Abstract
Radiation-based therapies aided by nanoparticles have been developed for decades, and can be primarily categorized into two main platforms. First, delivery of payload of photo-reactive drugs (photosensitizers) using the conventional nanoparticles, and second, design and development of photo-triggerable nanoparticles (primarily liposomes) to attain light-assisted on-demand drug delivery. The main focus of this review is to provide an update of the history, current status and future applications of photo-triggerable lipid-based nanoparticles (light-sensitive liposomes). We will begin with a brief overview on the applications of liposomes for delivery of photosensitizers, including the choice of photosensitizers for photodynamic therapy, as well as the currently available light sources (lasers) used for these applications. The main segment of this review will encompass the details of strategies used to develop photo-triggerable liposomes for their drug delivery function. The principles underlying the assembly of photoreactive lipids into nanoparticles (liposomes) and photo-triggering mechanisms will be presented. We will also discuss factors that limit the applications of these liposomes for in vivo triggered drug delivery and emerging concepts that may lead to the biologically viable photo-activation strategies. We will conclude with our view point on the future perspectives of light-sensitive liposomes in the clinic.
Introduction
Nano-drug delivery systems coupled with site-specific targeting ligands constitute a promising system to boost efficacy and bioavailability of existing drugs and pharmaceuticals (Allen Citation1997, Allen & Cullis Citation2004, Torchilin Citation2007, Yang et al. Citation2007, Wang et al. Citation2009). Optimal drug delivery systems feature multifunctional nanoparticles with imaging molecules, a pay-load of drugs, targeting ligands, destabilization elements as well as sensors that probe the efficacy of the drug in real time (Allen & Cullis Citation2004, Alonso Citation2004, Torchilin Citation2005, Alaouie & Sofou Citation2008, Ganta et al. Citation2008, Zhou Citation2008). Some widely examined nanocarriers aimed at delivering either DNA, pharmaceuticals and/or imaging agents include dendrimers (Tomalia et al. Citation2007, Majoros et al. Citation2008), nano-gold shells (Liao Citation2007, Bakri et al. Citation2008), nano-emulsions (Tiwari & Amiji Citation2006), drug-polymer conjugates (Reddy & Low Citation1998, Leamon & Low Citation2001), drug-antibody conjugates (Jaracz et al. Citation2005), and quantum dots (Cormode et al. Citation2009, Park et al. Citation2009, Rzigalinski & Strobl Citation2009). Each of these nanotechnology platforms entails unique fabrication components that rely on self-assembly of the structural motifs, while accommodating the pharmaceutical agent and the targeting ligand. The latest developments for some of these platforms are discussed in this issue (Nanoemulsions by Ganta, Amiji, Micelles, Sawant, and Torchilin (pp. 260–273), and Nanoparticulate systems by R. N. Saha (pp. 215–231)).
Liposome drug delivery: general considerations
Liposomes are amongst the longest-studied nanoparticles and these primarily consist of phospholipids (major components of biological membranes) (Fenske et al. Citation2008, Fenske & Cullis Citation2008). It is notable to mention that Doxil/caylex (a liposome-based formulation of an anticancer drug Doxorubicin, Ortho-Biotech), was the first formulation approved for its application in the clinic and hence may be regarded as an honorary nanoparticle for patient care (Batist et al. Citation2001, Davis et al. Citation2008). Historically, the important milestones that led to the research and development of clinically suitable liposome formulations can be summed up in two major technological achievements; (i) inclusion of pegylated lipids in the liposomes to bypass the reticulo-endothelial system, resulting in preferential accumulation in tumours (Papahadjopoulos et al. Citation1991, Gabizon & Martin Citation1997), and (ii) successful development of a remote drug loading process based on ammonium sulfate gradient method to achieve significantly high quantities of doxorubicin (as well as other weak bases) in the interior of the liposomes (Haran et al. Citation1993, Haran et al. Citation1994). We have recently provided a detailed overview of lipid-based nanoparticles elsewhere (Puri et al. Citation2009). To date, a number of liposome formulations are currently used in the clinic while others await clinical trials. However, two important features, namely targeting potential and on-demand drug release properties (Triggering) of liposomes are the subject of current and future considerations to further improve patient's treatment.
Targeting potential
Although liposomes bearing various targeting ligands (such as antibodies, peptides, affibody etc) appear to be promising candidates, the clinical benefits associated with targeting are under intense debate and are beyond the scope of this article (please refer to contributions by Omolola Eniola-Adefeso, Raymond Schiffelers and Robert Lee in this issue).
Triggering
It can be envisioned that modalities resulting in selective release of drugs from liposomes (once they have reached their target site) will have a significant impact on therapeutic index of the drugs. Towards this end several strategies have been described in the literature that can be broadly classified into internal and external triggers. The examples of internal triggers typically include either exploitation of low pH in the endosome or use of enzymes that are overexpressed in diseased states (the reader is referred to contributions by Yana Reshetnyak and Andresen/Kaasgaard in this issue). Examples of external triggers that are used to destabilize liposomes are heat and light, the two forces of nature. It is important to note that thermosensitive liposomes (currently in Phase 3 clinical trials and first described in the late 1970s) are thus far the best studied example of triggerable nanoparticles (Needham & Dewhirst Citation2001, Negussie et al. Citation2010).
Since conventional liposomes (that are not sensitive to light per se) have been used to deliver photoreactive molecules for radiation-based therapies, the information gained from these data presents a foundation for developing light-triggerable liposomes. Therefore, we will first provide an update of conventional liposome formulations used for the high concentration of delivery of various photosensitizers (photodynamic therapy, PDT). The main part of this review will deal with the design principles of photo-triggerable lipids and molecular mechanisms that result in photo-destabilization of liposome membrane. We will also discuss alternate mechanisms of photo-triggering of liposome entrapped drugs (from conventional liposomes) that are based on unique light absorption properties of metal ions such as gold nanoparticles (AuNP). Lastly, we will present our view on opportunities that may lead to a successful application of photo-triggerable liposomes in the clinic.
Liposomes in photodynamic therapy
Since the 1980s, photodynamic therapy (PDT) has served as a promising treatment protocol and involves the use of a photosensitizing agent coupled with an appropriate light source. A recent review by Juarranz et al. (Juarranz et al. Citation2008) provides a detailed description of the activation mechanisms of the photosensitizing agent used in PDT. Light-mediated activation of photosensitizing agents results in the generation of radical oxygen species (ROS) which destroys the target tissues or cells (Donnelly et al. Citation2008, Sibani et al. Citation2008). Typically photosensitizing agents absorb photons and are excited to a triplet state, which generates ROS (examples include superoxide anion, hydroxyl radical, hydrogen peroxide) or can transfer energy to a ground-state molecular oxygen, resulting in the production of a highly reactive singlet oxygen. It is the latter reaction that is thought to be the primary agent behind cell death in PDT, although both mechanisms may play a role depending on numerous variables, such as photosensitizing agent type, localization, and levels of oxygen. In animal models, tumour regression is well-documented using PDT, which presumably occurs by killing of cells, damage to tumour vasculature, and/or stimulation of immune responses that cause cell death (Juarranz et al Citation2008, Robertson et al. Citation2009).
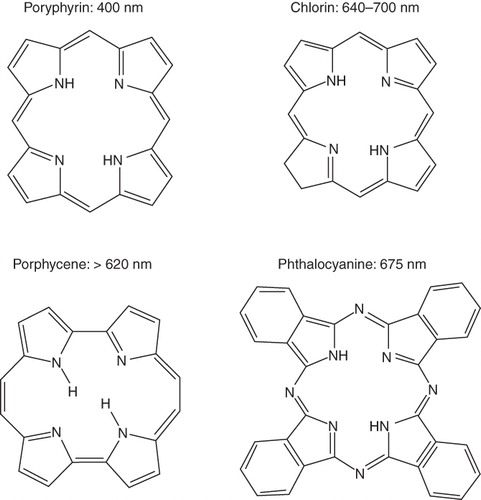
Although numerous photosensitizer formulations have been tested in laboratory settings, only a few have made it in to the clinic. The majority of photosensitizing agents fall into one of four categories: porphyrin derivatives, chlorins, phthalocyanines, and porphycenes () (De Rosa & Bentley Citation2000). Porphryins have traditionally been the most extensively studied and used. The first clinically available photosensitizer for PDT of multiple cancers was Photofrin, which may be classified as a hematoporphryin derivative. The second type, chlorins, contains compounds derived from chlorophyll and porphryins, including Benzoporphyrin derivative monoacid ring A (BPD-MA) which has been found effective in treating some forms of carcinomas. Second-generation PSA, phthalocyanines feature a diamagnetic metal ion, reduced phototoxic side effects, and have shown high potential in PDT in animal tumour models. The last group, porphycenes, has also proven to be effective in reducing tumour size through their efficient generation of singlet oxygen ().
Figure 1. Chemical structures of various photosensitizing chemical agents. Majority of photosensitizing chemical agents may be classified into four main categories according to their chemical structures, including porphyrins, chlorins, porphycenes, and phthalocyanines. Each PSA features a peak absorbance wavelength, typically in the red light region of the spectrum.
Despite advances made by clinically available photosensitizing agents, there are still several drawbacks limiting their use. For example the hydrophobic nature of most photosensitizing agent makes them highly susceptible to aggregation in aqueous solutions, and therefore also limits their efficiency and delivery in the body (Chen et al. Citation2005a, Chen et al. Citation2005b). Additionally, an inadequate affinity by most photosensitizers to tumour sites also results in some damage of normal tissue following PDT in patients. To resolve these issues and curb potential side effects, alternative photosensitizer formulations are required to achieve greater solubility and selective delivery to tumour sites. Liposomes have the ability to encapsulate the mostly hydrophobic molecules and avoid any aggregation issues associated with interactions in the aqueous environment (Donnelly et al. Citation2008). The inclusion of PEG-lipids within the liposomes also greatly enhances their efficacy and biodistribution through the avoidance of the reticuloendothelial system (Allen Citation1994). Recent advances in the liposomal field with regard to the conjugation of ligands to the nanoparticles' surface (for targeting) may even further enhance the potential of conventional liposomes as photosensitizer delivery vehicles for PDT. Below we review recent studies from the literature and provide specific examples of targeted liposomal formulations.
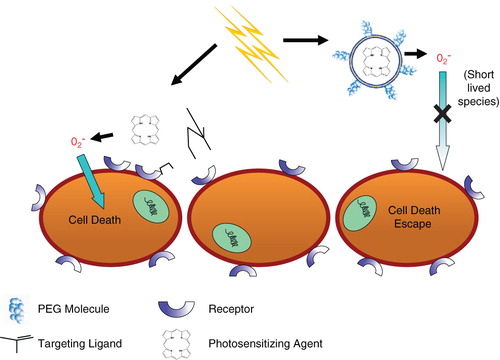
Recently, Oku and Ishii (Oku & Ishii Citation2009) suggested that actively targeted liposomes for PDT is the most efficient delivery system, since binding and internalization of the nanoparticles will be greater than that of control, or non-targeted PEG-coated liposomes. As the PEG-lipid molecule decreases interactions with cells, they are less likely to stay in close enough proximity to deliver an effective dose of singlet oxygen species generated from the photosensitizers. Since the reactive oxygen species are short-lived, the onset of photodynamic reaction (in close proximity to the cells and/or tissues) at the cancer site is important. This phenomenon is shown in a cartoon form ().
Figure 2. The effects of targeting on the delivery and cytotoxicity of liposome-encapsulated photosensitizers. The addition of targeting ligands to liposomes encapsulating photosensitizers improves cytotoxicity compared to non-targeted, PEG-coated liposomes. Exterior PEG-molecules do not allow for close contact between liposomes and cells, whereas targeted formulations induce binding between the two. This close interaction is required for efficient delivery of short-lived radical oxygen species, which are generated from the light-activated encapsulated photosensitizers.
Using the photosensitizer, benzoporphryin derivative monoacid ring A (BPD-MA), the same group studied tumor regression in a Meth-A sarcoma mouse model with a 689 nm diode-laser system (SP689) as the irradiation source. They modified a PEG-Liposomal BPD-MA formulation to include the APRPG peptide, which is known to target angiogenic endothelial cells, and found that the APRPG-targeted PEG-Liposomal BPD-MA displayed a 5 fold difference in tumor reduction compared to the non-targeted PEG-Liposomal BPD-MA. It was hypothesized that the close interaction of the APRPG-targeted formulation with endothelial cells resulted in greater cytotoxicity as the generated oxygen species need not travel far to induce cell death (Oku & Ishii Citation2009) (). Additionally, another recent study investigated the delivery of photosensitizer agents by targeted liposomes in vitro and in vivo. Derycke and colleagues (Derycke et al. Citation2004) took advantage of the upregulation of transferrin receptors on tumor cells, and conjugated the transferrin molecule to PEGylated liposomes loaded with the photosensitizer aluminum phthalocyanine tetrasulfonate (AlPCS4) (Derycke et al. Citation2004). Utilizing a 1000W halogen lamp system with a filter (center wavelength = 651 nm), researchers measured in vitro photodynamic effects of the transferrin-conjugated AlPCS4 liposomes on a rat bladder carcinoma cell line and tumor model. The targeted formulation displayed a cell killing greater than 3-fold in the log scale compared to the control, non-targeted AlPCS4 liposomes. Moreover, in the tumour model, accumulation of PSA, AlPCS4, was shown to be significantly enhanced by the conjugation of transferrin to the PEGylated liposomes. Hence, targeted liposomes may be of benefit over non-targeted liposomes.
In conclusion, pre-clinical studies using a variety of photosensitizers (ex: Photofrin, Visudyne, aminolevulinic acid) indicate that PDT may be a suitable treatment for a number of diseases, including several cancer types. However, alternate formulations appear to be required to increase the bio-availability of the photosensitizer at target sites and reduce adverse side effects. Liposomal encapsulation of photosensitizers may achieve these goals especially with targeted liposomes, which can be brought into close contact with target cells enabling rapid transport of short-lived ROS from the irradiated photosensitizer within the liposome to nearby target cells (). To make these more effective light-triggerable platforms that release the photoreactive agent have been designed (described below).
Light-sensitive liposomes: background
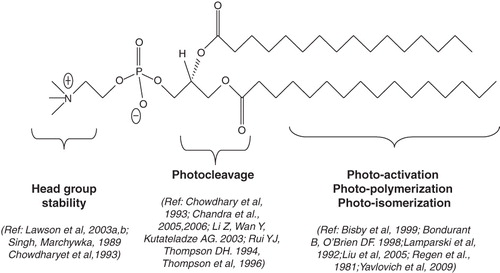
Light-sensitive liposomes (explored since early 1980s) have lately re-gained attention. Photoreactive lipid molecules provide an opportunity to generate stable nanodelivery vehicles for sustained release of drugs in circulation together with photo-triggerable systems for localized drug delivery. Triggerable liposomes are generally based on the principle of membrane destabilization (resulting in creation of local defects) for release of liposome-entrapped drugs and pharmaceuticals. Among other triggerable formulations, thermosensitive liposomes depend on transition release (PTR) properties of conventional phospholipids (or lipid mixtures), primarily phosphatidylcholines (PC), that are used to prepare liposomes. However, light-triggerable liposomes are developed by introducing photoreactive groups in the phospholipid molecule (‘designer lipids’) usually by chemical synthesis. One exception to this notion is plasmalogen (a natural ether phospholipid, typically found in tissues such as heart) that reacts with reactive oxygen species (ROS) to generate lysolipid, which promotes leakage from liposomes. There are several designer lipids synthesized to date, each of which utilizes a defined mechanism for photo-activation (). The PC molecule can be divided into three major parts, head group, glycerol backbone and fatty acyl chains ().
Table I. A partial list of synthetic photo-sensitive phospholipids.
Figure 3. Sites for chemical modifications in phospholipids (photoreactive lipids). Three major parts of phospholipids that can be chemically modified to generate photosensitive molecules. The lipid parts: head group, glycerol backbone and fatty acyl chains are described with their proposed modifications. The references correspond to the currently available designer lipids respectively.
Each of these regions has been modified either by the introduction of additional groups or modification of existing chemical bonds such as polymerizable moieties (Singh & Marchywka Citation1989, Yavlovich et al. Citation2009) to produce light-sensitive liposomes. The fatty acyl chain length and degree of unsaturation are important factors that govern bilayer packing properties of liposomes. Fatty acyl chains of the phospholipids have been further modified with light-sensitive groups and the resulting phospholipids have yielded photoactivable liposomes (Shum et al. Citation2001, varez-Lorenzo et al. Citation2009). Currently available photoactivable liposome systems are discussed below (see ).
Head-group polymerizable liposomes as candidates for sustained drug delivery
In late 1970s, the drug delivery potential of liposomes was realized. However, soon after, the issues related to the stability of liposomes became evident. The primary factors involved in destabilization of liposomes resulted from interactions between liposomal lipids with serum components (Kronberg et al. Citation1990). In addition, preferential uptake of liposomes by the reticuloendothelial system (RES) limited their in vivo applications (Papahadjopoulos et al. Citation1991, Gabizon & Martin Citation1997). Research efforts to generate stable liposomes include formulating compositions including lipids containing poly(ethylene glycol) (PEG) in their headgroup (Papahadjopoulos et al. Citation1991, Gabizon & Martin Citation1997). This is currently the most widely applicable method and has led to highly significant milestones in liposome drug delivery research (prolonged circulation in blood) (reviewed in Puri et al. Citation2009). Since the efficiency of pegylated liposomes is less than optimal in vivo, alternate approaches to generate stable liposomes are warranted. Towards these efforts, the stabilization of liposomes through chemically synthesized photoreactive lipids has been reported recently (Lawson et al. Citation2003a, Lawson et al. Citation2003b). The light-induced reaction in these liposomes involves photo-crosslinking (light-induced polymerization) under relatively mild conditions and often a water soluble free radical initiator is used to begin this reaction. The latter system is potentially versatile as the monomer-crosslinking methods (in preformed liposomes) may find applications for both biological and non-biological systems. The choice of monomer functionalities and the flexibility to place these monomers in the liposome membrane prior to cross-linking are attractive aspects of the polymerization approach to generate stable vesicles. However, an important consideration in developing the photoreactive monomers (such as photopolymerizable lipids) is to maintain the integrity of the liposome membrane as well as entrapped contents (such as pharmaceutical agents) during the photopolymerization step. Since the photo-activation of fatty acyl chains in the phospholipid molecules () will result in perturbations in the liposome membrane, such modifications have been implicated for photo-triggering to accomplish localized drug delivery from liposome formulations (discussed in sections below). It can be envisioned that the introduction of a photoreactive moiety in the head group of the phospholipid (see ) will be a smart choice; as such modifications will result in polymerized liposomes while sustaining their membrane properties. In the phospholipid realm, there are only a few examples of head-group photopolymerizable molecules ().
Figure 4. Light sensitive head-group polymerizable lipids. Chemical structures of currently available head group polymerizable lipids.
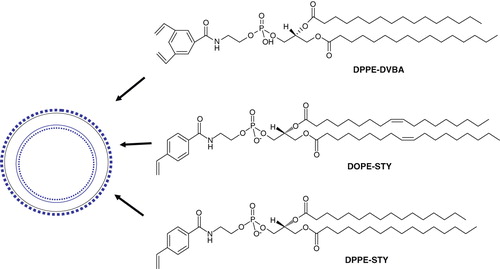
The synthesis of these phospholipids is based on the generation of N-acyl phosphatidylethanolamines (N-acyl PEs) that readily form liposomes. The chemical structures of PEs containing a 3,5-divinylbenzoyl functionality (Lawson et al. Citation2003b) and N-(4-Vinylbenzoyl) head group (Lawson et al. Citation2003a) are shown in . Polymerization in liposomes (prepared from these phospholipids) has been demonstrated to photo-crosslink in the presence of UV light without compromising the activity of entrapped enzymes. Although biophysical studies demonstrate that these head-group polymerizable lipids are potential candidates for generating stable liposomes (Lawson et al. Citation2003a), further studies are needed to evaluate the merit of these lipids for sustained drug delivery.
Phototriggerable liposomes
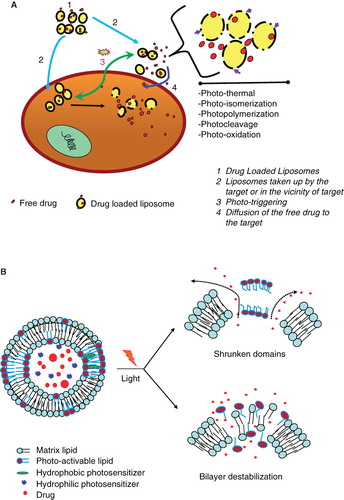
Photo-activation is an attractive option for triggering release of liposomal contents since it provides a very broad range of adjustable parameters (e.g., wavelength, duration, intensity) that can be optimized. The range of light necessary for tissue penetration is discussed below. The cartoon presented in shows drug-loaded photo-triggerable liposomes (), that either accumulates in the interior of the cells (tumor area) or in the vicinity of the disease cells/tissues (). Treatment with a suitable light source () will trigger drug release from these liposomes upon photo-activation. The internalized liposomes will deliver drug directly in the interior of the cell. Liposomes within the vicinity of cells/tumors will be also destabilized (upon light treatment), and the released drug will eventually be taken up by passive diffusion (). The mechanisms by which light-triggered defects in the liposome membrane are created encompass(s) several modalities (shown in the zoomed version of cartoon, see arrows, , right).
Figure 5. (A) Mechanisms of release of liposome-encapsulated drugs by photo-triggering. The diagram describes various mechanisms of light induced destabilization of liposomes and intracellular localization of released drugs. (B) A cartoon presenting the mechanisms of light triggered release from photo-sensitive liposomes. Two known conformational changes in photo-reactive liposome segments due to light treatment are presented. Light radiation can result in a shrunken domain that leads to leakage between domain boundaries (right, top). Light radiation can also create changes in the liposome bilayer resulting in destabilization and the release of the liposomal contents (right, top).
Light-triggered release of liposomal drugs involves modified phospholipid molecules, that undergo either photopolymerization (Regen et al. Citation1981), photosensitization by membrane anchored hydrophobic (Chowdhary et al. Citation1993, Bisby et al. Citation1999a, Bisby et al. Citation2000b, Lavi et al. Citation2002, Chandra et al. Citation2005, Chandra et al. Citation2006), or water soluble probes (Yavlovich et al. Citation2009), photo-isomerization (Morgan et al. Citation1995), photooxidation (Shum et al. Citation2001), or the degradation of photocleavable lipids (Chandra et al. Citation2005, Chandra et al. Citation2006). Approaches to promote photo-induced drug delivery from liposomes also include the use of wavelength-specific photosensitizers (primarily lipidic and/or hydrophobic in nature) in conjunction with photoactivable lipids. It may be noted that light-induced effects result in irreversible changes in the majority of liposome systems with the exception of molecules that under go cis-trans isomerization. The detailed mechanisms of light-triggered chemical changes in photo-reactive segments of some of these molecules were reviewed about 10 years ago (Shum et al. Citation2001), and are shown in in a cartoon form. Since then, some progress has been made in this field and here we will mainly focus on latest developments.
Photooxidation
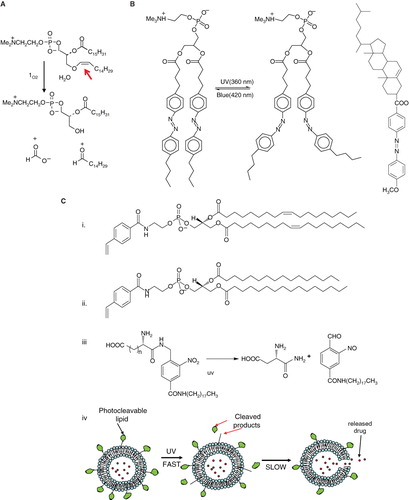
In the early 1990s, researchers explored photo-oxidation of lipids containing vinyl ether linkages (bearing at least one double bond) by reactive oxygen species (generated by a series of photosensitizers) (). Significant efforts were put forward by Thompson and colleagues to design and synthesize plasmenylcholine molecules (Rui & Thompson Citation1994, Rui & Thompson Citation1996). Photo-oxidation was initiated by several light-responsive molecules such as Zn-phthalocyanine, octabutoxyphthalocyanine, and bacterio-chlorophyll-α. These sensitizing agents absorb between 630–820 nm photo-triggering of liposomes and release of contents (Thompson et al. Citation1996). The use of the near-infrared sensitizer bacteriochlorophyll led to 100% calcein release in less than 20 min when irradiated at 800 nm, indicating that these formulations may have the advantage for deep tissue penetration depth for small animal model studies (for discussion, please see later in the article) (Gerasimov et al. Citation1997, Shum et al Citation2001). Vinyl ether linkages in plasmenylcholine and diplasmenylcholine being susceptible to photooxidation were proposed as a novel targets for triggering.
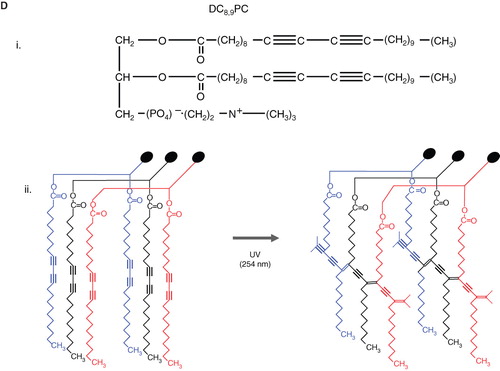
Figure 6. Schematic diagrams of light-mediated chemical reactions in radiation-sensitive lipids. (A) Photo-oxidation of lipids containing vinyl ether linkages by reactive oxygen species generated by a series of photo sensitizers (Adapted from Thompson et al., BBA, Citation1996). (B) Photo isomerization of azobenzene groups undergo wavelength-specific manner radiation leading to cis-trans conformational changes. (Adapted from Morgan et al., FEBS Letters, Citation1995 and Liu et al., BBA, Citation2005). The structrue of photoisomerizable cholesterol is also shown on the right (adapted from Liu et al, Citation2005). (C) Photo cleavable lipids (i–iii); the structure of representative lipids that are subject to cleavage upon light treatment. (iv) The photocleaved breakdown products lead to destabilization of liposome membrane. (D) (i) Chemical structure of a photo-polymerizable phospholipid DC8,9PC. (ii) A cartoon depicting photo-crosslinking of DC8,9PC upon UV treatment. (Adapted from Singh A & Gaber BP, Citation1988).
Cis-trans isomerization
As mentioned above, most of the light-activated liposome formulations result in irreversible changes in the phospholipids, and hence represent one-time delivery protocols. However, formulations with an ability to reversibly regulate light-triggered drug release will have significant advantage in the clinics. Keeping this in mind, phospholipids containing the azobenzene group were designed by Bisby and colleagues in 1990s. The azobenzene groups undergo cis-trans isomerization (420/360nm) in a wavelength-specific manner (), resulting in transient/programmed release of entrapped solutes from the liposomes. It was demonstrated that DPPC liposomes containing a photochromic lipid ‘Bis-Azo PC’ (6 %mol) with addition of cholesterol (up to 25 mol%) released their contents in response to visible light in the region of 470 nm. A photo stationary state is dominated by bulky cis form after photo isomerization created by UV radiation (360 nm) which interferes in the liposomes bilayer packing allowing rapid leakage of encapsulated agents. The acyl chains are compact and organized in closely packed liposomes in the trans form that is thermodynamically preferable (Bisby et al. Citation2000a, Bisby et al. Citation2000b). In 2005, Liu and colleagues reported the synthesis of a series of photo-isomerizable cholesterol derivatives (); these molecules may have potential for light-triggered delivery, although no biological data have been reported (Liu et al. Citation2005).
Photocleavable liposomes
The concept of photocleavable lipids has been in existence for decades (Zhang & Smith Citation1999, Mueller et al. Citation2000, Shum et al. Citation2001, varez-Lorenzo et al. Citation2009). In recent years, photo-labile lipid molecules (other than phospholipids) have been reported for their potential applications in drug delivery (Li et al. Citation2003, Chandra et al. Citation2005, Chandra et al. Citation2006). These molecules () were designed on the basis of their susceptibility to light (typically in the UV range) resulting in the breakdown products that can destabilize liposome membrane. Li et al. synthesized the dihydroxybenzophenone-based amphiphiles as the photolabile lipids using the dithiane-based modular approach. The same group also synthesized nitropyridine-based self-sensitized photolabile amphiphiles () (Li et al. Citation2003). These molecules also included the possibility of the addition of hydrogen-bond elements for further modification of hydrophilic portion of the lipids. Biophysical studies revealed that these amphiphiles could be used in conventional liposome formulations for phototriggering. Chandra et al. also aimed at synthesizing photocleavable amphiphilic lipids () by connecting stearyl amine (as the non-polar tail) and charged amino acids (as polar heads) via the o-nitrobenzyl derivatives (Chandra et al. Citation2005, Chandra et al. Citation2006). Their efforts were focused on using the chemical approaches that will produce photocleavable lipids in high yields and easy purifications steps.
Using these photocleavable lipids, the investigators showed that light-induced leakage occurred in a two step process, first the fast reaction of photolysis followed by a slow release of entrapped contents (shown in cartoon ). Therefore, it is possible that reorganization of the lipids occurs before all entrapped dye is released. Incidentally, photocleavable lipids described here are activated only by short-wave lengths and their in vivo application remains to be examined.
Photopolymerizable liposomes
The systems described above (5b, i–iii) are primarily based on light-induced perturbations in the liposome membrane, either via the irreversible modification (photo-cleavage) of the photoreactive lipids (5b, i & iii) or by reversible conformational changes in the lipids (5b, ii). This mode of phototriggering is shown in (lower panel, right, membrane destabilization). However, it is possible to use photoreactive lipids that can undergo photo-crosslinking within the liposome bilayer (photopolymerization). It can be assumed that the synergized molecular packing of photopolymerizable lipids is a crucial element to this effect. The phenomenon is shown in the cartoon in (top, right panel, shrunken domains). We will discuss the properties and drug delivery potential of two photopolymerizable phospholipid molecules namely bis-SorbPC and DC8,9PC.
Bis-SorbPC
Elegant studies by O'Brien and colleagues previously reported that UV light-induced photopolymerization of liposomes containing bis-sorbyl phosphatidylcholine (bis-SorbPC) results in leakage of liposome-entrapped contents (Lamparski et al. Citation1992, Bondurant & O'Brien Citation1998, Mueller et al. Citation2000). Interestingly, inclusion of a cationic dye, 1,1'-didodecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI, as a hydrophobic photo-sensitizer) in these liposomes also triggered destabilization of the liposomal membranes when treated with visible light (550 nm). The light at this wavelength is only absorbed by the DiI and not by the lipids. The photo-activation of DiI produces oxygen radicals in the presence of oxygen which can initiate the polymerization of bis-SorbPC resulting in creation of domain boundaries (shrunken domains, , top right). One can predict that the packing properties of photoactivable lipid (such as bis-SorbPC) and the hydrophobic photosensitiser (such as DiI) in the liposome bilayer should be in concert to produce oxygen radical-mediated photoreaction. Therefore, lipid-photosensitizer packing will be a major determinant of this photopolymerization mechanism. Our recent studies show that the entrapment of a water soluble photosensitizer in liposomes containing a diacetylenic lipid promotes a similar outcome, but resulting from an independent mechanism unrelated to photopolymerization (unpublished). These results are discussed below.
DC8,9PC
The photopolymerizable phospholipid (1,2 bis(tricosa-10,12-diynoyl)-sn-glycero-3-phosphocholine (DC8,9PC, ), is present in lower organisms () (Pakhomov et al. Citation2003), and has been shown to uniquely assemble into the lipid bilayer due to the presence of triple bonds in the fatty acyl chains. UV (254 nm)-induced photopolymerization of DC8,9PC, as well as the analysis of chemical modifications in this molecule upon UV treatment has been well documented since the 1980s () (Regen et al. Citation1981, Singh Citation1990). Photo-crosslinking properties of DC8,9PC have been exploited for potential biological applications that include functionalized polymerized vesicles for vascular targeted molecular imaging (Li & Bednarski Citation2002), candidates for oral vaccine preparations (Alonso-Romanowski et al. Citation2003), and DNA delivery (Zarif Citation2002, Chiaramoni et al. Citation2007).
Recently, we have examined in situ light-triggered drug release properties of DC8,9PC liposomes (Yavlovich et al. Citation2009). According to our hypothesis, DC8,9PC is likely to form aggregates (self-assemble) in the bilayer of phospholipids containing saturated acyl chains, and this packing is prone to create phase boundary defects in lipid model membranes (). In support of this hypothesis, we demonstrated that UV (254 nm)-triggered calcein release occurs from liposomes containing a mixture of saturated phospholipids and DC8,9PC. The UV-triggered mechanism of calcein release was due to the photopolymerization of DC8,9PC.
We are currently pursuing DC8,9PC formulations for their drug delivery applications. We have demonstrated that visible light (514 nm) treatment of liposomes containing photo-sensitizer results in release of contents in a wave-length specifc manner. It is evident that the visible-light triggered mechanism of solute release is unrelated to photopolymerization. The DC8,9PC formulations appear promising candidates for drug delivery because 514-nm triggered release of doxorubicin (an anticancer drug) from these liposomes improves cytotoxicity in cell culture experiments. We are hopeful that our formulations can be considered as the next-generation of light-sensitive liposomes for drug delivery applications in the clinic (discussed below).
Limitations of phototriggerable liposomes for biological applications
Although a number of light-triggerable formulations have been examined to date (), none of the formulations developed so far have been successful for in vivo applications presumably due to the lack of adequate photon energy produced by the radiation source(s) or inability of radiation to penetrate into biological tissues. We believe that the development of innovative strategies to combine unique chemistry of photoactivable lipids with ‘the helper’ components (such as metal ions) is needed to acquire high energy radiations. One should keep in mind that the light source(s) used should have minimal effects on the biology of normal cells and tissues. The visible and/or infrared light sources currently in use for PDT could also be considered for light-triggered drug delivery applications. In this regard, alternate strategies to promote light-mediated drug release from the liposomes include use of metal ions with unique characteristics. One such metal, gold has been recently tested (Chithrani et al. Citation2010). The studies are summarized in the next section below.
Metal ions as alternate strategies for phototriggering of liposomes
Nanostructures bearing noble metals are considered smart choices for targeting, and imaging because their properties deviate from the bulk materials (Lewis Citation1993, Klabunde et al. Citation1996, Pileni Citation2001, Schmid et al. Citation1999, Weller & Moser Citation1999). Among various metal ion-nanostructures, gold nanostructures (AuNSTs) have been extensively investigated as a model platform for biomedical research (Bergen et al. Citation2006b). The size and shape of AuNSTs can be customized in the range of 2–100 nm and their surface properties are amenable to the introduction of functional groups for conjugation of targeting ligands (Tkachenko et al. Citation2004, Souza et al. Citation2006, Berry et al. Citation2007, Oyelere et al. Citation2007, Jiang et al. Citation2008, Nativo et al. Citation2008) (see ). AuNSTs are also biocompatible and, therefore, may serve as good tools for in vivo cell imaging, biosensing, and drug and/or gene delivery (Markowitz et al. Citation1999, Sandhu et al. Citation2002, Sokolov et al. Citation2003, Regev et al. Citation2004, Shukla et al. Citation2005, Bergen et al. Citation2006a, Han et al. Citation2006, Kneipp et al. Citation2006, Kumar et al. Citation2007, Chithrani et al. Citation2010). Currently studied AuNSTs can be categorized into gold nanoparticles (AuNPs, ) or gold nanoshells (HGNs, ).
Table II. A summary of AuNSTs-liposome systems.
Figure 7. Gold nanostuctures-liposome systems. Diagram showing AuNPs entrapment in different regions of liposomes. (a) Hydrophilic Au-MSA in the aqueous core, (b) Hydrophobic Au-C6SH in the lipid bilayer, (c) DPPE-Nanogold® tethered to lipid bilayer.
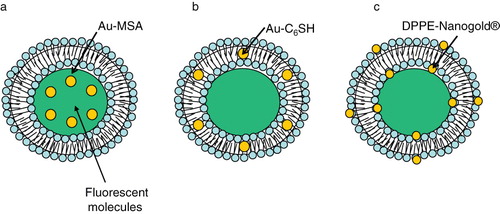
Figure 8. Gold nanostuctures-liposome systems. Diagram showing HGNs entrapment in different regions of liposomes. (a) HGNs in the aqueous core, (b) Au-SH-PEG-lipid tethered to lipid bilayer, (c) HGNs suspended freely outside.
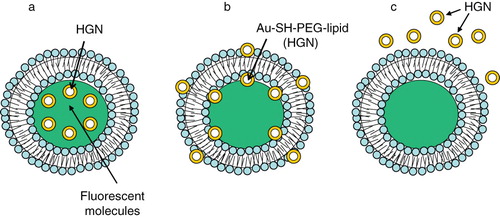
Gold nanostructures (AuNP/HGNs) can be encapsulated into other nanoparticles such as liposomes (Markowitz et al. Citation1999, Faure et al. Citation2003, Regev et al. Citation2004, Park et al. Citation2006) and polymeric matrices (Mayer & Mark Citation1997, Cole et al. Citation1999, Gittins & Caruso Citation2001) making them suitable for biological applications. Recently it was reported that the incorporation of hydrophobic stearylamine coated AuNPs (3–4 nm) into the lipid bilayer of thermosensitive liposomes (DPPC formulation) increased the fluidity of the membranes above their phase transition temperature. This effect correlated with a significant increase in efflux of entrapped contents in contrast to non-AuNP loaded liposomes (Park et al. Citation2006). The observed effect may be attributed to the altered packing properties of the liposomes. This observation indicates that the thermosensitive properties of liposomes coupled with an increase in AuNP-mediated fluidity may have an advantage in delivering a payload of drugs at a particular temperature. These studies, however, were conducted using only biophysical assays, and their application in biological systems remains to be evaluated.
AuNSTs exhibit surface plasmon resonance (Mulvaney Citation1996), as they absorb energy at a characteristic wavelength. Most of the absorbed energy is converted to heat while a part of it is emitted as photoluminescence (Wilcoxon et al. Citation1998, Harris et al. Citation2006). Therefore, light-induced heating of AuNSTs -liposome systems by UV, visible and near-infrared to release liposome-entrapped drugs and pharmaceuticals is drawing considerable interest (Paasonen et al. Citation2007, Wu et al. Citation2008a, Anderson et al. Citation2010). It may be noted that irradiated AuNSTs serve as energy collectors and work as localized heat sources by absorbing the energy of radiation. The heat is then transferred in a highly selective manner to the immediate microenvironment, without dissipating heat to the bulk suspension. Therefore, light-triggered thermal properties of AuNST-liposomes are distinct than the ‘classical’ thermosensitive liposomes (that use local hyperthermia). We will briefly discuss various AuNSTs/liposome systems that have been developed for light-triggered drug delivery using the UV, VIS or near IR (described below).
AuNP-liposomes for triggered release
Paasonen et al. (Paasonen et al. Citation2007) for the first time described a novel method for light-induced contents release from AuNPs-incorporated liposomes. Preferential partitioning of AuNP into the aqueous milieu, hydrophilic surface or the lipid bilayer of liposomes is modulated by physico-chemical properties of the AuNPs being examined. In this study, hydrophobic hexanethiol-capped AuNPs (Au-C6SH) were embedded into the lipid bilayer, negatively charged hydrophilic mercaptosuccinic acid AuNPs (Au-MSA) were encapsulated in the core of DPPC liposomes, and lipid functionalized AuNPs (DPPE-Nanogold®) were localized on the inner and the outer surface of the liposomes (). These AuNPs loaded liposomes released calcein, upon light irradiation of 250 nm. 2–3 nm small AuNPs were used because bigger particles could impede with the packing of the liposome bilayer and cause uninvited leak of contents.
The proximity of AuNP to the lipid bilayer was an important parameter for the calcein release kinetics. Since Au-C6SH and DPPE-Nanogold® are in direct contact with the lipid bilayer, heat is conducted more efficiently to the lipid molecules, thereby inducing the phase transition and calcein release whereas Au-MSA particles located in the core of the liposomes exhibited very slow kinetics of calcein release upon UV treatment. Higher AuNP concentrations showed maximum calcein release in all the liposomal formulations in comparison to lower concentration, however, the stability of liposomes was compromised at higher AuNP concentrations. In all the formulations examined, longer treatments with UV light (up to 30 minutes) resulted in maximum calcein release (). Although no biological data were reported, this system deserves a closer look especially in combination with photoactivable lipids (described in section 5b).
HGNs as nano-‘sonicator’ for liposome triggering
UV-triggered release of liposomal contents (described above) is limited only for topical treatments (such as skin), therefore AuNP mediated triggering mechanisms using light sources compatible with deep tissue penetrating wavelengths are desired. Near IR light penetrates into the tissue up to 10 cm, and bears additional advantages (please see section 7 later for details). Near IR-based drug release (using the AuNP) has been demonstrated for polymeric carriers (Radt et al. Citation2004, Goodwin et al. Citation2005, Skirtach et al. Citation2005, Das et al. Citation2007), and these systems are currently in the developmental stage (Portney & Ozkan Citation2006). However, near IR-based liposome-AuNP systems have not been fully explored presumably due to the technical limitations in synthesizing AuNPs particles susceptible to near IR light sources (Chen et al. Citation2007).
An alternate approach using the HGN was developed by Wu et al. (Wu et al. Citation2008b). The design of HGNs is shown in . This group synthesized 33 nm HGNs, either tethered to the liposome bilayer with a Au-SH-PEG-lipid linker or encapsulated within liposomes by an interdigitation-fusion method (Boyer & Zasadzinski Citation2007). Almost complete contents release was achieved within seconds (‘burst’ kinetics) from liposomes by irradiating the liposomes tethered with Au-SH-PEG-lipid linker by femtosecond pulses of the near IR laser. Since irradiation of HGNs is likely to produce localized heating within the vicinity of HGN, this temperature gradient was proposed to produce unstable vapor microbubbles leading to transient cavitation by the mechanical and thermal effects (Pecha & Gompf Citation2000, Popinet & Zaleski Citation2002). Therefore, these laser-heated HGNs act as optically triggered nano-‘sonicators’ for triggered release. Of note, when HGN were encapsulated within the liposomes, the near IR light-triggered release was not significant.
AuNP-liposomes, plasmonic nanobubble triggered release
The principle of ‘nano-sonication’ pertains to the generation of microbubbles by near IR-induced local heating in HGNs. Anderson and co-workers (Anderson et al. Citation2010) devised a new method of optically guided controlled release from liposomes-AuNPs by tunable plasmonic nanobubbles (PNBs). In this study, fluorescent proteins (R-phycoerythrin (PE) or allophycocyanin (APC)) were released by PNBs generated by irradiating 80 nm AuNPs (possibly trapped inside the liposome) with a single visible light short laser pulse. Upon absorption of a pulsed laser, AuNPs quickly evaporate the surrounding liquid medium to produce transient PNBs which can mechanically disrupt the liposome to release the entrapped content. PNBs besides inducing mechanical disruption and contents (drugs) release from liposomes, bears other significant advantages of being a non-thermal process in which the thermal energy is concentrated within PNBs (Lapotko Citation2009a, Lapotko Citation2009b), and thus protects the entrapped load within the liposome. PNBs have outstanding optical scattering properties that surpass those of AuNPs and fluorescent markers and can serve to control the release process.
Electromagnetic radiation: General considerations for therapy
Steady advancements in the laser-technology field have yielded devices with marked improvement in the wavelength range, output of light intensity, beam diameter regulation, and light responsiveness. These improvements have served as an added asset in the development of light-triggered treatment modalities including photo-triggerable nano-particles and PDT (Weissleder & Ntziachristos Citation2003).
It is evident that light-guided therapy is subject to the use of adequate light sources that can penetrate the tissues for drug delivery and therapeutic applications. Wavelength sources below 700 nm cannot penetrate deeply into tissues, because of scattering and a high level of endogenous absorbers, such as oxy- and deoxy-hemoglobin, lipids and water (Klohs et al. Citation2008). Near-infrared wavelengths (700–2500 nm) penetrate more than 1 cm depth into human skin and blood and have a low absorption coefficient with water and lipids and hemoglobin (the principal absorber of visible light) (Weissleder & Ntziachristos Citation2003). Therefore current light-guided therapy technologies have proved to be successful for skin-related diseases (Donnelly et al. Citation2006a,Mccoy et al. Citation2007) as well for oral treatments (Donnelly et al. Citation2006b), primarily because tissue depth was not a limitation. Since high energy/long wavelength sources are a requirement for treatment of deep tissues (Ackermann et al. Citation2002), currently available UV/visible light-photoactivable drug delivery systems (described in section 5b) are not suitable for clinical applications. Therefore, a key strategy will be to develop near IR-sensitive phototriggerable nanoparticles. Near IR is considered innocuous and does not cause a significant heating in the area of its application, therefore, such light source bears merit for triggered drug delivery and PDT (Bisby et al. Citation1999b). Interestingly, the type of organs (and tissues) as well as their location in the body also determines the success of light-guided therapies. For example, bladder is relatively more translucent than other human tissues and therefore has great potential for photodynamic therapy. Shackley and colleagues (Shackley et al. Citation1999, Shackley et al. Citation2000) reported that laser sources with wavelengths of 633–693 nm were potentially applicable for bladder PDT.
Future perspectives of photosensitive lipid-based nanoparticles in drug delivery
It can be imagined that nanotechnology platforms coupled with localized drug delivery technology will have significant impact on cancer therapy and other related diseases. Our understanding of the photochemistry of molecules as well as light-induced modifications in biological systems including various cellular processes provides a strong basis to develop nanoparticles that can be modulated by distinct sources of light in vivo. To date, various light-triggerable nanoparticles have been developed for their applications in drug delivery. Each of these systems stands on its own merit and has served as a sound proof of principle using the in vitro systems. However, the applications of these platforms for patient treatment are dependent on light-triggerable nanoparticles that will use tissue-penetrating light sources. The progress in laser technology is a definite asset toward success in this direction. Currently available photosensitive molecules are primarily responsive to UV or visible wavelengths. The combination of wavelength-specific photosensitizers with these molecules has been explored to enhance light-sensitive properties of the nanoparticles. We propose that a closer look at the chemical and physical properties of biocompatible metal ions (such as gold) to activate currently available photosensitive molecules at tissue-penetrating wavelengths is likely to be successful in future drug delivery applications.
Acknowledgements
We thank Drs Alok Singh and Jae-Ho Lee for critical reading of the manuscript.
Declaration of interest: This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- Ackermann G, Hartmann M, Scherer K, Lang EW, Hohenleutner U, Landthaler M, Baumler W. 2002. Correlations between light penetration into skin and the therapeutic outcome following laser therapy of port-wine stains. Lasers Med Sci 17:70–78.
- Alaouie AM, Sofou S. 2008. Liposomes with triggered content release for cancer therapy. J Biomed Nanotechnol 4:234–244.
- Allen TM. 1994. Long-circulating (sterically stabilized) liposomes for targeted drug-delivery. Trends Pharmacolog Sci 15:215–220.
- Allen TM. 1997. Liposomes. Opportunities in drug delivery. Drugs 54(Suppl. 4):8–14.
- Allen TM, Cullis PR. 2004. Drug delivery systems: entering the mainstream. Science 303(5665):1818–1822.
- Alonso MJ. 2004. Nanomedicines for overcoming biological barriers. Biomed Pharmacother 58:168–172.
- Alonso-Romanowski S, Chiaramoni NS, Lioy VS, Gargini RA, Viera LI, Taira MC. 2003. Characterization of diacetylenic liposomes as carriers for oral vaccines. Chem.Phys Lipids 122:191–203.
- Anderson LJE, Hansen E, Lukianova-Hleb EY, Hafner JH, Lapotko DO. 2010. Optically guided controlled release from liposomes with tunable plasmonic nanobubbles. J Control Release 144:151–158.
- Bakri SJ, Pulido JS, Mukherjee P, Marler RJ, Mukhopadhyay D. 2008. Absence of histologic retinal toxicity of intravitreal nanogold in a rabbit model. Retina 28:147–149.
- Batist G, Ramakrishnan G, Rao CS, Chandrasekharan A, Gutheil J, Guthrie T, Shah P, Khojasteh A, Nair MK, Hoelzer K, Tkaczuk K, Park YC, Lee LW. 2001. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J Clin Oncol 19:1444–1454.
- Bergen JM, Von Recum HA, Goodman TT, Massey AP, Pun SH. 2006a. Gold nanoparticles as a versatile platform for optimizing physicochemical parameters for targeted drug delivery. Macromolec Biosci 6:506–516.
- Bergen JM, Von Recum HA, Goodman TT, Massey AP, Pun SH. 2006b. Gold nanoparticles as a versatile platform for optimizing physicochemical parameters for targeted drug delivery. Macromolec Biosci 6:506–516.
- Berry CC, de la Fuente JM, Mullin M, Chu SW, Curtis AS. 2007. Nuclear localization of HIV-1 tat functionalized gold nanoparticles. IEEE Trans Nanobiosci 6:262–269.
- Bisby RH, Mead C, Mitchell AC, Morgan CG. 1999a. Fast laser-induced solute release from liposomes sensitized with photochromic lipid: effects of temperature, lipid host, and sensitizer concentration. Biochem Biophys Res Commun 262:406–410.
- Bisby RH, Mead C, Morgan CG. 1999b. Photosensitive liposomes as ‘cages’ for laser-triggered solute delivery: the effect of bilayer cholesterol on kinetics of solute release. FEBS Lett 463:165–168.
- Bisby RH, Mead C, Morgan CG. 2000a. Active uptake of drugs into photosensitive liposomes and rapid release on UV photolysis. Photochem Photobiol 72:57–61.
- Bisby RH, Mead C, Morgan CG. 2000b. Wavelength-programmed solute release from photosensitive liposomes. Biochem Biophys Res Commun 276:169–173.
- Bondurant B, O'Brien DF. 1998. Photoinduced destabilization of sterically stabilized liposomes. J Am Chem Soc 120:13541–13542.
- Boyer C, Zasadzinski JA. 2007. Multiple lipid compartments slow vesicle contents release in lipases and serum. Acs Nano 1:176–182.
- Chandra B, Mallik S, Srivastava DK 2005. Design of photocleavable lipids and their application in liposomal ‘uncorking’. Chem.Commun.(Camb.) (24):3021–3023.
- Chandra B, Subramaniam R, Mallik S, Srivastava DK. 2006. Formulation of photocleavable liposomes and the mechanism of their content release. Org Biomol Chem 4:1730–1740.
- Chen JY, Wang DL, Xi JF, Au L, Siekkinen A, Warsen A, Li ZY, Zhang H, Xia YN, Li XD. 2007. Immuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancer cells. Nano Lett 7:1318–1322.
- Chen YH, Gryshuk A, Achilefu S, Ohulchansky T, Potter W, Zhong TX, Morgan J, Chance B, Prasad PN, Henderson BW, Oseroff A, Pandey RK. 2005a. A novel approach to a bifunctional photosensitizer for tumor imaging and phototherapy. Bioconj Chem 16:1264–1274.
- Chen YH, Miclea R, Srikrishnan T, Balasubramanian S, Dougherty TJ, Pandey RK. 2005b. Investigation of human serum albumin (HSA) binding specificity of certain photosensitizers related to pyropheophorbide-a and bacteriopurpurinimide by circular dichroism spectroscopy and its correlation with in vivo photosensitizing efficacy. Bioorg Med Chem Lett 15:3189–3192.
- Chiaramoni NS, Speroni L, Taira MC, Alonso SV. 2007. Liposome/DNA systems: correlation between association, hydrophobicity and cell viability. Biotechnol Lett 29:1637–1644.
- Chithrani DB, Dunne M, Stewart J, Allen C, Jaffray DA. 2010. Cellular uptake and transport of gold nanoparticles incorporated in a liposomal carrier. Nanomed Nanotechnol Biol Med 6:161–169.
- Chowdhary RK, Green CA, Morgan CG. 1993. Dye-sensitized destabilization of liposomes bearing photooxidizable lipid head groups. Photochem Photobiol 58:362–366.
- Cole DH, Shull KR, Baldo P, Rehn L. 1999. Dynamic properties of a model polymer/metal nanocomposite: gold particles in poly(tert-butyl acrylate). Macromolecules 32:771–779.
- Cormode DP, Skajaa T, Fayad ZA, Mulder WJ. 2009. Nanotechnology in medical imaging: probe design and applications. Arterioscler Thromb Vasc Biol 29:992–1000.
- Das M, Sanson N, Fava D, Kumacheva E. 2007. Microgels loaded with gold nanorods: hotothermally triggered volume transitions under physiological conditions. Langmuir 23:196–201.
- Davis ME, Chen ZG, Shin DM. 2008. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov 7:771–782.
- De Rosa FS, Bentley MV. 2000. Photodynamic therapy of skin cancers: sensitizers, clinical studies and future directives. Pharm Res 17:1447–1455.
- Derycke AS, Kamuhabwa A, Gijsens A, Roskams T, De VD, Kasran A, Huwyler J, Missiaen L, De Witte PA. 2004. Transferrin-conjugated liposome targeting of photosensitizer AlPcS4 to rat bladder carcinoma cells. J Natl Cancer Inst 96:1620–1630.
- Donnelly RF, Ma LW, Juzenas P, Iani V, McCarron PA, Woolfson AD, Moan J. 2006a. Topical bioadhesive patch systems enhance selectivity of protoporphyrin IX accumulation. Photochem Photobiol 82:670–675.
- Donnelly RF, McCarron PA, Ma LW, Juzenas P, Iani V, Woolfson AD, Zawislak AA, Moan J. 2006b. Facilitated delivery of ALA to inaccessible regions via bioadhesive patch systems. J Environ Pathol Toxicol Oncol 25:389–402.
- Donnelly RF, McCarron PA, Morrow DI, Sibani SA, Woolfson AD. 2008. Photosensitiser delivery for photodynamic therapy. Part 1: Topical carrier platforms. Expert Opin Drug Del 5:757–766.
- Faure C, Derre A, Neri W. 2003. Spontaneous formation of silver nanoparticles in multilamellar vesicles. J Phys Chem B 107:4738–4746.
- Fenske DB, Chonn A, Cullis PR. 2008. Liposomal nanomedicines: an emerging field. Toxicol Pathol 36:21–29.
- Fenske DB, Cullis PR. 2008. Liposomal nanomedicines. Expert Opin Drug Deliv 5:25–44.
- Gabizon A, Martin F. 1997. Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours. Drugs 54(Suppl. 4):15–21.
- Ganta S, Devalapally H, Shahiwala A, Amiji M. 2008. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Rel 126:187–204.
- Gerasimov OV, Qualls M, Rui Y, Thompson DH 1997. Intracellular drug delivery using pH- and light-activated diplasmenylcholine liposomes. Abstr Papers Am Chem Soc 213:303-MSE.
- Gittins DI, Caruso F. 2001. Tailoring the polyelectrolyte coating of metal nanoparticles. J Phys Chem B 105:6846–6852.
- Goodwin AP, Mynar JL, Ma YZ, Fleming GR, Frechet JMJ. 2005. Synthetic micelle sensitive to IR light via a two-photon process. J Am Chem Soc 127:9952–9953.
- Han G, You CC, Kim BJ, Forbes NS, Martin CT, Rotello VM 2006. Light-regulated DNA transcription and delivery using photolabile gold nanoparticles. Abstr Papers Am Chem Soc 231.
- Haran G, Cohen R, Bar LK, Barenholz Y. 1993. Transmembrane ammonium-sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim Biophys Acta 1151:201–215.
- Haran G, Cohen R, Bar LK, Barenholz Y. 1994. Transmembrane ammonium-sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases (Vol 1151, Pg 201, 1993). Biochim Biophys Acta – Biomembr 1190:197.
- Harris N, Ford MJ, Cortie MB. 2006. Optimization of plasmonic heating by gold nanospheres and nanoshells. J Phys Chem B 110:10701–10707.
- Jaracz S, Chen J, Kuznetsova LV, Ojima I. 2005. Recent advances in tumor-targeting anticancer drug conjugates. Bioorg Med Chem 13:5043–5054.
- Jiang W, Kim BYS, Rutka JT, Chan WCW. 2008. Nanoparticle-mediated cellular response is size-dependent. Nature Nanotechnol 3:145–150.
- Juarranz A, Jaen P, Sanz-Rodriguez F, Cuevas J, Gonzalez S. 2008. Photodynamic therapy of cancer. Basic principles and applications. Clin Translat Oncol 10:148–154.
- Klabunde KJ, Stark J, Koper O, Mohs C, Park DG, Decker S, Jiang Y, Lagadic I, Zhang DJ. 1996. Nanocrystals as stoichiometric reagents with unique surface chemistry. J Phys Chem 100:12142–12153.
- Klohs J, Wunder A, Licha K. 2008. Near-infrared fluorescent probes for imaging vascular pathophysiology. Basic Res Cardiol 103:144–151.
- Kneipp J, Kneipp H, McLaughlin M, Brown D, Kneipp K. 2006. In vivo molecular probing of cellular compartments with gold nanoparticles and nanoaggregates. Nano Lett 6:2225–2231.
- Kronberg B, Dahlman A, Carlfors J, Karlsson J, Artursson P. 1990. Preparation and evaluation of sterically stabilized liposomes V colloidal stability, serum stability, macrophage uptake, and toxicity. J Pharmaceut Sci 79:667–671.
- Kumar S, Harrison N, Richards-Kortum R, Sokolov K. 2007. Plasmonic nanosensors for imaging intracellular biomarkers in live cells. Nano Lett 7:1338–1343.
- Lamparski H, Liman U, Barry JA, Frankel DA, Ramaswami V, Brown MF, O'Brien DF. 1992. Photoinduced destabilization of liposomes. Biochemistry 31:685–694.
- Lapotko D. 2009a. Optical excitation and detection of vapor bubbles around plasmonic nanoparticles. Optics Express 17:2538–2556.
- Lapotko D. 2009b. Pulsed photothermal heating of the media during bubble generation around gold nanoparticles. Int J Heat Mass Transfer 52:1540–1543.
- Lavi A, Weitman H, Holmes RT, Smith KM, Ehrenberg B. 2002. The depth of porphyrin in a membrane and the membrane's physical properties affect the photosensitizing efficiency. Biophys J 82:2101–2110.
- Lawson GE, Lee Y, Singh A. 2003a. Formation of stable nanocapsules from polymerizable phospholipids. Langmuir 19:6401–6407.
- Lawson GW, Breen JJ, Marquez M, Singh A, Smith BD. 2003b. Polymerization of vesicles composed of N-(4-vinylbenzoyl)phosphatidylethanolamine. Langmuir 19:3557–3560.
- Leamon CP, Low PS. 2001. Folate-mediated targeting: from diagnostics to drug and gene delivery. Drug Discov Today 6:44–51.
- Lewis LN. 1993. Chemical catalysis by colloids and clusters. Chem Rev 93:2693–2730.
- Li KC, Bednarski MD. 2002. Vascular-targeted molecular imaging using functionalized polymerized vesicles. J.Magn Reson Imaging 16:388–393.
- Li Z, Wan Y, Kutateladze AG. 2003. Dithiane-based photolabile amphiphiles: toward photolabile liposomes1,2. Langmuir 19:6381–6391.
- Liao JY. 2007. Construction of nanogold hollow balls with dendritic surface as immobilized affinity support for protein adsorption. Colloids Surf B Biointerfaces 57:75–80.
- Liu XM, Yang B, Wang YL, Wang JY. 2005. Photoisomerisable cholesterol derivatives as photo-trigger of liposomes: effect of lipid polarity, temperature, incorporation ratio, and cholesterol. Biochim Biophys Acta (BBA) – Biomembranes 1720:28–34.
- Majoros IJ, Williams CR, Baker JR, Jr. 2008. Current dendrimer applications in cancer diagnosis and therapy. Curr Top Med Chem 8:1165–1179.
- Markowitz MA, Dunn DN, Chow GM, Zhang J. 1999. The effect of membrane charge on gold nanoparticle synthesis via surfactant membranes. J Colloid Interface Sci 210:73–85.
- Mayer ABR, Mark JE. 1997. Comparisons between cationic polyelectrolytes and nonionic polymers for the protection of palladium and platinum nanocatalysts. J Polymer Sci Part A –Polymer Chem 35:3151–3160.
- Mccoy CP, Rooney C, Edwards CR, Jones DS, Gorman SP. 2007. Light-triggered molecule-scale drug dosing devices. J Am Chem Soc 129:9572.
- Morgan CG, Bisby RH, Johnson SA, Mitchell AC. 1995. Fast solute release from photosensitive liposomes: an alternative to ‘caged’ reagents for use in biological systems. FEBS Lett 375:113–116.
- Mueller A, Bondurant B, O'Brien DF. 2000. Visible-light-stimulated destabilization of PEG-liposomes. Macromolecules 33:4799–4804.
- Mulvaney P. 1996. Surface plasmon spectroscopy of nanosized metal particles. Langmuir 12:788–800.
- Nativo P, Prior IA, Brust M. 2008. Uptake and intracellular fate of surface-modified gold nanoparticles. Acs Nano 2:1639–1644.
- Needham D, Dewhirst MW. 2001. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Advanced Drug Del Rev 53:285–305.
- Negussie AH, Miller JL, Reddy G, Drake SK, Wood BJ, Dreher MR. 2010. Synthesis and in vitro evaluation of cyclic NGR peptide targeted thermally sensitive liposome. J.Control Release 143:265–273.
- Oku N, Ishii T. 2009. Antiangiogenic photodynamic therapy with targeted liposomes. Methods Enzymol Liposomes Pt G 465:313–330.
- Oyelere AK, Chen PC, Huang XH, El-Sayed IH, El-Sayed MA. 2007. Peptide-conjugated gold nanorods for nuclear targeting. Bioconjugate Chem 18:1490–1497.
- Paasonen L, Laaksonen T, Johans C, Yliperttula M, Kontturi K, Urth A. 2007. Gold nanoparticles enable selective light-induced contents release from liposomes. J Control Rel 122:86–93.
- Pakhomov S, Hammer RP, Mishra BK, Thomas BN. 2003. Chiral tubule self-assembly from an achiral diynoic lipid. Proc Natl Acad Sci USA 100:3040–3042.
- Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, Lee KD, Woodle MC, Lasic DD, Redemann C. 1991. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci USA 88:11460–11464.
- Park H, Jung SH, Kim DC, Shin SC, Cho CW. 2009. Development and validation of HPLC assay of a new molecule, 6-methyl-3-phenethyl-3, 4-dihydro-1H-quinazoline-2-thione from solid lipid nanoparticles and its topical formulations. J Liquid Chromatogr Rel Technol 32:512–525.
- Park SH, Oh SG, Mun JY, Han SS. 2006. Loading of gold nanoparticles inside the DPPC bilayers of liposome and their effects on membrane fluidities. Colloids Surfaces B-Biointerfaces 48:112–118.
- Pecha R, Gompf B. 2000. Microimplosions: cavitation collapse and shock wave emission on a nanosecond time scale. Phys Rev Lett 84:1328–1330.
- Pileni MP. 2001. Magnetic fluids: fabrication, magnetic properties, and organization of nanocrystals. Advanced Functional Mat 11:323–336.
- Popinet S, Zaleski S. 2002. Bubble collapse near a solid boundary: a numerical study of the influence of viscosity. J Fluid Mechanics 464:137–163.
- Portney NG, Ozkan M. 2006. Nano-oncology: drug delivery, imaging, and sensing. Anal Bioanal Chem 384:620–630.
- Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E, Blumenthal R. 2009. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carrier Syst 26: 523–80.
- Radt B, Smith TA, Caruso F. 2004. Optically addressable nanostructured capsules. Advanced Mat 16:2184.
- Reddy JA, Low PS. 1998. Folate-mediated targeting of therapeutic and imaging agents to cancers. Crit Rev Ther Drug Carrier Syst 15:587–627.
- Regen SL, Singh A, Oehme G, Singh M. 1981. Polymerized phosphatidyl choline vesicles. Stabilized and controllable time-release carriers. Biochem Biophys Res Commun 101:131–136.
- Regev O, Backov R, Faure C. 2004. Gold nanoparticles spontaneously generated in onion-type multilamellar vesicles. Bilayers particle coupling imaged by Cryo-TEM. Chem Mat 16:5280–5285.
- Robertson CA, Evans DH, Abraharnse H 2009. Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J Photochem Photobiol B–Biol 961–8.
- Rui YJ, Thompson DH. 1994. Stereocontrolled synthesis of plasmalogen-type lipids from glyceryl ester precursors. J Organic Chem 59:5758–5762.
- Rui YJ, Thompson DH. 1996. Efficient stereoselective synthesis of plasmenylcholines. Chem-A Eur J 2:1505–1508.
- Rzigalinski BA, Strobl JS. 2009. Cadmium-containing nanoparticles: perspectives on pharmacology and toxicology of quantum dots. Toxicol Appl Pharmacol 238:280–288.
- Sandhu KK, McIntosh CM, Simard JM, Smith SW, Rotello VM. 2002. Gold nanoparticle-mediated transfection of mammalian cells. Bioconjugate Chem 13:3–6.
- Schmid G, Baumle M, Geerkens M, Helm I, Osemann C, Sawitowski T. 1999. Current and future applications of nanoclusters. Chem Soc Rev 28:179–185.
- Shackley DC, Whitehurst C, Clarke NM, Betts C, Moore JV. 1999. Photodynamic therapy. J Roy Soc Med 92:562–565.
- Shackley DC, Whitehurst C, Moore JV, George NJR, Betts CD, Clarke NW. 2000. Light penetration in bladder tissue: implications for the intravesical photodynamic therapy of bladder tumours. Bju Int 86:638–643.
- Shukla R, Bansal V, Chaudhary M, Basu A, Bhonde RR, Sastry M. 2005. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir 21:10644–10654.
- Shum P, Kim JM, Thompson DH. 2001. Phototriggering of liposomal drug delivery systems. Adv.Drug Deliv Rev 53:273–284.
- Sibani SA, McCarron PA, Woolfson AD, Donnelly RF. 2008. Photosensitiser delivery for photodynamic therapy. Part 2: systemic carrier platforms. Expert Opin Drug Del 5:1241–1254.
- Singh A. 1990. An efficient synthesis of phosphatidylcholines. J Lipid Res 31:1522–1525.
- Singh A, Marchywka S. 1989. Synthesis and characterization of head group modified 1,3 diacetylenic phospholipids. Polym Mat Sci Eng 61:675–678.
- Singh A, Gaber BP 1988. Influence of short chain lipid spacers on the properties of diacetylenic phosphotidylcholine bilayers in applied bioreactive polymers. In: Gebelein CC, editor. Applied bioactive polymers. New York: Plenum Press, 239–249.
- Skirtach AG, Dejugnat C, Braun D, Susha AS, Rogach AL, Parak WJ, Mohwald H, Sukhorukov GB. 2005. The role of metal nanoparticles in remote release of encapsulated materials. Nano Lett 5:1371–1377.
- Sokolov K, Follen M, Aaron J, Pavlova I, Malpica A, Lotan R, Richards-Kortum R. 2003. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res 63:1999–2004.
- Souza GR, Christianson DR, Staquicini FI, Ozawa MG, Snyder EY, Sidman RL, Miller JH, Arap W, Pasqualini R. 2006. Networks of gold nanoparticles and bacteriophage as biological sensors and cell-targeting agents. Proc Nat Acad Sci USA 103:1215–1220.
- Thompson DH, Gerasimov OV, Wheeler JJ, Rui Y, Anderson VC. 1996. Triggerable plasmalogen liposomes: improvement of system efficiency. Biochim Biophys Acta 1279:25–34.
- Tiwari SB, Amiji MM. 2006. Improved oral delivery of paclitaxel following administration in nanoemulsion formulations. J Nanosci Nanotechnol 6:3215–3221.
- Tkachenko AG, Xie H, Liu YL, Coleman D, Ryan J, Glomm WR, Shipton MK, Franzen S, Feldheim DL. 2004. Cellular trajectories of peptide-modified gold particle complexes: comparison of nuclear localization signals and peptide transduction domains. Bioconjugate Chem 15:482–490.
- Tomalia DA, Reyna LA, Svenson S. 2007. Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem Soc Trans 35(Pt 1):61–67.
- Torchilin VP. 2005. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov 4:145–160.
- Torchilin VP. 2007. Targeted pharmaceutical nanocarriers for cancer therapy and Imaging. Aaps J 9:E128–E147.
- varez-Lorenzo C, Bromberg L, Concheiro A. 2009. Light-sensitive intelligent drug delivery systems. Photochem Photobiol 85:848–860.
- Wang SX, Bao A, Phillips WT, Goins B, Herrera SJ, Santoyo C, Miller FR, Otto RA. 2009. Intraoperative therapy with liposomal drug delivery: retention and distribution in human head and neck squamous cell carcinoma xenograft model. Int J Pharm 373:156–164.
- Weissleder R, Ntziachristos V. 2003. Shedding light onto live molecular targets. Nat Med 9:123–128.
- Weller D, Moser A. 1999. Thermal effect limits in ultrahigh-density magnetic recording. IEE Transact Magnetics 35:4423–4439.
- Wilcoxon JP, Martin JE, Parsapour F, Wiedenman B, Kelley DF. 1998. Photoluminescence from nanosize gold clusters. J Chem Phys 108:9137–9143.
- Wu G, Mikhailovsky A, Khant HA, Fu C, Chiu W, Zasadzinski JA. 2008a. Remotely triggered liposome release by near-infrared light absorption via hollow gold nanoshells. J Am Chem Soc 130:8175–8177.
- Wu G, Mikhailovsky A, Khant HA, Fu C, Chiu W, Zasadzinski JA. 2008b. Remotely triggered liposome release by near-infrared light absorption via hollow gold nanoshells. J Am Chem Soc 130:8175–8177.
- Yang T, Choi MK, Cui FD, Lee SJ, Chung SJ, Shim CK, Kim DD. 2007. Antitumor effect of paclitaxel-loaded PEGylated immunoliposomes against human breast cancer cells. Pharmaceut Res 24:2402–2411.
- Yavlovich A, Singh A, Tarasov S, Capala J, Blumenthal R, Puri A. 2009. Design of liposomes containing photopolymerizable phospholipids for triggered release of contents. J Thermal Anal Calorimetry 98:97–104.
- Zarif L. 2002. Elongated supramolecular assemblies in drug delivery. J Control Rel 81:7–23.
- Zhang ZY, Smith BD. 1999. Synthesis and characterization of NVOC-DOPE, a caged photoactivatable derivative of dioleoylphosphatidylethanolamine. Bioconjugate Chem 10:1150–1152.
- Zhou Y. 2008. Lipid nanotubes: formation, templating nanostructures and drug nanocarriers. Crit Rev Solid State Materials Sci 33:183–196.