Abstract
Efficient and site-specific delivery of therapeutic drugs is a critical challenge in clinical treatment of cancer. Nano-sized carriers such as liposomes, micelles, and polymeric nanoparticles have been investigated for improving bioavailability and pharmacokinetic properties of therapeutics via various mechanisms, for example, the enhanced permeability and retention (EPR) effect. Further improvement can potentially be achieved by conjugation of targeting ligands onto nanocarriers to achieve selective delivery to the tumour cell or the tumour vasculature. Indeed, receptor-targeted nanocarrier delivery has been shown to improve therapeutic responses both in vitro and in vivo. A variety of ligands have been investigated including folate, transferrin, antibodies, peptides and aptamers. Multiple functionalities can be incorporated into the design of nanoparticles, e.g., to enable imaging and triggered intracellular drug release. In this review, we mainly focus on recent advances on the development of targeted nanocarriers and will introduce novel concepts such as multi-targeting and multi-functional nanoparticles.
Keywords::
Introduction
Cancer is a leading cause of death in the US and around the world. A majority of anticancer agents in clinical use today are chemotherapeutics given systemically. These are toxic not only to cancerous cells but also to proliferating normal cells, such as those of the bone marrow and the intestinal epithelium. This can lead to severe side-effects and treatment failure. Therefore, improving the therapeutic index by increasing therapeutic effects to tumour cells and decreasing toxicity to healthy tissues is a central issue in improving cancer therapy. One strategy towards this goal is to develop new drugs that specifically interfere with intracellular pathways exclusive to cancer cells (Neidle and Thurston Citation2005). For example, a tyrosine kinase inhibitor imatinib (Gleevec) targeting the BCR-ABL oncogene was successfully used in treating chronic myelogenous leukaemia (CML) (Allen Citation2002, Sawyers Citation2004). In addition, a number of therapeutic monoclonal antibodies have entered clinical use, e.g., trastuzumab (Herceptin) targeting HER2 receptor overexpressed in a subset of breast cancer (Allen Citation2002, Sawyers Citation2004). An alternative strategy for developing a targeted drug is to design a receptor-targeted delivery system, which either can be based on direct coupling of an anticancer drug to a ligand or based on encapsulation of the drug into a ligand-directed drug carrier (Arap et al. Citation1998, Allen Citation2002, Torchilin Citation2007). For example, Mylotarg, an anti-CD33-calicheamicin immunoconjugate, has been developed for the treatment of acute myelogenous leukemia (AML) (Sharkey and Goldenberg Citation2006, Citation2008). In general, however, immunoconjugates have a relatively low achievable drug/antibody ratio (usually 3 ∼ 10 drug molecules/antibody). Only a few extremely potent drugs can be targeted in this fashion, precluding the use of this delivery technology on most existing chemotherapeutic agents (Allen Citation2002, Sharkey and Goldenberg Citation2008). Furthermore, direct conjugation of drugs to the targeting ligand may adversely impact the targeting molecule by disrupting ligand/receptor recognition and potentially alter the cytotoxicity of the drug (Allen Citation2002, Noble et al. Citation2004). As an alternative, receptor-targeted nanocarrier drug delivery system is an emerging platform in development of cancer therapy (Arap et al. Citation1998, Allen Citation2002, Sapra and Allen Citation2003, Noble et al. Citation2004, CitationEl Bayoumi and Torchilin Citation2009).
Nano-sized carriers (10 ∼ 400 nm) are desirable as drug carriers because they possess the advantages of being capable of carrying large amount of drugs, having prolonged circulation time (especially when surface PEGylated), and facilitating selective tumour accumulation via the enhanced permeability and retention (EPR) effect (Jain Citation1999, Allen and Cullis Citation2004, Alexis et al. Citation2008, Soussan et al. Citation2009). Nanocarriers are also helpful in addressing other limitations of conventional drugs, including poor aqueous solubility, low bioavailability and/or unfavourable pharmacokinetic properties. In addition, delivery via nanocarriers has been reported to overcome multidrug resistance (MDR) caused by drug efflux transporters such as the P-glycoprotein, which are frequently overexpressed in cancer cells (Gottesman et al. Citation2002, Piddock Citation2006). To date, a variety of nanocarriers (e.g., liposomes, micelles, and polymeric nanoparitcles) have demonstrated efficacy both in vitro and in vivo (Allen and Cullis Citation2004, Duncan Citation2006, Peer et al. Citation2007, Davis et al. Citation2008, Jagur-Grodzinski Citation2009). To achieve higher specificity, nanocarriers can be surface modified with ligands that specifically recognize receptors on tumour cells. Combining passive and active targeting in a single platform may further improve the therapeutic index of nanocarrier delivered drugs.
A number of recent reviews have provided perspectives on the use of various types of nanocarriers as diagnostic tools and nanomedicines in cancer research (Allen Citation2002, Noble et al. Citation2004, Allen and Cullis Citation2004, Torchilin Citation2005b, Citation2006a, Wagner et al. Citation2006, Peer et al. Citation2007, Farokhzad and Langer Citation2009, Gindy and Prud'homme Citation2009, Riehemann et al. Citation2009, Yu et al. Citation2009). Here we will briefly discuss the various factors in designing targeted nanocarriers and commonly used ligands for these carriers, and summarize recent progress in this field.
Design of targeted nanocarriers
The feasibility of selective and efficient delivery of anticancer therapeutics using nanocarriers has been demonstrated in numerous studies. There are two major mechanisms: Passive targeting and active targeting. Important factors to be considered include carrier composition and selection of targeting ligand.
Passive versus active targeting
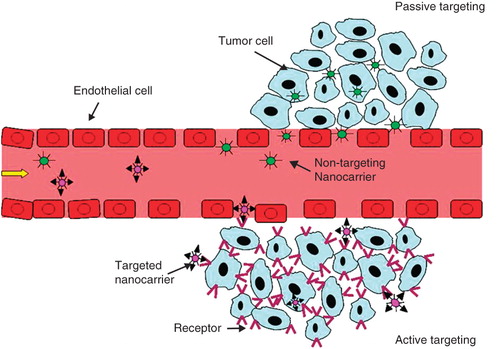
In contrast to normal tissues, many solid tumours possess unique structural features of hyperpermeable vasculature and impaired lymphatic drainage (Matsumura and Maeda Citation1986, Hobbs et al. Citation1998). As a result, tumour tissues are relatively permeable to macromolecules and nanocarriers (Jain Citation1999, Torchilin Citation2005b, Gu et al. Citation2007, Peer et al. Citation2007, Gullotti and Yeo Citation2009, Shi et al. Citation2009b). Passive targeting, therefore, refers to the selective extravasation and retention of long-circulating nanocarriers at tumour sites due to the enhanced permeability and retention (EPR) effect. In contrast, active targeting is based on specific interactions between the nanocarrier and receptors on the target cell, which may also promote internalization of nanocarriers through receptor mediated endocytosis ().
Figure 1. A schematic diagram representing the accumulation of nanocarriers in tumor sites by passive or active targeting. Both targeted nanocarriers and non-targeted nanocarriers reach tumors selectively through the leaky vasculature in the tumors. Upon arrival at tumor sites, targeted nanocarriers can bind to the target tumor cells or enter the cells via receptor mediated endocytosis.
To take full advantage of the EPR effect, it is critical to incorporate several properties into the design of nanocarriers. A key consideration is the need for long circulation time in the blood stream, required for extravasation. It has been shown that the threshold size for extravasation in tumours is ∼400 nm in diameter, and that nanocarriers with diameters of less than 200 nm are preferred (Yuan et al. Citation1995, Hobbs et al. Citation1998). On the other hand, it is known that the kidneys are capable of filtering particles significantly smaller than 10 nm (about 70,000 Da) (Caliceti and Veronese Citation2003, Alexis et al. Citation2008). Therefore, the particle size of nanocarrier should be between 10 and 200 nm for tumour delivery. Surface charge of nanocarriers is another important parameter. Both highly positive and highly negative charged nanocarriers are susceptible to rapid clearance by the reticuloendothelial system (RES) (Li and Huang Citation2008). Thus, it is important to design nanocarriers with either a neutral or a slight negative zeta potential. In addition, a common method for reducing the recognition of nanocarriers by the RES is to coat their surfaces with polyethylene glycol (PEG) (Caliceti and Veronese Citation2003, Alexis et al. Citation2008). Due to steric effect of the hydrophilic PEG, the binding of nanocarriers to opsonins, which promotes RES clearance, is significantly reduced, resulting in prolonged circulation time and increased accumulation at the tumour sites via EPR.
Passive targeting only facilitates the efficient localization of nanocarriers in the tumour interstitium. It cannot further promote their uptake by cancer cells. For this reason, receptor-based active targeting strategies are being investigated for nanocarriers. In addition to specific interactions between the ligands on the surface of nanocarriers and receptors expressed on the tumour cells, this may trigger receptor-mediated endocytosis. Furthermore, active targeting has shown the potential to suppress multidrug resistance (MDR) via bypassing of P-glycoprotein-mediated drug efflux (Gottesman et al. Citation2002, Gu et al. Citation2007, Peer et al. Citation2007).
Interestingly, many studies have revealed no significant differences in the overall levels of tumour accumulation between non-targeted nanocarriers and receptor targeted nanocarriers (Alexis et al. Citation2008, Gullotti and Yeo Citation2009). This is because both types of nanocarriers can selectively reach tumour sites via EPR. The targeting ligands may not play a role until the targeted nanocarriers extravasate at the tumour sites. Nevertheless, the therapeutic efficacy of anticancer drugs in targeted nanocarriers can be improved dramatically due to receptor-mediated internalization and intracellular drug release. Notable exceptions to this include when the target is on the tumour endothelial cells rather than the tumour cells, and when the targeted cells reside in the vascular compartment or in tissues with high accessibility to the vasculature. In these situations, targeting occurs relatively quickly and does not require extravasation of the nanocarriers. As an example of vasculature targeting, there have been numerous reports of nanocarriers conjugated to cyclic RGD peptide (targeting neovasculature marker αvβ3 integrin), VEGF or anti-VEGFR (targeting VEGFR), or antibody or ligand targeting the prostate specific membrane antigen (PSMA). These have shown significant improvements in therapeutic efficacy in vivo. Meanwhile, various types of leukemia constitute examples of target cells having high accessibility from the circulation without the requirement for extravasation due to the vascular localization of the leukemia cells. In fact, most therapeutic antibodies approved to date have been targeting leukemias (e.g., rituxan, zevalin, bexxar, campath, mylotarg).
Commonly used nanocarriers
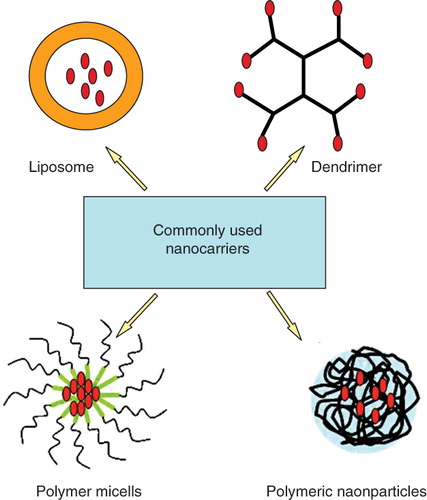
Nanocarriers refer to nano-sized particles that are capable of carrying drugs. Several classes of materials have been developed for nanocarriers, including lipids (liposomes), biocompatible polymers (e.g., polymeric nanoparticles), and surfactants (micelles) (Duncan Citation2003, Allen and Cullis Citation2004, Torchilin Citation2005b, Duncan Citation2006, Farokhzad and Langer Citation2009, Soussan et al. Citation2009) (). Drugs can be encapsulated in a vesicle, entrapped in a matrix, or solubilized within a hydrophilic or a hydrophobic component (Bonacucina et al. Citation2009, Mishra et al. Citation2010).
Liposomes are self-assembling vesicles composed of lipid bilayers surrounding an aqueous compartment. Hydrophilic drugs are readily encapsulated in the aqueous core while lipophilic drugs are solubilized within the lipid bilayer (Torchilin Citation2005b, Abu Lila et al. Citation2009). Liposomes carrying chemotherapeutic drugs such as doxorubicin (Doxil®) and daunorubicin (DaunoXome®) have been approved by FDA since the mid-1990s (Lammers et al. 2008). Micelles are composed of surfactants such as amphiphilic block-copolymers that self-assemble into nano-aggregates (5–50 nm). Drugs can be entrapped in the core of micelles (Torchilin Citation2005a, Aliabadi and Lavasanifar Citation2006, Peer et al. Citation2007). SP1049C, a micelle formulation of doxorubicin comprising pluronics, and NK911, another micelle formulation of doxorubicin comprising polyethylene glycol-poly(aspartic acid) block copolymer have been studied in clinical trials (Danson et al. Citation2004, Matsumura et al. Citation2004). Dendrimers are well-defined branched macromolecules with high molecular uniformity and narrow polydispersity. Hydrophobic drugs can be entrapped in the hydrophobic interior of dendrimers to promote solubilization. Drugs can also be covalently conjugated onto the surface of the dendrimer (Duncan and Izzo Citation2005, Nanjwade et al. Citation2009). Polymeric nanoparticles are generally nanosized polymeric matrix by which drug can be physically entrapped via the association between the drug and polymer or chemically conjugated through the covalent bond between the drug and polymer (Duncan Citation2006, Jagur-Grodzinski Citation2009). Alternative to polymeric nanocarriers, several polymeric macromolecular conjugates, such as Oncaspar (PEG-L-asparaginase), PEG-INTRON (PEG-α-interferon 2b), and Zinostatin (Styrene maleic anhydride), have been clinically approved for the treatment of various types of cancers (Duncan Citation2003).
Targeting ligands for nanocarriers
Various types of targeting ligands have been exploited for constructing targeted nanocarriers (), as discussed in recent reviews (Allen Citation2002, Sapra and Allen Citation2003, Noble et al. Citation2004, Gu et al. Citation2007, Phillips et al. Citation2008, Vives et al. Citation2008, Yan and Levy Citation2009, Veiseh et al. Citation2010). The targeted receptor can either be tumour-specific or tissue-specific. Tumour-specific ligands also include receptors upregulated in tumour-associated vasculature. It may not be very feasible to identify unique receptors expressed only on tumours but not on normal cells. Therefore, tissue-specific ligands or ligands overexpressed on tumour cells are frequently used, which are designed to reduce side-effects to other normal tissues. For examples, galactose derivatives, which targets the asialoglycoprotein receptor (ASGPR) (Wu et al. Citation2002) (expressed on hepatocytes and on hepatoma cells), rituximab, which targets CD20 (Allen Citation2002) (expressed on normal and malignant B cells), and transferrin (Tf), which targets the Tf receptor (Qian et al. Citation2002, Daniels et al. Citation2006) (expressed on normal tissues and, often at elevated levels on tumour cells) have proven to be useful ligands for tumour targeting. Other important factors to consider include expression level of receptor on tumours, internalization capacity and rate, receptor binding affinity, ligand sizes, immunogenicity, as well as availability. In general, nanocarriers with internalizing antibody show better delivery efficiency than those with non-internalizing antibody (Allen Citation2002). However, in some cases, a non-internalizing antibody that only binds on cell surface may have the benefit of promoting greater bystander effects and enabling immunological mechanisms, such as antibody-dependent cellular cytotoxicity (ADCC) (Clynes et al. Citation2000). Examples of potential targeting ligand are folate, transferrin, antibodies and peptides. Folate receptor (FR) is frequently overexpressed in a number of human tumours such as ovarian, colorectal and breast cancer, while not expressed in most normal tissues (Low et al. Citation2008, Zhao et al. Citation2008). Thus, FR is a highly selective tumour marker. Linking folic acid to liposomes or polymers has been shown to facilitate tumour-specific delivery of anticancer drugs. Similar to other low molecular weight ligands, folic acid has advantages of simple conjugation chemistry, non-immunogenicity and low cost. Transferrin receptor (TfR) is highly upregulated on many tumour cells due to their higher demand for iron (Daniels et al. Citation2006). TfR-mediated delivery can be obtained via the chemical conjugation of Tf or anti-TfR antibody or antibody fragments onto nanocarriers. Due to the high specificity and affinity of antibody-antigen interaction, Ab mediated delivery is an attractive means for designing targeted nanocarriers. Besides whole Abs, antibody fragments such as Fab and scFv can be used for constructing targeted nanocarriers (Allen Citation2002). Potential problems include immunogenicity, high cost and large ligand size. As an alternative to antibodies, peptides have gained much attention as targeting ligands because of their small size, lower immunogenicity and higher stability as well as easy manufacture (Vives et al. Citation2008). For instance, cyclic versions of the arginine-glycine-aspartic acid (RGD) peptide that targets to αvβ3 integrin receptor has been used as a targeting ligand to tumour vasculature (Sipkins et al. Citation1998, Ruoslahti et al. Citation2010). The αvβ3 integrin is highly expressed in tumour vasculature and various metastatic cancer cells (Ruoslahti Citation2002, Desgrosellier and Cheresh Citation2010). The use of new screening technologies such as phage display has led to the discovery of novel peptides with high binding affinity and specificity towards various cells, tissues and organs (Balestrieri and Napoli Citation2007, Vives et al. Citation2008).
Table I. Commonly used targeting ligands for targeted nanocarriers.
Conjugation strategies in targeted nanocarriers
The construction of targeted nanocarriers involves the association of targeting ligands on the nanocarriers through either chemical conjugation or physical interaction. Chemical conjugation provides a stable linkage between targeting ligands and nanocarriers. The conjugation can be performed either before or after nanocarrier formation. Thioether, disulfide, and amide covalent linkages are frequently incorporated into the chemical conjugation of ligands on the surface of nanocarriers (Nobs et al. Citation2004). To maximize the targeting efficiency, PEG is usually used as a linker to increase the distance between nanocarriers and targeting ligands, thereby reducing the steric interference of nanocarriers to receptor binding. A streptavidin-biotin affinity-based non-covalent conjugation strategy also has frequently been used in targeted nanocarriers (Nobs et al. Citation2004, Shi et al. Citation2009b).
Recent advances in targeted nanocarriers
New tumour targets and tumour vasculature targets are being discovered and developed for nanocarrier targeting based on the use of novel technologies such as protein engineering, phage display and aptamer screening. A number of novel concepts have been investigated in developing targeted nanocarriers, e.g., a dual receptor targeting strategy that combines two ligands on the same nanocarriers (Laginha et al. Citation2005, Saul et al. Citation2006), and an organelle-targeted delivery strategy (Torchilin Citation2006b, Yousif et al. Citation2009). Moreover, novel designs have integrated more functions in nanocarriers, including simultaneous delivery of anticancer drugs and nucleic acids (e.g., siRNA), imaging agents (e.g., quantum dots), and stimuli-responsive components (e.g., light, temperature and pH-sensitive materials) (Torchilin Citation2006a, 2009, Ganta et al. Citation2008, McCarthy and Weissleder Citation2008, Farokhzad and Langer Citation2009, Patil et al. Citation2010, Veiseh et al. Citation2010). These multifunctional nanocarriers can be used to visualize their distribution in the body by non-invasive imaging methods, and can possibly monitor treatment response in real time. The combination of diagnostic and therapeutic functions in nanoparticles (theranostic nanoparticles) represents a new trend in academic research in the area.
Development of novel ligands for targeted nanocarriers
Novel ligands for tumour and tumour vasculature targeting
Representative novel ligands for targeted nanocarriers and their application in anticancer therapy are listed in . Generally, receptors for endogenous ligands consist of transferrin receptor, carbohydrates (lectins) receptors, hormone receptors, mannose receptor, asialoglycoprotein (galactose) receptor, lipoprotein receptors, macroglobulin receptor, epidermal growth factor (EGF) receptor, platelet-derived growth factor (PDGF) receptor, vasculature receptors and insulin receptor. Antibodies are often used for construction of targeted nanocarriers. As illustrated in , some novel Abs like anti-Tf, A7 antibody and 5D4 antibody et al. are used in targeted nanocarriers (Sharkey and Goldenberg Citation2008). Whole antibodies have poor tumour penetration and may promote clearance via FcR by macrophages (Carter Citation2001). Therefore, antibody fragments including antigen-binding fragments (Fab) and single-chain variable fragment (scFv) can be used. For example, a low-affinity scFv against the ErbB2 has been shown to enhance tumour localization of nanocarriers in a mouse tumour model (De Lorenzo et al. Citation2002). More recently, newer classes of engineered proteins or protein-like molecules have been developed as targeting ligands for targeted nanocarriers. These include affibody (a small protein domain) (Wikman et al. Citation2004), avimers (small protein acting as antibody) (Silverman et al. Citation2005) and nanobody (a heavy-chain evolved from antibody) (Cortez-Retamozo et al. Citation2004). In comparison with intact antibodies, these smaller molecules have been shown as enhanced tumour penetration and lower immunogenicity. Derived from the phage display technology, a 7 kD affibody molecule is selected to bind EGFR receptor with sub-nanomolar affinity (Wikman et al. Citation2004). Beuttler et al. (Citation2009) reported that anti-EGFR affibody molecule can be easily conjugated on liposomes to obtain EGFR targeted nanocarriers.
Table II. Examples of novel ligands for targeted nanocarriers.
Aptamers are short single-stranded DNA or RNA oligonucleotides (6 ∼ 26 kDa) that fold into well-defined 3D structures that recognize a variety of biological molecules including transmembrane proteins, sugars and nucleic acids with high affinity and specificity (Phillips et al. Citation2008, Yan and Levy Citation2009). A technology called “‘systematic evolution of ligands by exponential enrichment’ (SELEX)” has been used to identify optimal aptamers for targets on cancer cells (Levy-Nissenbaum et al. Citation2008, Fang and Tan Citation2010). Aptamers have been conjugated to nanocarriers for targeted drug delivery. For example, the 2′-fluorinated A10 RNA aptamer, which recognizes the extracellular domain of the prostate-specific membrane antigen (PSMA), were conjugated on docetaxel nanoparticles and shown to have high selectivity for tumour and therapeutic efficacy in vivo (Farokhzad et al. Citation2006).
Peptides (10 ∼ 15 amino acids) are able to bind to target proteins, cells and tissues in a specific manner. Furthermore, they are characterized by reduced immunogenicity, high stability, and easy synthesis, scale-up, and chemical conjugations to nanocarriers. Phage display is emerging as the most popular approach (Paschke Citation2006), which has successfully isolated peptide ligands for somatostatin receptors, hormone receptors (LHRH receptors) (Norberto and Kakar Citation2009, Sundaram et al. Citation2009), and markers for the tumour vasculature (Balestrieri and Napoli Citation2007). In addition to targeting cell surface receptors, effective peptide ligands also have been developed for extracellular matrix (ECM) receptors such as heparin sulphate and hyaluronan (HA), which are overexpressed in tumours (Peer and Margalit Citation2004a, Citation2004b). HA targeted liposomes showed significantly improved circulation time and uptake by HA receptor-expressing tumours in vivo (Peer and Margalit Citation2004a).
Dual-ligand targeting strategies
To improve targeting efficiency, dual-ligand directed nanocarriers have been proposed. In biological systems, cell-cell recognition typically involves a multitude of recognition events, e.g., interactions between an antigen presenting cell and a T-cell. Similarly, simultaneous targeting of two receptors on cell surface could lead to greater affinity and specificity for nanocarriers. Indeed, several studies using dual-ligand directed nanocarriers have demonstrated significantly improved therapeutic efficiency in comparison with individual ligand conjugated nanocarriers. For example, Saul et al. (Citation2006) conjugated folic acid and mAb225 to nanocarriers for targeted delivery to KB cells. In another study, Laginha et al. (Citation2005) synthesized dual-Ab nanocarriers by post-insertion of internalizing (anti-CD19) and non-internalizing (anti-CD20) antibodies into liposomes for B-cell targeting. The interaction of CD19 and CD20 antibodies on the same liposome led to synergistic cytotoxicity, by simultaneously engaging two different antigen sites in close proximity on the same cell (Laginha et al. Citation2005). Dual-peptide nanocarriers against two receptors have also been reported recently (Kluza et al. Citation2010, Murase et al. Citation2010).
Intracellular organelle targeting
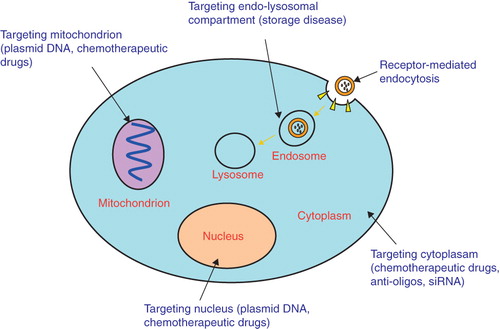
Many molecular drug targets are associated with specific subcellular compartments () (Mukhopadhyay and Weiner Citation2007, Yousif et al. Citation2009, Rajendran et al. Citation2010). Therefore, specifically directing therapeutic agents to an individual organelle is an attractive strategy for drug delivery. Nanocarriers can be designed as efficient delivery vehicles to intracellular organelles, including early-late endosomes, lysosomes, cytoplasm, mitochondria and nucleus. The sequestration of drug nanocarriers within endosomes/lysosomes following endocytosis is one of the most critical bottlenecks for cytoplasmic drug delivery, especially for high molecular weight drugs such as plasmid DNA and siRNAs. Strategies have been designed to increase endosome/lysosome escape through controlled membrane destabilization (e.g., triggered by acidic pH or reducing environment, or proteases). This can be accomplished, e.g., by incorporation of a fusogenic peptide (Kakudo et al. Citation2004, Futaki et al. Citation2005). Alternately, nanocarriers can be designed to bypass the endosomal route. This strategy can be achieved by conjugating cell-penetrating peptides to nanocarriers (Brooks et al. Citation2005, Vives et al. Citation2008, Zhang et al. Citation2009). In order to direct drug to mitochondria or the nucleus, specific trafficking signals may be attached on the nanocarriers, e.g., a nuclear localization signal (NLS) (Tkachenko et al. Citation2003, Misra and Sahoo Citation2010) and a mitochondrial targeting signal peptide (MTS).
Figure 3. Schematic illustration of the basic intracellular targets including endo-lysosomal compartment, mitochondrion, cytoplasm, nucleus for drug, gene and oligo deliveries.
Mitochondrion is a promising therapeutic drug target due to its important role in energy supply and cell death regulation. A variety of methods have been developed to enhance mitochondrial accumulation of drugs (Mukhopadhyay and Weiner Citation2007, Yamada and Harashima Citation2008, Yousif et al. Citation2009). Harashima and Yamada (2008) described a lipid-based carrier multifunctional envelope-type nano-device (MEND) and MITO-Porter. MITO-Porter consists of liposomes conjugated to octaarginine (R8) peptides, which facilitate uptake of the entire assembly into cells by macropinocytosis. Following endosomal escape, MITO-Porter is able to further fuse with the mitochondrial outer membrane due to fusogenic lipids in the liposome (Yamada et al. Citation2008). The dequalinium (DQA)-based liposomes constitute another class of mitochondrial delivery nanocarriers. DQA is a symmetrical delocalized lipophilic divalent cation, which promotes efficient mitochondrial localization (Weissig et al. Citation1998, D'Souza et al. Citation2003). Furthermore, surface modification of liposomes with mitochondriotropic triphenylphosphonium (TPP) cations has been reported to promote the efficient subcellular delivery of a model drug to mitochondria both in vitro and in vivo (Boddapati et al. Citation2008).
Novel delivery strategies for targeted nanocarriers
Novel drug nanocarrier compositions
In parallel to developing novel targeting ligands, novel nanocarrier compositions have also been extensively investigated in the past decade, e.g., minicells (MacDiarmid et al. Citation2007a, 2007b), apotransferrin (Krishna et al. Citation2009) and synthetic HDL or LDL lipid nanoparticles (Nikanjam et al. Citation2007, Thaxton et al. Citation2009). Moreover, inorganic drug delivery systems such as nanodiamond and single wall carbon nanotube also demonstrated some potential (Bhirde et al. Citation2009, Lam and Ho Citation2009). However, among the drug nanocarriers reported, natural and synthetic polymers and lipids remain the dominant materials for constructing drug delivery nanocarriers due to their proven biocompatibility.
MacDiarmid et al. (Citation2007a, Citation2007b, Citation2009) described a new type of nanocarrier known as minicells. These are ∼400 nm in diameter and are derived from achromosomal bacterial cells. They are immunostimulatory, can stably encapsulate a variety of chemotherapeutics or siRNAs and be specifically targeted using antibodies (MacDiarmid et al. Citation2007b). Greater tumour growth inhibition was observed with minicell delivery of doxorubicin (DOX) or paclitaxel than with the treatment of ∼1875-fold and ∼8000-fold higher amounts of their respective free drugs (MacDiarmid et al. Citation2007a, 2007b).
Nanoparticles made of human serum albumin (HSA) possess several specific advantages including biocompatibility, ease of preparation and covalent modification with targeting ligands. Enrichment of the HSA nanoparticles in tumour tissue may occur by passive or active targeting mechanisms (Hawkins et al. Citation2008). HAS-based drug delivery has been successfully translated into the clinic. For example, Abraxane, an albumin-nanoparticle formulation of paclitaxel, was approved by FDA in 2005 for the treatment of metastatic breast cancer (Dranitsaris et al. Citation2009, Miele et al. Citation2009).
Several other nanocarriers have shown great promises for tumour-targeted delivery. For example, low-density lipoproteins (LDL) or high-density lipoproteins (HDL) have been investigated as nanocarriers for cancer therapeutics due to high LDL/HDL receptor expression on tumours, and their presumed biocompatibility and non-immunogenicity (Nikanjam et al. Citation2007, Thaxton et al. Citation2009). Recent studies have shown the feasibility of synthesizing drug loaded LDL/HDL nanparticles. Zhang et al. (Citation2009, Citation2010) and Chen et al. (Citation2007) extended the application of synthetic LDL/HDL nanoparticles to cancer diagnostics and therapeutics by conjugating tumour-specific ligands (e.g., folate, EGFR) to reroute LDL/HDL nanoparticles away from their native receptors. Amphiphilic macrocyclic molecules and nanomaterials such as cyclodextrins (CDs) and their derivatives have been investigated as drug nanocarriers based on their ability to encapsulate hydrophobic drugs. Cucurbit[6]uril (CB[6]), a member of the macrocyclic host family cucurbit[n]uril (CB[n]), has a hydrophobic cavity similar to that of α-CD. Recently, Cucurbituril-based nanoparticles (CB[6]NPs) has been shown as new efficient nanocarriers for delivery of hydrophobic drugs (Park et al. Citation2009). Furthermore, substantial work has been done with polymeric nanoparticles (Shi et al. Citation2009a). For example, anti-HER2 conjugated doxorubicin immuno-nanoparticles enhanced intracellular delivery of the drug to HER2-overespressing SKBR-3 (Shi et al. Citation2009a).
Multifunctional nanocarriers
Recently, multifunctional nanocarriers have attracted much interest (Torchilin Citation2006a, 2009, Gindy and Prud'homme Citation2009). These can integrate therapeutic agents, targeting ligands, imaging agents (e.g., magnetic nanoparticles or quantum dots), cell penetration peptide and stimulus-sensitive components (e.g., pH, temperature or photo-sensitive) into the design of a single nanoparticle. Several recent studies showed that co-delivery of anticancer drugs and MDR targeting siRNA overcomed tumour drug resistance. For example, paclitaxel and P-gp targeted siRNA were engineered in the same PLGA-PEI nanoparticles. As a result of silencing of P-gp, paclitaxel delivery efficiency was significantly improved and the tumour drug resistance was overcome both in vitro and in vivo (Patil et al. Citation2010). Combination of therapeutic drugs and imaging agents (theranostic) has become an important strategy in nanoparticle research. Using imaging agents, the nanoparticles can be tracked in vivo in real-time. Magnetic nanoparticles (MNPs) represent a class of non-invasive imaging agents that can be monitored through magnetic resonance (MR) imaging (McCarthy and Weissleder Citation2008). MNPs mainly include metallic, bimetallic, and superparamagnetic iron oxide nanoparticles (SPIONs). MNPs have been combined with several anticancer drugs, including paclitaxel, doxorubicin, and methotrexate (MTX) (McCarthy and Weissleder Citation2008, Dilnawaz et al. Citation2010). For example, Yang et al. (Citation2007) developed an anti-HER targeted multifunctional magneto-polymeric nanohybrids (HER-MMPNs) for breast cancer, which combined magnetic nanocrystals (for MRI) and the anticancer drug doxorubicin. The resultant HER-MMPNs demonstrated excellent inhibition of tumour growth and ultrasensitive targeted detection by MRI in animal model.
Nanocarriers with stimuli-responsive elements can be triggered to release drug at the tumour sites by the changes in temperature, pH, light, ultrasound, and magnetic field as well as redox potential (Ganta et al. Citation2008). Nanocarriers (e.g., liposomes, micelles, or polymeric nanoparticles) are able to obtain the stimuli-responsive features via stimuli-sensitive components. For example, Ong et al. (Citation2008). reported stable liposomes (∼100 nm in diameter) made from quinone-dioleoyl phosphatidylethanolamine (Q-DOPE) can be triggered by the redox activation of the quinine headgroup to rapidly release their payload.
Concluding remarks
Nanocarriers have played an increasing role in the cancer therapy over the last decade. Compared to free drugs, nanocarrier-encapsulated drugs preferentially accumulate in the tumour sites through the EPR effects, thereby improving therapeutic outcomes and reducing side-effects. Targeting of nanocarrier can further improve the efficiency and specificity of drug delivery. A wide variety of targeted nanocarriers have been developed and demonstrated efficacy in vivo. However, to date there have been no FDA approved targeted nanocarriers and only a handful of them has reached clinical trials. FCE28069, a conjugate of HPMA copolymer, doxorubicin and galactose, is the first receptor-targeted nanoparticle to reach the clinic (Seymour et al. Citation2002, Duncan Citation2009). In addition, CALAA-01 (a polymeric nanoparticle containing siRNA), MBP-426 (an oxaliplatin-encapsulated liposome), and SGT-53 (a liposome containing a plasmid DNA against p53 gene) are currently under clinical investigation. All these three kinds of nanoparticles are conjugated by a targeting ligand against the transferrin receptor (Heath and Davis Citation2008). Enormous efforts have been invested in developing novel targeted nanocarriers and the associated therapeutic strategies. The development of complimentary technologies promises to identify more high specificity ligands such as aptamers and peptides. In addition, several novel nanocarriers such as minicells and synthetic LDL/HDL nanoparticles hold great promise for future clinical development. The recent advances in multifunctional targeted nanocarriers further expand the potential of nanocarriers. However, the complexity of novel delivery systems would be substantially increased, which may introduce difficulties in the scale-up production, quality control and gaining regulatory approval. In conclusion, there have been a large volume of work published demonstrating the potential of targeted nanocarriers in cancer therapy and relatively few examples of clinical trials of viable products. Future efforts should be focused on clinical translation of novel targeted nanocarriers.
Acknowledgements
This work was support in part by NSF grant EEC-0425626, NIH grants R21 CA131832, R21EB008247, R01 CA095673 and R01 CA135243.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abu Lila AS, Ishida T, Kiwada H. 2009. Recent advances in tumor vasculature targeting using liposomal drug delivery systems. Expert Opin Drug Deliv 6:1297–1309.
- Alexis F, Pridgen E, Molnar LK, Farokhzad OC. 2008. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm 5:505–515.
- Aliabadi HM, Lavasanifar A. 2006. Polymeric micelles for drug delivery. Expert Opin Drug Deliv 3:139–162.
- Allen TM. 2002. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer 2:750–763.
- Allen TM, Cullis PR. 2004. Drug delivery systems: Entering the mainstream. Science 303:1818–1822.
- Arap W, Pasqualini R, Ruoslahti E. 1998. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 279:377–380.
- Balestrieri ML, Napoli C. 2007. Novel challenges in exploring peptide ligands and corresponding tissue-specific endothelial receptors. Eur J Cancer 43:1242–1250.
- Beuttler J, Rothdiener M, Muller D, Frejd FY, Kontermann RE. 2009. Targeting of epidermal growth factor receptor (EGFR)-expressing tumor cells with sterically stabilized affibody liposomes (SAL). Bioconjug Chem 20:1201–1208.
- Bhirde AA, Patel V, Gavard J, Zhang G, Sousa AA, Masedunskas A, Leapman RD, Weigert R, Gutkind JS, Rusling JF. 2009. Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano 3:307–316.
- Boddapati SV, D'Souza GG, Erdogan S, Torchilin VP, Weissig V. 2008. Organelle-targeted nanocarriers: Specific delivery of liposomal ceramide to mitochondria enhances its cytotoxicity in vitro and in vivo. Nano Lett 8:2559–2563.
- Bonacucina G, Cespi M, Misici-Falzi M, Palmieri GF. 2009. Colloidal soft matter as drug delivery system. J Pharm Sci 98:1–42.
- Brooks H, Lebleu B, Vives E. 2005. Tat peptide-mediated cellular delivery: Back to basics. Adv Drug Deliv Rev 57:559–577.
- Caliceti P, Veronese FM. 2003. Pharmacokinetic and biodistribution properties of poly(ethylene glycol)-protein conjugates. Adv Drug Deliv Rev 55:1261–1277.
- Carter P. 2001. Improving the efficacy of antibody-based cancer therapies. Nat Rev Cancer 1:118–129.
- Chang DK, Lin CT, Wu, CH, Wu HC. 2009. A novel peptide enhances therapeutic efficacy of liposomal anti-cancer drugs in mice models of human lung cancer. PLoS One 4:e4171.
- Chen J, Corbin IR, Li H, Cao W, Glickson JD, Zheng G. 2007. Ligand conjugated low-density lipoprotein nanoparticles for enhanced optical cancer imaging in vivo. J Am Chem Soc 129:5798–5799.
- Clynes R, Towers T, Presta L, Ravetch J. 2000. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med 6:443–446.
- Cortez-Retamozo V, Backmann N, Senter PD, Wernery U, De Baetselier P, Muyldermans S, Revets H. 2004. Efficient cancer therapy with a nanobody-based conjugate. Cancer Res 64:2853–2857.
- D'Souza GG, Rammohan R, Cheng SM, Torchilin VP, Weissig V. 2003. DQAsome-mediated delivery of plasmid DNA toward mitochondria in living cells. J Control Release 92:189–197.
- Daniels TR, Delgado T, Helguera G, Penichet ML. 2006. The transferrin receptor part II: Targeted delivery of therapeutic agents into cancer cells. Clin Immunol 121:159–176.
- Danson S, Ferry D, Alakhov V, Margison J, Kerr D, Jowle D, Brampton M, Halbert G, Ranson M. 2004. Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer. Br J Cancer 90:2085–2091.
- Davis ME, Chen ZG, Shin DM. 2008. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat Rev Drug Discov 7:771–782.
- De Lorenzo C, Palmer DB, Piccoli R, Ritter MA, D'Alessio G. 2002. A new human antitumor immunoreagent specific for ErbB2. Clin Cancer Res 8:1710–1719.
- Desgrosellier JS, Cheresh DA. 2010. Integrins in cancer: Biological implications and therapeutic opportunities. Nat Rev Cancer 10:9–22.
- Di Nenedetto M, Starzec A, Vassy R, Perret GY, Crepin M. 2008. Distinct heparin binding sites on VEGF165 and its receptors revealed by their interaction with a non sulfated glycoaminoglycan (NaPaC). Biochim Biophys Acta 1780:723–732.
- Dilnawaz F, Singh A, Mohanty C, Sahoo SK. 2010. Dual drug loaded superparamagnetic iron oxide nanoparticles for targeted cancer therapy. Biomaterials 31:3694–3706.
- Dranitsaris G, Cottrell W, Spirovski B, Hopkins S. 2009. Economic analysis of albumin-bound paclitaxel for the treatment of metastatic breast cancer. J Oncol Pharm Pract 15:67–78.
- Duncan R. 2003. The dawning era of polymer therapeutics. Nat Rev Drug Discov 2:347–360.
- Duncan R. 2006. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer 6:688–701.
- Duncan R. 2009. Development of HPMA copolymer-anticancer conjugates: Clinical experience and lessons learnt. Adv Drug Deliv Rev 61(13):1131–1148.
- Duncan R, Izzo L. 2005. Dendrimer biocompatibility and toxicity. Advanced Drug Delivery Reviews 57:2215–2237.
- El Bayoumi TA, Torchilin VP. 2009. Tumor-targeted nanomedicines: Enhanced antitumor efficacy in vivo of doxorubicin-loaded, long-circulating liposomes modified with cancer-specific monoclonal antibody. Clin Cancer Res 15:1973–1980.
- Fang X, Tan W. 2010. Aptamers generated from cell-SELEX for molecular medicine: A chemical biology approach. Acc Chem Res 43:48–57.
- Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, Richie JP, Langer R. 2006. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci USA 103:6315–6320.
- Farokhzad OC, Langer R. 2009. Impact of nanotechnology on drug delivery. Acs Nano 3:16–20.
- Futaki S, Masui Y, Nakase I, Sugiura Y, Nakamura T, Kogure K, Harashima H. 2005. Unique features of a pH-sensitive fusogenic peptide that improves the transfection efficiency of cationic liposomes. J Gene Med 7:1450–1458.
- Ganta S, Devalapally H, Shahiwala A, Amiji M. 2008. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release 126:187–204.
- Garanger E, Boturyn D, Coll JL, Favrot MC, Dumy P. 2006. Multivalent RGD synthetic peptides as potent alphaVbeta3 integrin ligands. Org Biomol Chem 4:1958–1965.
- Gindy ME, Prud'homme RK. 2009. Multifunctional nanoparticles for imaging, delivery and targeting in cancer therapy. Expert Opin Drug Deliv 6:865–878.
- Gosk S, Vermehren C, Storm G, Moos T. 2004. Targeting anti-transferrin receptor antibody (OX26) and OX26-conjugated liposomes to brain capillary endothelial cells using in situ perfusion. J Cereb Blood Flow Metab 24:1193–1204.
- Gottesman MM, Fojo T, Bates SE. 2002. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat Rev Cancer 2:48–58.
- Gu FX, Karnik R, Wang AZ, Alexis F, Levy-Nissenbaum E, Hong S, Langer RS, Farokhzad OC. 2007. Targeted nanoparticles for cancer therapy. Nano Today 2:14–21.
- Gullotti E, Yeo Y. 2009. Extracellularly activated nanocarriers: A new paradigm of tumor targeted drug delivery. Mol Pharm 6:1041–1051.
- Hawkins MJ, Soon-Shiong P, Desai N. 2008. Protein nanoparticles as drug carriers in clinical medicine. Adv Drug Deliv Rev 60:876–885.
- Heath JR, Davis ME. 2008. Nanotechnology and cancer. Annu Rev Med 59:251–265.
- Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. 1998. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc Natl Acad Sci USA 95:4607–4612.
- Huang YF, Shangguan D, Liu H, Phillips JA, Zhang X, Chen Y, Tan W. 2009. Molecular assembly of an aptamer-drug conjugate for targeted drug delivery to tumor cells. Chembiochem 10:862–868.
- Jagur-Grodzinski J. 2009. Polymers for targeted and/or sustained drug delivery. Polymers for Adv Technol 20:595–606.
- Jain RK. 1999. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng 1:241–263.
- Kakudo T, Chaki S, Futaki S, Nakase I, Akaji K, Kawakami T, Maruyama K, Kamiya H, Harashima H. 2004. Transferrin-modified liposomes equipped with a pH-sensitive fusogenic peptide: An artificial viral-like delivery system. Biochemistry 43:5618–5628.
- Kirpotin D, Park JW, Hong K, Zalipsky S, Li WL, Carter P, Benz CC, Papahadjopoulos D. 1997. Sterically stabilized anti-HER2 immunoliposomes: Design and targeting to human breast cancer cells in vitro. Biochemistry 36:66–75.
- Kluza E, van der Schaft DW, Hautvast PA, Mulder WJ, Mayo KH, Griffioen AW, Strijkers GJ, Nicolay K. 2010. Synergistic targeting of alphavbeta3 integrin and galectin-1 with heteromultivalent paramagnetic liposomes for combined MR imaging and treatment of angiogenesis. Nano Lett 10:52–58.
- Krishna AD, Mandraju RK, Kishore G, Kondapi AK. 2009. An efficient targeted drug delivery through apotransferrin loaded nanoparticles. PLoS One 4:e7240.
- Laginha K, Mumbengegwi D, Allen T. 2005. Liposomes targeted via two different antibodies: Assay, B-cell binding and cytotoxicity. Biochim Biophys Acta 1711:25–32.
- Laginha KM, Moase EH, Yu N, Huang A, Alien TM. 2008. Bioavailability and therapeutic efficacy of HER2 scFv-targeted liposomal doxorubicin in a murine model of HER2-overexpressing breast cancer. J Drug Target 16:605–610.
- Lam R, Ho D. 2009. Nanodiamonds as vehicles for systemic and localized drug delivery. Expert Opin Drug Deliv 6:883–895.
- Lapalombella R, Yu B, Triantafillou G, Liu Q, Butchar JP, Lozanski G, Ramanunni A, Smith LL, Blum W, Andritsos L, Wang DS, Lehman A, Chen CS, Johnson AJ, Marcucci G, Lee RJ, Lee LJ, Tridandapani S, Muthusamy N, Byrd JC. 2008. Lenalidomide down-regulates the CD20 antigen and antagonizes direct and antibody-dependent cellular cytotoxicity of rituximab on primary chronic lymphocytic leukemia cells. Blood 112:5180–5189.
- Leuschner C, Kumar CS, Hansel W, Soboyejo W, Zhou J, Hormes J. 2006. LHRH-conjugated magnetic iron oxide nanoparticles for detection of breast cancer metastases. Breast Cancer Res Treat 99:163–176.
- Levy-Nissenbaum E, Radovic-Moreno AF, Wang AZ, Langer R, Farokhzad OC. 2008. Nanotechnology and aptamers: Applications in drug delivery. Trends Biotechnol 26:442–449.
- Li SD, Huang L. 2008. Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm 5:496–504.
- Loomis K, Smith B, Feng Y, Garg H, Yavlovich A, Campbell-Massa R, Dimitrov DS, Blumenthal R, Xiao X, Puri A. 2010. Specific targeting to B cells by lipid-based nanoparticles conjugated with a novel CD22-ScFv. Exp Mol Pathol 88:238–249.
- Lopes de Menezes DE, Pilarski LM, Allen TM. 1998. In vitro and in vivo targeting of immunoliposomal doxorubicin to human B-cell lymphoma. Cancer Res 58:3320–3330.
- Low PS, Henne WA, Doorneweerd DD. 2008. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc Chem Res 41:120–129.
- MacDiarmid JA, Amaro-Mugridge NB, Madrid-Weiss J, Sedliarou I, Wetzel S, Kochar K, Brahmbhatt VN, Phillips L, Pattison ST, Petti C, Stillman B, Graham RM, Brahmbhatt H. 2009. Sequential treatment of drug-resistant tumors with targeted minicells containing siRNA or a cytotoxic drug. Nat Biotechnol 27:643–651.
- MacDiarmid JA, Madrid-Weiss J, Amaro-Mugridge NB, Phillips L, Brahmbhatt H. 2007a. Bacterially-derived nanocells for tumor-targeted delivery of chemotherapeutics and cell cycle inhibitors. Cell Cycle 6:2099–2105.
- MacDiarmid JA, Mugridge NB, Weiss JC, Phillips L, Burn AL, Paulin RP, Haasdyk JE, Dickson KA, Brahmbhatt VN, Pattison ST, James AC, Al Bakri G, Straw RC, Stillman B, Graham RM, Brahmbhatt H. 2007b. Bacterially derived 400 nm particles for encapsulation and cancer cell targeting of chemotherapeutics. Cancer Cell 11:431–445.
- Matsui M, Shimizu Y, Kodera Y, Kondo E, Ikehara Y, Nakanishi H. 2010. Targeted delivery of oligomannose-coated liposome to the omental micrometastasis by peritoneal macrophages from patients with gastric cancer. Cancer Sci 101:1670–1677.
- Matsumura Y, Maeda H. 1986. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 46:6387–6392.
- Matsumura Y, Hamaguchi T, Ura T, Muro K, Yamada Y, Shimada Y, Shirao K, Okusaka T, Ueno H, Ikeda M, Watanabe N. 2004. Phase I clinical trial and pharmacokinetic evaluations of NK911, a micelle-encapsulated doxorubicin. Br J Cancer 91:1775–1781.
- McCarthy JR, Weissleder R. 2008. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv Drug Deliv Rev 60:1241–1251.
- Miele E, Spinelli GP, Tomao F, Tomao S. 2009. Albumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancer. Int J Nanomed 4:99–105.
- Mishra B, Patel BB, Tiwari S. 2010. Colloidal nanocarriers: A review on formulation technology, types and applications toward targeted drug delivery. Nanomedicine 6:9–24.
- Misra R, Sahoo SK. 2010. Intracellular trafficking of nuclear localization signal conjugated nanoparticles for cancer therapy. Eur J Pharm Sci 39:152–163.
- Mukhopadhyay A, Weiner H. 2007. Delivery of drugs and macromolecules to mitochondria. Adv Drug Deliv Rev 59:729–738.
- Murase Y, Asai T, Katanasaka Y, Sugiyama T, Shimizu K, Maeda N, Oku N. 2010. A novel DDS strategy, ‘dual-targeting’, and its application for antineovascular therapy. Cancer Lett 287:165–171.
- Nanjwade BK, Bechra HM, Derkar GK, Manvi FV, Nanjwade VK. 2009. Dendrimers: Emerging polymers for drug-delivery systems. Eur J Pharmaceut Sci 38:185–196.
- Negussie AH, Miller JL, Reddy G, Drake SK, Wood BJ, Dreher MR. 2010. Synthesis and in vitro evaluation of cyclic NGR peptide targeted thermally sensitive liposome. J Control Release 143:265–273.
- Neidle S, Thurston DE. 2005. Chemical approaches to the discovery and development of cancer therapies. Nat Rev Cancer 5:285–296.
- Ng EW, Shima DT, Calias P, Cunningham ET, Guyer DR, Adamis AP. 2006. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov 5:123–132.
- Nikanjam M, Blakely EA, Bjornstad KA, Shu X, Budinger TF, Forte TM. 2007. Synthetic nano-low density lipoprotein as targeted drug delivery vehicle for glioblastoma multiforme. Int J Pharm 328:86–94.
- Noble CO, Kirpotin DB, Hayes ME, Mamot C, Hong K, Park JW, Benz CC, Marks JD, Drummond DC. 2004. Development of ligand-targeted liposomes for cancer therapy. Expert Opin Ther Targets 8:335–353.
- Nobs L, Buchegger F, Gurny R, Allemann E. 2004. Current methods for attaching targeting ligands to liposomes and nanoparticles. J Pharm Sci 93:1980–1992.
- Norberto SE, Kakar SS. 2009. The use of nanoparticles in LHRH receptor targeted therapy for cancer. Exp Mol Pathol.
- Ong W, Yang Y, Cruciano AC, McCarley RL. 2008. Redox-triggered contents release from liposomes. J Am Chem Soc 130:14739–14744.
- Park JW, Hong K, Kirpotin DB, Colbern G, Shalaby R, Baselga J, Shao Y, Nielsen UB, Marks JD, Moore D, Papahadjopoulos D, Benz CC. 2002. Anti-HER2 immunoliposomes: Enhanced efficacy attributable to targeted delivery. Clin Cancer Res 8:1172–1181.
- Park KM, Suh K, Jung H, Lee DW, Ahn Y, Kim J, Baek K, Kim K. 2009. Cucurbituril-based nanoparticles: A new efficient vehicle for targeted intracellular delivery of hydrophobic drugs. Chem Commun (Camb):71–73.
- Paschke M. 2006. Phage display systems and their applications. Appl Microbiol Biotechnol 70:2–11.
- Pastorino F, Brignole C, Marimpietri D, Cilli M, Gambini C, Ribatti D, Longhi R, Allen TM, Corti A, Ponzoni M. 2003. Vascular damage and anti-angiogenic effects of tumor vessel-targeted liposomal chemotherapy. Cancer Res 63:7400–7409.
- Patil YB, Swaminathan SK, Sadhukha T, Ma L, Panyam J. 2010. The use of nanoparticle-mediated targeted gene silencing and drug delivery to overcome tumor drug resistance. Biomaterials 31:358–365.
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. 2007. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2:751–760.
- Peer D, Margalit R. 2004a. Loading mitomycin C inside long circulating hyaluronan targeted nano-liposomes increases its antitumor activity in three mice tumor models. Int J Cancer 108:780–789.
- Peer D, Margalit R. 2004b. Tumor-targeted hyaluronan nanoliposomes increase the antitumor activity of liposomal Doxorubicin in syngeneic and human xenograft mouse tumor models. Neoplasia 6:343–353.
- Phillips JA, Lopez-Colon D, Zhu Z, Xu Y, Tan W. 2008. Applications of aptamers in cancer cell biology. Anal Chim Acta 621:101–108.
- Piddock LJV. 2006. Multidrug-resistance efflux pumps – not just for resistance. Nature Rev Microbiol 4:629–636.
- Qian ZM, Li H, Sun H, Ho K. 2002. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev 54:561–587.
- Rajendran L, Knolker HJ, Simons K. 2010. Subcellular targeting strategies for drug design and delivery. Nat Rev Drug Discov 9:29–42.
- Riehemann K, Schneider SW, Luger TA, Godin B, Ferrari M, Fuchs H. 2009. Nanomedicine – challenge and perspectives. Angewandte Chemie-Int Ed 48:872–897.
- Ruoslahti E. 2002. Specialization of tumour vasculature. Nat Rev Cancer 2:83–90.
- Ruoslahti E, Bhatia SN, Sailor MJ. 2010. Targeting of drugs and nanoparticles to tumors. J Cell Biol 22(188):759–768.
- Sapra P, Allen TM. 2003. Ligand-targeted liposomal anticancer drugs. Prog Lipid Res 42:439–462.
- Saul JM, Annapragada AV, Bellamkonda RV. 2006. A dual-ligand approach for enhancing targeting selectivity of therapeutic nanocarriers. J Control Release 114:277–287.
- Sawant RM, Cohen MB, Torchilin VP, Rokhlin OW. 2008. Prostate cancer-specific monoclonal antibody 5D4 significantly enhances the cytotoxicity of doxorubicin-loaded liposomes against target cells in vitro. J Drug Targeting 16:601–604.
- Sawyers C. 2004. Targeted cancer therapy. Nature 432:294–297.
- Seymour LW, Ferry DR, Anderson D, Hesslewood S, Julyan PJ, Poyner R, Doran J, Young AM, Burtles S, Kerr DJ. 2002. Hepatic drug targeting: Phase I. Evaluation of polymer bound doxorubicin. J Clin Oncol 20:1668–1676.
- Sharkey RM, Goldenberg DM. 2006. Targeted therapy of cancer: New prospects for antibodies and immunoconjugates. CA Cancer J Clin 56:226–243.
- Sharkey RM, Goldenberg DM. 2008. Use of antibodies and immunoconjugates for the therapy of more accessible cancers. Adv Drug Deliv Rev 60:1407–1420.
- Shi M, Ho K, Keating A, Shoichet MS. 2009a. Doxorubicin-conjugated lmmuno-nanoparticles for intracellular anticancer drug delivery. Adv Functio Mater 19:1689–1696.
- Shi M, Lu J, Shoichet MS. 2009b. Organic nanoscale drug carriers coupled with ligands for targeted drug delivery in cancer. J Mater Chem 19:5485–5498.
- Silverman J, Liu Q, Bakker A, To W, Duguay A, Alba BM, Smith R, Rivas A, Li P, Le H, Whitehorn E, Moore KW, Swimmer C, Perlroth V, Vogt M, Kolkman J, Stemmer WP. 2005. Multivalent avimer proteins evolved by exon shuffling of a family of human receptor domains. Nat Biotechnol 23:1556–1561.
- Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC. 1998. Detection of tumor angiogenesis in vivo by alphaVbeta3-targeted magnetic resonance imaging. Nat Med 4:623–626.
- Soussan E, Cassel S, Blanzat M, Rico-Lattes I. 2009. Drug delivery by soft matter: Matrix and vesicular carriers. Angew Chem Int Ed Engl 48:274–288.
- Sugahara K, Teesalu T, Karmali P, Kotamraju V, Agemy L, Greenwald D, Ruoslahti E. 2010. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science 328:1031–1035.
- Sundaram S, Durairaj C, Kadam R, Kompella UB. 2009. Luteinizing hormone-releasing hormone receptor-targeted deslorelin-docetaxel conjugate enhances efficacy of docetaxel in prostate cancer therapy. Mol Cancer Ther 8:1655–1665.
- Thaxton CS, Daniel WL, Giljohann DA, Thomas AD, Mirkin CA. 2009. Templated spherical high density lipoprotein nanoparticles. J Am Chem Soc 131:1384–1385.
- Tkachenko AG, Xie H, Coleman D, Glomm W, Ryan J, Anderson MF, Franzen S, Feldheim DL. 2003. Multifunctional gold nanoparticle-peptide complexes for nuclear targeting. J Am Chem Soc 125:4700–4701.
- Toma A, Otsuji E, Kuriu Y, Okamoto K, Ichikawa D, Hagiwara A, Ito H, Nishimura T, Yamagishi H. 2005. Monoclonal antibody A7-superparamagnetic iron oxide as contrast agent of MR imaging of rectal carcinoma. Br J Cancer 93:131–136.
- Torchilin VP. 2009. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur J Pharm Biopharm 71:431–444.
- Torchilin VP. 2005a. Lipid-core micelles for targeted drug delivery. Curr Drug Deliv 2:319–327.
- Torchilin VP. 2005b. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov 4:145–160.
- Torchilin VP. 2006a. Multifunctional nanocarriers. Adv Drug Deliv Rev 58:1532–1555.
- Torchilin VP. 2006b. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu Rev Biomed Eng 8:343–375.
- Torchilin VP. 2007. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. Aaps J 9:E128–147.
- Torchilin VP, Lukyanov AN, Gao Z, Papahadjopoulos-Sternberg B. 2003. Immunomicelles: Targeted pharmaceutical carriers for poorly soluble drugs. Proc Nat Acad Sci USA 100:6039–6044.
- Veiseh O, Gunn JW, Zhang M. 2010. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv Drug Deliv Rev 62:284–304.
- Vives E, Schmidt J, Pelegrin A. 2008. Cell-penetrating and cell-targeting peptides in drug delivery. Biochim Biophys Acta 1786:126–138.
- Voinea M, Manduteanu I, Dragomir E, Capraru M, Simionescu M. 2005. Immunoliposomes directed toward VCAM-1 interact specifically with activated endothelial cells – a potential tool for specific drug delivery. Pharm Res 22:1906–1917.
- Wagner S, Rothweiler F, Anhorn MG, Sauer D, Riemann I, Weiss EC, Katsen-Globa A, Michaelis M, Cinatl J Jr, Schwartz D, Kreuter J, von Briesen H, Langer K. 2010. Enhanced drug targeting by attachment of an anti alphav integrin antibody to doxorubicin loaded human serum albumin nanoparticles. Biomaterials 31:2388–2398.
- Wagner V, Dullaart A, Bock AK, Zweck A. 2006. The emerging nanomedicine landscape. Nat Biotechnol 24:1211–1217.
- Weissig V, Lasch J, Erdos G, Meyer HW, Rowe TC, Hughes J. 1998. DQAsomes: A novel potential drug and gene delivery system made from Dequalinium. Pharm Res 15:334–337.
- Wikman M, Steffen AC, Gunneriusson E, Tolmachev V, Adams GP, Carlsson J, Stahl S. 2004. Selection and characterization of HER2/neu-binding affibody ligands. Protein Eng Des Sel 17:455–462.
- Wu J, Nantz MH, Zern MA. 2002. Targeting hepatocytes for drug and gene delivery: Emerging novel approaches and applications. Front Biosci 7:d717–725.
- Yamada Y, Akita H, Kamiya H, Kogure K, Yamamoto T, Shinohara Y, Yamashita K, Kobayashi H, Kikuchi H, Harashima H. 2008. MITO-Porter: A liposome-based carrier system for delivery of macromolecules into mitochondria via membrane fusion. Biochim Biophys Acta 1778:423–432.
- Yamada Y, Harashima H. 2008. Mitochondrial drug delivery systems for macromolecule and their therapeutic application to mitochondrial diseases. Adv Drug Deliv Rev 60:1439–1462.
- Yan AC, Levy M. 2009. Aptamers and aptamer targeted delivery. RNA Biol 6:316–320.
- Yang J, Lee CH, Ko HJ, Suh JS, Yoon HG, Lee K, Huh YM, Haam S. 2007. Multifunctional magneto-polymeric nanohybrids for targeted detection and synergistic therapeutic effects on breast cancer. Angew Chem Int Ed Engl 46:8836–8839.
- Yousif LF, Stewart KM, Kelley SO. 2009. Targeting mitochondria with organelle-specific compounds: Strategies and applications. Chembiochem 10:1939–1950.
- Yu B, Zhao X, Lee LJ, Lee RJ. 2009. Targeted delivery systems for oligonucleotide therapeutics. Aaps J 11:195–203.
- Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK. 1995. Vascular permeability in a human tumor xenograft: Molecular size dependence and cutoff size. Cancer Res 55:3752–3756.
- Zhang Z, Cao W, Jin H, Lovell JF, Yang M, Ding L, Chen J, Corbin I, Luo Q, Zheng G. 2009. Biomimetic nanocarrier for direct cytosolic drug delivery. Angew Chem Int Ed Engl 48:9171–9175.
- Zhang Z, Chen J, Ding L, Jin H, Lovell JF, Corbin IR, Cao W, Lo PC, Yang M, Tsao MS, Luo Q, Zheng G.. 2010. HDL-mimicking peptide-lipid nanoparticles with improved tumor targeting. Small 6:430–437.
- Zhao X, Li H, Lee RJ. 2008. Targeted drug delivery via folate receptors. Expert Opin Drug Deliv 5:309–319.