2% fetal bovine serum. Then, the cells were fixed, washed and incubated with mouse anti-Myc or rabbit anti-HA antibody for 1 h. After incubation the cells were washed twice with chilled DMEM containing 2% fetal bovine serum. Then, goat anti-mouse antibody conjugated with Alexa 488 or donkey anti-rabbit antibody conjugated with Cy3 (Molecular Probes, Eugene, Or, USA) was used to probe mouse anti-Myc or rabbit anti-HA antibody, respectively. The cells were washed and analyzed by using FACSortTM flow cytometer (Becton-Dickinson, USA).
Expression studies in MDCK cells
Cell culture
MDCK cells stably expressing kAE1 proteins were generated as described previously [Citation18]. Briefly, HEK 293T cells were cotransfected with three retroviral plasmids pVpack-GP, pVpack-VSVG (vesicular stomatitis virus glycoprotein), and pFBNeo-kAE1 HA557 using FuGene 6 Transfection reagent (Roche Diagnostics, Indianapolis, IN). The virus-containing supernatant was collected 24–36 h later and added to 30–50% confluent MDCK cells, in the presence of 8 μg/ml of polybrene (Sigma, St. Louis, MO). The infected cells were selected with 1 mg/ml geneticin (G418, Sigma). Polarized MDCK cells were obtained by growing confluent infected cells on Transwell polycarbonate filters (Corning Headquarters, Corning, NY) for 4–5 days. As previously described, the viral infection results in a heterogenous population of kAE1-expressing cells [Citation19].
Western blot analysis
SDS-PAGE and Western blotting were performed as previously described [Citation20]. Briefly, infected MDCK cells were lysed in PBS containing 1% C12E8 detergent and protease inhibitors leupeptin (1 μM), aprotinin (1 μM), PMSF (200 μM), and pepstatin A (1 μM). After centrifugation to remove insoluble material, 15 μg of total protein per lane were loaded and resolved on 8% SDS-PAGE and transferred to a nitrocellulose membrane. The blotted proteins were detected with a mouse anti-HA monoclonal antibody (Covance, Princeton, NJ), followed by an anti-mouse antibody coupled to horseradish peroxidase.
Co-immunoprecipitation
Co-immunoprecipitations were performed as previously described [Citation19]. Briefly, co-infected MDCK cells expressing HA- and Myc-tagged proteins were lysed in 0.5 ml of PBS containing 1% C12E8 and centrifuged to remove insoluble materials. An aliquot was saved as total fraction while the remaining supernatant was incubated with 10 μl rabbit anti-Myc antibody followed by 40 μl protein G-Sepharose. After three washes, the protein was eluted with Laemmli buffer, resolved by SDS-PAGE, blotted and detected with a mouse anti-HA antibody followed by an anti-mouse antibody coupled to horseradish peroxidase.
Immunocytochemistry
MDCK cells were grown on glass coverslips, or on semipermeable Transwell polycarbonate filters (Corning Headquarters, Corning, NY) to confluency for 4–5 days. Cells were fixed using 3.8% formaldehyde for 15 min, then washed once with 100 mM glycine, permeabilized with 0.2% Triton X-100 for 15 min and blocked for 30 min with 1% BSA. A 1/1,000 mouse anti-HA antibody (Covance) and 1/500 rabbit anti-Myc antibody (Stressgen Biotechnologies, San Diego, CA) in 1% BSA were added to the sample for 30 min. After several washes, 1/1,000 of Alexa 488-conjugated anti-mouse antibody (Molecular Probes, Eu- gene, OR) or Cy3-conjugated anti-rabbit antibody (Jackson Immunoresearch Laboratories, West Grove, PA) was added for 30 min before observation using a Zeiss laser confocal microscope LSM 510. For co-expression experiments, the co-infected cells were fixed and permeabilized using the same protocol as above described. The samples were then incubated with 1/1,000 mouse anti-HA antibody (Covance, Princeton, NJ) and 1/500 rabbit polyclonal anti-Myc antibody (Santa Cruz Biotechnologies, Santa Cruz, CA), followed, after several washes, by 1/1,000 of Alexa 488-conjugated anti-mouse antibody (Molecular Probes, Eugene, OR) and Cy3-conjugated anti-rabbit antibody (Jackson Immunoresearch Laboratories). Samples were examined using a Zeiss laser confocal microscope LSM 510.
Results
We have recently identified two pediatric patients in a Thai family with dRTA caused by novel compound heterozygous SLC4A1 G701D/A858D mutations [Citation14]. Here, we have conducted in vitro studies to examine the molecular defect associated with this compound heterozygous condition. HEK 293T and MDCK cells were transfected with recombinant plasmids expressing tagged versions (HA, Myc, His) of wild-type kAE1, kAE1 G701D or kAE1 A858D, or co-transfected with all pairs of plasmids. Protein expression was examined by Western blotting; protein dimerization by affinity co-purification and co-immunoprecipitation; and subcellular localization by immunofluorescence staining and confocal microscopy. Additionally, cell surface expression of wild-type and mutant kAE1 tagged with either Myc or HA epitope was analyzed by flow cytometry.
HEK 293T cells
Expression and interaction of wild-type and mutant kAE1 proteins in HEK 293T cells
HEK 293T cells transfected with the recombinant plasmids expressed His-, Myc- or HA-tagged kAE1 WT, kAE1 G701D, or kAE1 A858D produced similar levels of immuno-reactive proteins with the estimated molecular weight (MW) of ∼96 kDa (). Thus, the dRTA mutants are not targeted for rapid degradation in transfected HEK cells.
Figure 1. (A) Western blot analysis of wild-type and mutant kAE1 proteins, tagged with 6xHis or Myc or HA, expressed in HEK 293T cells, (B) affinity co-purification, and (C) co-immunoprecipitation of wild-type and mutant kAE1 proteins in HEK 293T cells. HEK 293T cells transfected or co-transfected with the plasmid constructs expressing kAE1-His and kAE1-HA were lysed and 6xHis-tagged oligomers were purified by Co2+ resin or immunoprecipitated (IP) by mouse anti-His antibody followed by Protein G-sepharose. The bound protein was eluted by 2× Laemmli buffer and detected by Western blot analysis using rabbit anti-HA antibody. Lanes 1–2 are individually expressed kAE1 WT-HA and kAE1 WT-His, respectively. Lanes 3–5 are kAE1 WT-His co-expressed with kAE1 WT-HA, kAE1 G701D-HA, and kAE1 A858D-HA, respectively. Lane 6 is kAE1 G701D-His co-expressed with kAE1 A858D-HA. Lane 7 is kE1 A858D-His co-expressed with kAE1 G701D-HA. The results show that kAE1 WT-His could interact with either kAE1 WT-HA, kAE1 G701D-HA, or kAE1 A858D-HA. In addition, kAE1 G701D-His could interact with kAE1 A858D-HA, and kAE1 A858D-His could interact with kAE1 G701D-HA.
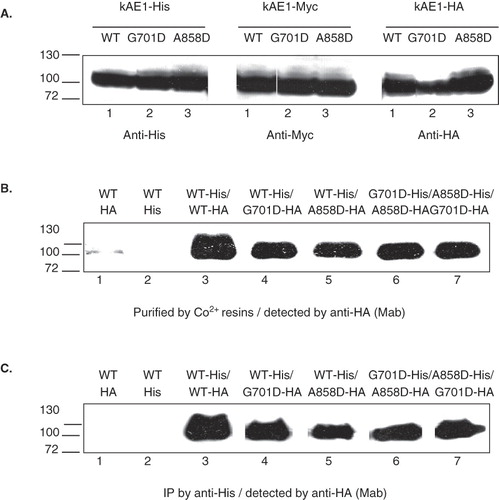
The interactions between kAE1 WT and kAE1 G701D or kAE1 A858D, and between kAE1 G701D and kAE1 A858D co-expressed in HEK 293T cells were examined by affinity co-purification and co-immunoprecipitation. His-tagged kAE1 WT or mutants were purified using Co2+ resins and interacting HA-tagged proteins were detected by immunoblot using anti-HA antibody (). The purified fraction from control HEK 293T cells expressing only kAE1 WT-HA or kAE1 WT-His did not show any kAE1 WT bands on the HA blot as expected (, lanes 1 and 2). The purified fraction from HEK 293T cells co-expressing kAE1 WT-His and kAE1 WT-HA showed heterodimer formation between the two tagged kAE1 proteins (, lane 3). When kAE1 WT-His was co-expressed with kAE1 G701D-HA or kAE1 A858D-HA, it could form heterodimers with the dRTA mutants (, lanes 4 and 5).
To mimic the compound heterozygous kAE1 G701D and kAE1 A858D mutations, the cells were co-transfected to express kAE1 G701D-His and kAE1 A858D-HA or kAE1 A858D-His and kAE1 G701D-HA. Co-expression of the two dRTA mutants showed that they could interact with each other (, lanes 6 and 7). The pull-down experiments show that kAE1 WT is able to form heterodimers with the dRTA mutants, but also that the dRTA mutants can form heterodimers.
Co-immunoprecipitation experiments showed the same results when kAE1 proteins were immunoprecipitated with anti-His antibody and associated HA-tagged kAE1 proteins were detected by immunoblotting with anti-HA antibody (). The co-purification and co-immunoprecipitation results indicate that the two dRTA mutations do not seriously affect the protein folding and interactions responsible for forming kAE1 dimers.
Sub cellular localization of wild-type and mutant kAE1 dRTA proteins in HEK 293T cells
The subcellular localization of wild-type and mutant kAE1 proteins in HEK 293T cells was examined by using immunofluorescence and confocal microscopy. Transfected HEK 293T cells expressing either tagged kAE1 WT-Myc or kAE1 WT-HA showed predominant expression at the cell surface () as previously described [Citation16,Citation21,Citation22]. The kAE1 G701D was mainly localized intracellularly with little or no cell surface localization () as previously reported [Citation18,Citation19]. kAE1 A858D-Myc or kAE1 A858D-HA was observed both at the cell surface and intracellularly (). These results indicate that kAE1 WT can traffic efficiently to the cell surface in transfected HEK 293T cells. The kAE1 A858D mutant can also traffic to the cell surface, but not as efficiently as the wild-type kAE1. In contrast, the kAE1 G701D mutant fails to traffic to the cell surface.
Figure 2. (A) Immunofluorescence staining of kAE1 WT, kAE1 G701D, and kAE1 A858D, tagged with Myc or HA, expressed in HEK 293T cells, (B) immunofluorescence staining of kAE1 WT-Myc co-expressed with either kAE1 WT-HA, kAE1 G701D-HA, or kAE1 A858D-HA, and (C) immunofluorescence staining of kAE1 G701D-Myc co-expressed with kAE1 A858D-HA, and kAE1 A858D-Myc co-expressed with kAE1 G701D-HA in HEK 293T cells. Transiently transfected or co-transfected HEK 293T cells expressing kAE1-Myc or kAE1-HA were fixed and permeabilized and then stained with anti-Myc or anti-HA antibody followed by anti-mouse IgG-Alexa 488 antibodies (green) or anti-rabbit IgG-Cy3 (red). Yellow color indicates co-localized proteins. Immunofluorescence images were captured by using Zeiss LSM510 META confocal microscopy. (This figure is reproduced in colour in Molecular Membrane Biology online.)
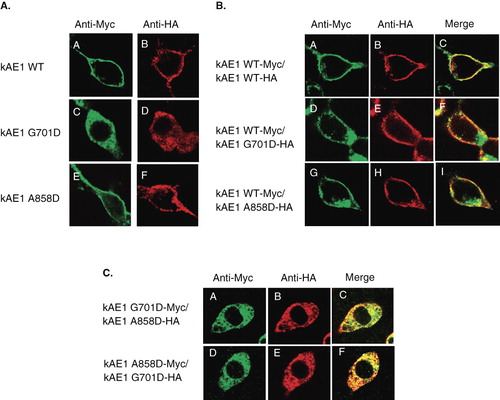
Co-expression and localization of wild-type and mutant kAE1 proteins in HEK 293T cells
We next determined the effect of the dRTA mutants on the wild-type kAE1 trafficking and localization and vice versa, and the effect of the two dRTA mutants on each other using co-expression studies. Co-expression of kAE1 WT-Myc with kAE1 WT-HA showed cell surface expression and co-localization of both kAE1 proteins as expected (). Co-expression of kAE1 WT-Myc with kAE1 G701D-HA or kAE1 WT-Myc with kAE1 A858D-HA showed cell surface expression and co-localization of wild-type and mutant kAE1 proteins (). There was a strong co-localization of kAE1 WT with the kAE1 A858D mutant at the cell surface with little intracellular staining of the kAE1 A858D mutant. While the kAE1 A858D mutant can traffic to the cell surface when expressed alone, its trafficking is facilitated when co-expressed with kAE1 WT. There was also co-localization of kAE1 WT with the kAE1 G701D mutant at the cell surface, indicating that kAE1 WT could rescue the cell surface trafficking of the kAE1 G701D mutant, In contrast to the kAE1 A858D mutant, the kAE1 G701D mutant retained a predominant intracellular staining even when co-expressed with kAE1 WT. These results indicate that kAE1 WT facilitates the trafficking of kAE1 A858D and kAE1 G701D to the cell surface, but the rescue of kAE1 G701D is less complete. This rescue effect likely occurs through the formation of heterodimers in the endoplasmic reticulum. The heterodimers that contain a wild-type subunit can exit the ER more efficiently and traffic to the cell surface than the mutant homodimers.
To mimic the compound heterozygous SLC4A1 G701D and A858D mutations found in the patients, kAE1 G701D-Myc and kAE1 A858D-HA or A858D-myc and kAE1 G701D-HA were co-expressed in HEK 293T cells. The two dRTA mutants were largely expressed intracellularly with poor localization at the plasma membrane (). Unlike wild-type kAE1, the kAE1 A858D mutant that could traffic to the cell surface when expressed alone, but was unable to rescue kAE1 G701D trafficking to the cell surface. In this case the kAE1 G701D had a dominant effect on the kAE1 A858D mutant, retaining it intracellularly. These results indicate that the wild-type kAE1 could rescue both mutant dRTA kAE1 proteins from intracellular retention to the plasma membrane but that kAE1 G701D and kAE1 A858D fail to rescue each other.
Analysis of individual expression and co-expression of kAE1-Myc and kAE1-HA tagged protein by flow cytometry in HEK 293T cells
Myc or HA epitopes were inserted in the third extracellular loop at the position 557 of kAE1 to allow immuno-detection of intact cells expressing the protein at the cell surface. The HEK 293T cells that individually expressed kAE1 WT-Myc, kAE1 G701D-Myc or kAE1 A858D-Myc had mean fluorescence intensities of 34.57 ± 2.24, 1.14 ± 0.35 and 7.08 ± 1.12, respectively (). This confirms the low level of cell surface expression of the dRTA mutants, especially the G701D mutant, relative to wild-type kAE1.
Figure 3. Cell-surface expression of Myc557-tagged kAE1 in transfected HEK 293T cells measured by flow cytometry. The transfected cells were incubated with mouse anti-Myc antibody followed by anti-mouse immunoglobulin G antibody conjugated with Alexa 488. Fluorescence intensity was detected by flow cytometry with kAE1-positive cells showing heterogeneous expression above background levels. (A) HEK 293T expressing cells kAE1 WT-Myc (green), kAE1 G701D-Myc (pink), and kAE1 A858D-Myc (blue). (B) HEK 293T cells expressing kAE1 WT-Myc and kAE1 WT-HA (orange), kAE1 WT-Myc and kAE1 G701D-HA (blue), kAE1 WT-Myc and kAE1 A858D-HA (red), kAE1 G701D-HA and kAE1 A858D-HA (purple), and kAE1 A858D-Myc and kAE1 G701D-HA (greenish blue). The experiments were conducted in triplicates and percentages of cell-surface expression of Myc557-tagged kAE1 (mean ± SD) were shown. (This figure is reproduced in colour in Molecular Membrane Biology online.)
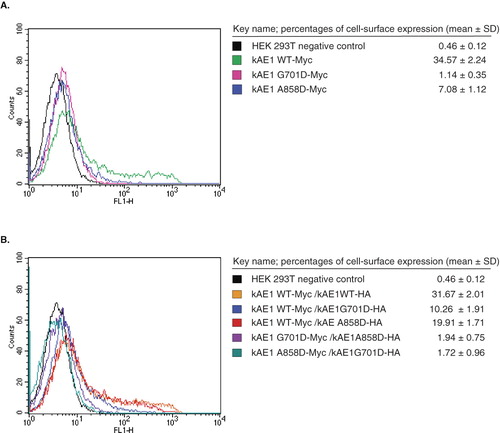
HEK 293T cells co-expressing kAE1 WT-Myc with either kAE1 G701D-HA (10.26 ± 1.91) or kAE1 A858D-HA (19.91 ± 1.71) showed higher levels of cell surface expression of the mutants than when expressed alone. This indicates that kAE1 WT is able to rescue the cell surface expression of both dRTA mutants. The level of cell surface expression was still less than that of kAE1 WT-HA (31.67 ± 2.01) (), indicating that the rescue was not complete, especially in the case of the kAE1 G701D. The transfected cells co-expressing both kAE1 G701D-Myc and kAE1 A858D-HA or A858D-myc and kAE1 G701D-HA presented very low signal levels (1.94 ± 0.75 and 1.72 ± 0.96) when compared to when the mutants were co-expressed with kAE1 WT (). These results indicate that kAE1 A858D has a more severe trafficking impairment when co-expressed with kAE1 G701D than when expressed alone. Furthermore, the kAE1 A858D mutant could not rescue kAE1 G701D trafficking to the cell surface. In contrast, kAE1 WT could rescue both kAE1 G701D and kAE1 A858D mutant proteins to the cell surface with more efficient rescue of the kAE1 A858D mutant.
MDCK cells
Expression and heterodimerization of wild-type and mutant kAE1 proteins in MDCK cells
To determine the expression of kAE1 G701D and kAE1 A858D mutant proteins tagged with HA in MDCK epithelial cells, immunoblot analyses of whole cell extracts using anti-HA antibody () or anti-kNt-AE1 antibody () were performed. The immunoblot in shows the HA-tagged proteins, while that in shows the full complement of expressed kAE1 proteins using an anti-kNt-kAE1 antibody. The immunoblot results show that the wild-type and mutant kAE1 proteins could be expressed and detected in MDCK cells with the wild-type protein showing higher expression levels relative to the dRTA mutants. The wild-type kAE1 protein ran as two closely-spaced bands ( and B, lanes 1), a major upper band and a minor lower band. Enzymatic deglycosylation experiments (not shown) confirmed previous results [Citation19] that the upper band contained complex oligosaccharide that had exited the ER, while the lower band contained high mannose oligosaccharide. The kAE1 G701D contained about equal amounts of the two glyco-forms, indicating impaired exit from the ER. The kAE1 A858D contained a higher proportion of complex oligosaccharide than the kAE1 G701D mutant, suggesting a less severe impairment in ER exit.
Figure 4. Western blot analysis of total cell extracts of HA557- or Myc557-kAE1 WT, G701D, and A858D (A), and co-immunoprecipitation studies (B and C). MDCK cells were infected or co-infected for 12 days and lysed by 1% C12E8 buffer. The cell extracts were loaded onto 8% SDS-PAGE and transferred to nitrocellulose membrane. For co-immunoprecipitation, the cell extract was incubated with mouse anti-HA or rabbit anti-Myc antibodies followed by Protein G-Sepharose. The bound proteins were eluted with 2× Laemmli buffer and detected by Western blot. (A) The total expressed kAE1 protein was detected by mouse anti-HA followed by anti-mouse IgG-HRP antibodies. (B) The total expressed kAE1 protein was detected by rabbit anti-kNt-AE1 antibody. (C) HA557-kAE1 WT (lane 1), G701D (lane 2) and A858D (lane 3) were co-immunoprecipitated (Co-IP) by mouse anti-HA antibody and detected by rabbit anti-kNt-AE1 antibody followed anti-rabbit IgG-HRP conjugated antibody. (D) Co-IP of Myc557-kAE1 WT with kAE1 G701D-HA (lane 4), Myc557-kAE1 WT with kAE1 A858D-HA (lane 5), kAE1 A858D-Myc with kAE1 G701D-HA (lane 6), kAE1 G701D-HA with kAE1 A858D-Myc (lane 7). The samples of lanes 4–6 were co-immunoprecipitated by rabbit anti-Myc, detected by mouse anti-HA and followed by anti mouse IgG-HRP antibody. The sample of lane 7 was co-immunoprecipitated by mouse anti-HA, detected by rabbit anti-myc, and followed by anti rabbit IgG-HRP antibody.
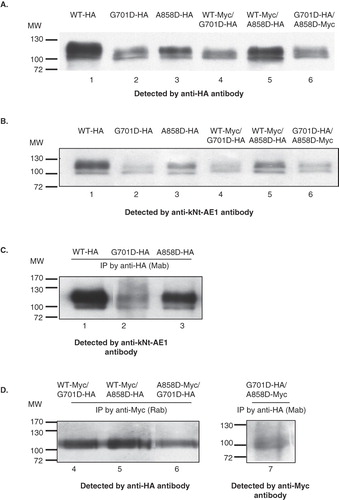
To mimic the heterozygous state kAE1 WT-Myc was co-expressed with kAE1 G701D-HA or kAE1 A858D-HA in MDCK cells. To mimic the compound heterozygous state found in the patients with dRTA the kAE1 G701D-HA was co-expressed with kAE1 A858D-Myc ( and ). shows a control experiment where HA-tagged protein immunoprecipitated using the anti-HA antibody could be detected by the anti-Nt-kAE1 antibody. shows that two dRTA kAE1 mutants could interact with the wild-type kAE1, and importantly, with each other. The kAE1 WT-Myc immunoprecipitated using the anti-Myc antibody was associated with the kAE1 G701D-HA and A858D-HA as detected by anti-HA antibody (, lanes 4 and 5). The kAE1 G701D-HA was co-immunoprecipitated with the kAE1 A858D-Myc (, lane 6) and in reverse (, lane 7), showing that the two mutants could interact with each other in MDCK cells. Interestingly, the immunoblots () showed only the upper complex band. Thus, kAE1 WT can interact with the kAE1 G701D and A858D mutants and exit the ER. The lack of detection of high mannose forms in the co-immunoprecipitation experiments () that are present in the control immunoprecipitation experiment () indicates that the ER-retained forms are likely unstable and degraded. This is consistent with the lower levels of expression observed for the dRTA mutants relative to the wild-type protein when expressed in MDCK cells.
Examination of cellular localization of dRTA kAE1 mutants in MDCK cells
The wild-type and dRTA mutant proteins or the two mutants, carrying a HA or Myc epitope to discriminate the different proteins, were expressed alone or together and the relative location of the tagged proteins were determined by immunofluorescence and confocal microscopy in both non-polarized and polarized MDCK cells. We were particularly interested in the effect of co-expression of different forms of kAE1 on their mutual localization. The wild-type protein when expressed alone was predominantly found at the cell surface of the non-polarized MDCK cells ( left panel) and at the basolateral membrane after polarization ( right panel and ). In contrast, the kAE1 G701D was predominantly found intracellularly in non-polarized and polarization MDCK cells. The kAE1 A858D also showed expression at the cell surface and basolateral membrane, however there was also considerable intracellular localization ().
Figure 5. (A) Immunofluorescence staining of kAE1 WT-HA557, kAE1 G701D-HA557 and kAE1 A858D-HA557, and (B) co-immunofluorescence staining of kAE1 WT-Myc557 and kAE1 G701D-HA557, (C) kAE1 WT-Myc557 and kAE1 A858D-HA557, and (D) kAE1 G701D-HA557 and kAE1 A858D-Myc557 in MDCK cells. MDCK cells were fixed, permeabilized and incubated with mouse anti-HA followed by anti-mouse IgG-Alexa488 antibodies (green) or rabbit anti-myc and mouse anti-HA followed by anti-rabbit IgG-cy3 (red). Yellow color indicated co-localization of the proteins. The images were observed by Zeiss LSM510 confocal microscope. Bar represents 10 μm. X–Y shows the top view of middle section and X–Z shows the side view of the polarized cells. (This figure is reproduced in colour in Molecular Membrane Biology online.)
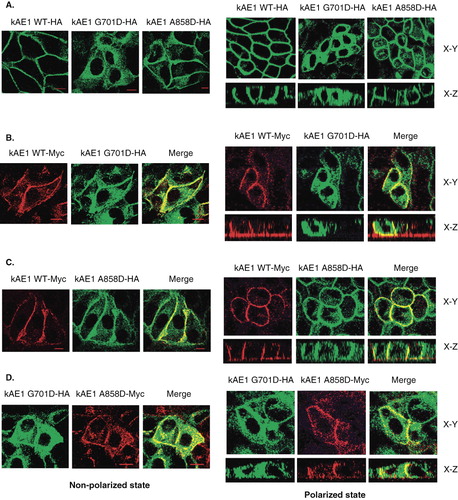
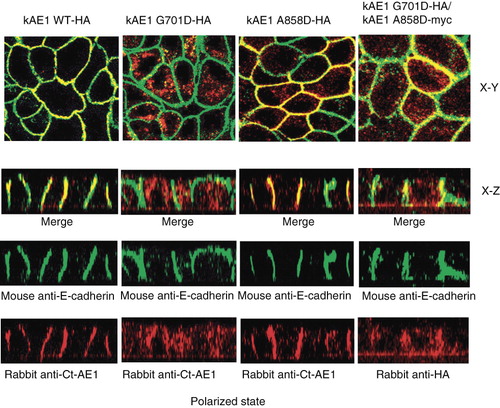
Figure 6. Co-immunofluorescence staining of individually expressed kAE1 WT-HA557, kAE1 G701D-HA557, or kAE1 A858D-HA557, and co-expressed kAE1 G701D-HA557 and kAE1 A858D-Myc557 in MDCK cells. Viral infected MDCK cells were grown on semipermeabilized filter (polarized state) for 6 days until polarized. Then cells were fixed, permeabilized and incubated with mouse anti-E-cadherin (epithelia basolateral marker) followed by anti mouse-Alexa488 (green), rabbit anti-Ct of AE1, or rabbit anti-HA (for co-expression only to detect kAE1 G701D) followed by anti rabbit IgG-Cy3 (red) antibodies. Yellow color indicates co-localization of the kAE1 proteins with E-cadherin at the basolateral membrane. The cells were observed by using the Zeiss LSM510 confocal microscope. X–Y shows the top view of the middle section and X–Z shows the side view of the polarized cells. (This figure is reproduced in colour in Molecular Membrane Biology online.)
When kAE1 WT was co-expressed with the kAE1 G701D in the same cells, both proteins were detected at the cell surface () however, with strong intracellular staining for kAE1 G701D. When the kAE1 WT was co-expressed with the kAE1 A858D (), both proteins were localized to the cell surface, with some intracellular staining for the kAE1 A858D mutant (). These results suggest that the co-expressed wild-type kAE1 could partially rescue both mutant kAE1 proteins from intracellular retention to the plasma membrane. There was however a pool of mutant protein that was still localized intracelluarly even when co-expressed with the wild-type kAE1, with no evidence of a major pool of intracellular wild-type kAE1.
The results of co-expressed kAE1 G701D and kAE1 A858D showed that kAE1 G701D was co-localized with kAE1 A858D intracellularly but also at the cell surface in non-polarized and polarized MDCK cells (). The kAE1 A858D could partially rescue the kAE1 G701D to cell surface, however there was still strong intracellular staining observed for the kAE1 G701D even in the presence of the kAE1 A858D mutant.
To confirm cell surface expression, shows localization of the kAE1 protein and E-cadherin, a basolateral marker. The kAE1 WT strongly co-localized with E-cadherin as did kAE1 A858D, which also showed some intracellular staining. In contrast, kAE1 G701D was very strongly localized intracellularly and there was very little co-localization with E-cadherin at the basolateral membrane. When kAE1 G701D was co-expressed with the kAE1 A858D; however, some co-localization of the kAE1 G701D mutant with E-cadherin was observed. Thus, the kAE1 A858D could only poorly facilitate trafficking of the kAE1 G701D to the basolateral membrane.
Discussion
Novel compound heterozygous SLC4A1 G701D/A858D mutations causing dRTA have recently been identified in two pediatric patients from a Thai family [Citation14]. The interactions between wild-type kAE1 and the two mutant kAE1 (G701D and A858D) associated with dRTA and between each other were examined by co-immunoprecipitation and affinity co-purification. The wild-type kAE1 protein could form heterodimers with kAE1 G701D and kAE1 A858D proteins, while the two mutant proteins could form heterodimers with each other. This indicates that these two mutations do not seriously affect the regions of kAE1 involved in the formation of dimers.
When expressed alone, kAE1 WT was found at the cell surface of HEK 293T and non-polarized MDCK cells and the basolateral membrane of polarized MDCK cells indicating efficient trafficking of kAE1 in the cell lines, particularly polarized MDCK cells. The kAE1 A858D mutant was also found at the cell surface, with some intracellular localization, suggesting a mild trafficking defect. In contrast, the kAE1 G701D mutant was very poorly expressed at the cell surface and predominantly retained intracellularly as noted previously [Citation16,Citation21].
In this paper, we have examined the cellular localization of kAE1 in the heterozygous and compound heterozygous G701D/A858D conditions by co-expression of wild-type kAE1 with either kAE1 G701D or A858D, and by co-expression of both mutant proteins to mimic the circumstance in α-intercalated kidney cells of the patients. The results showed that wild-type kAE1 was able to rescue trafficking kAE1 A858D and partially rescue kAE1 G701D to the cell surface. In contrast, kAE1 A858D could only poorly rescue kAE1 G701D, which maintained a predominant intracellular localization in non-polarized MDCK and polarized MDCK cells. Heterozygous patients expressing the wild-type protein and the dRTA mutants are able to express the proteins at the cell surface due to rescue by the wild-type protein. Co-expression of kAE1 G701D and kAE1 A858D however would result in impaired anion transport activity in the α-intercalated cells of the distal nephron of the patients with the compound heterozygous G701D/A858D due to their poor cell surface expression.
Acknowledgements
PY is a Senior Research Scholar of Thailand Research Fund (TRF) and Higher Education Commission (HEC).
Declaration of interest: DU is financially supported by the Faculty of Medicine Siriraj Hospital and WU by Siriraj Graduate Scholarship. The research carried out by JL and RAFR was supported by an operating grant (FRN15266) from the Canadian Institutes of Health Research. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jay DG. 1996. Role of band 3 in homeostasis and cell shape. Cell 86(6):853–854.
- Tanner MJ. 2002. Band 3 anion exchanger and its involvement in erythrocyte and kidney disorders. Curr Opin Hematol 9(2):133–139.
- Tanner MJ. 1997. The structure and function of band 3 (AE1): recent developments (review). Mol Membr Biol 14(4):155–165.
- Wrong O, 2002. Band 3 mutations, distal renal tubular acidosis, and Southeast Asian ovalocytosis. Kidney Int 62(1):10–19.
- Yenchitsomanus P. 2003. Human anion exchanger1 mutations and distal renal tubular acidosis. Southeast Asian J Trop Med Public Health 34(3):651–658.
- Yenchitsomanus P, 2005. Molecular mechanisms of autosomal dominant and recessive distal renal tubular acidosis caused by SLC4A1 (AE1) mutations. J Mol Genet Med 1(2):49–62.
- Bruce LJ, 2000. Band 3 mutations, renal tubular acidosis and South-East Asian ovalocytosis in Malaysia and Papua New Guinea: loss of up to 95% band 3 transport in red cells. Biochem J 350 Pt 1:41–51.
- Choo KE, 2006. Recessive distal renal tubular acidosis in Sarawak caused by AE1 mutations. Pediatr Nephrol 21(2):212–217.
- Ribeiro ML, 2000. Severe hereditary spherocytosis and distal renal tubular acidosis associated with the total absence of band 3. Blood 96(4):1602–1604.
- Sritippayawan S. 2003. A de novo R589C mutation of anion exchanger 1 causing distal renal tubular acidosis. Pediatr Nephrol 18(7):644–648.
- Tanphaichitr V, 1998. Novel AE1 mutations in recessive distal renal tubular acidosis. Loss-of-function is rescued by glycophorin A. J Clin Invest 102(12):2173–2179.
- Vasuvattakul S. 1999. Autosomal recessive distal renal tubular acidosis associated with Southeast Asian ovalocytosis. Kidney Int 56(5):1674–1682.
- Yenchitsomanus P, 2002. Autosomal recessive distal renal tubular acidosis caused by G701D mutation of anion exchanger 1 gene. Am J Kidney Dis 40(1):21–29.
- Khositseth S, 2007. Distal renal tubular acidosis associated with anion exchanger 1 mutations in children in Thailand. Am J Kidney Dis 49(6):841–850 e1.
- Khositseth S, 2008. Hematological abnormalities in patients with distal renal tubular acidosis and hemoglobinopathies. Am J Hematol 83(6):465–471.
- Sawasdee, N, 2006. Trafficking defect of mutant kidney anion exchanger 1 (kAE1) proteins associated with distal renal tubular acidosis and Southeast Asian ovalocytosis. Biochem Biophys Res Commun 350(3):723–230.
- Cordat E, Li J, Reithmeier, RA. 2003. Carboxyl-terminal truncations of human anion exchanger impair its trafficking to the plasma membrane. Traffic 4(9):642–651.
- Cordat E, Reithmeier RA. 2006. Expression and interaction of two compound heterozygous distal renal tubular acidosis mutants of kidney anion exchanger 1 in epithelial cells. Am J Physiol Renal Physiol 291(6):F1354–1361.
- Cordat E, 2006. Dominant and recessive distal renal tubular acidosis mutations of kidney anion exchanger 1 induce distinct trafficking defects in MDCK cells. Traffic 7(2):117–128.
- Kittanakom S, Cordat E, Reithmeier RA. 2008. Dominant-negative effect of Southeast Asian ovalocytosis anion exchanger 1 in compound heterozygous distal renal tubular acidosis. Biochem J 410(2):271–281.
- Kittanakom S, 2004. Trafficking defects of a novel autosomal recessive distal renal tubular acidosis mutant (S773P) of the human kidney anion exchanger (kAE1). J Biol Chem 279(39):40960–40971.
- Quilty JA, Reithmeier RA. 2000. Trafficking and folding defects in hereditary spherocytosis mutants of the human red cell anion exchanger. Traffic 1(12):987–998.