Abstract
The exchange of proteins and lipids between the trans-Golgi network (TGN) and the endosomal system requires multiple cellular machines, whose activities are coordinated in space and time to generate pleomorphic, tubulo-vesicular carriers that deliver their content to their target compartments. These machines and their associated protein networks are recruited and/or activated on specific membrane domains where they select proteins and lipids into carriers, contribute to deform/elongate and partition membrane domains using the mechanical forces generated by actin polymerization or movement along microtubules. The coordinated action of these protein networks contributes to regulate the dynamic state of multiple receptors recycling between the cell surface, endosomes and the TGN, to maintain cell homeostasis as exemplified by the biogenesis of lysosomes and related organelles, and to establish/maintain cell polarity. The dynamic assembly and disassembly of these protein networks mediating the exchange of membrane domains between the TGN and endosomes regulates cell-cell signalling and thus the development of multi-cellular organisms. Somatic mutations in single network components lead to changes in transport dynamics that may contribute to pathological modifications underlying several human diseases such as mental retardation.
Introduction
The trans-Golgi network (TGN) is the last sorting station of the secretory pathway from which a plethora of soluble or membrane proteins and lipids are sorted into distinct domains for subsequent transport to different destinations: the cell surface (apical and basolateral in polarized cells), the endosomal system, secretory granules in endocrine cells, synaptic domains in neurons.
Two major classes of transmembrane proteins are sorted in the TGN for delivery to the endosomal system. The first class comprises transmembrane proteins cycling between the TGN and endosomes, such as the two mannose-6-phosphate receptors (MPRs), which deliver their bound lysosomal enzymes to endosomes in a mannose 6-phosphate (M6P) dependent manner (Munier-Lehmann et al. Citation1996), (Ghosh et al. Citation2003). After unloading their bound ligands in endosomes, they return either to the cell surface or to the TGN, a retrieval pathway also used by several endocytosed molecules. In the TGN, VPS10 family receptors (e.g., sortilin or neurotensin receptor 3) or LIMP-2 (Reczek et al. Citation2007) may also account for the M6P-independent delivery of some soluble lysosomal enzymes to the endosomal system. The second class includes transmembrane proteins destined to reside in lysosomes or in lysosome-related organelles in pigmented cells, such as lysosomal membrane glycoproteins (LAMPs and LIMPs) and melanosomal proteins. The cytoplasmic domains of these transmembrane proteins contain single or multiple sorting motifs of different types: tyrosine based motifs (YxxØ) and variations of acidic clusters combined with di-Leucine motifs ([D/E]xxxL[L/I] or DxxLL) (Bonifacino and Traub Citation2003), (Braulke and Bonifacino Citation2009). Here, we will give a general overview of the machineries and mechanisms regulating the sorting and the exchange of these cargos between the TGN and endosomes.
Sorting machineries mediating post-TGN transport to endosomes
Heterotetrameric coat protein complexes known as adaptor proteins (AP-1, AP-2, AP-3 and AP-4) decode the sorting motifs in cargo tails, thereby segregating cargos into transport carriers. Similar to other APs, the ubiquitous AP-1A adaptor protein complex consists of four subunits (β1, γ, μ1a and σ1); μ1 recognizes tyrosine-based-motifs whereas a γ/σ1 hemicomplex binds [D/E]xxxL[L/I] signatures (Bonifacino and Traub Citation2003), (Janvier et al. Citation2003). A class of monomeric clathrin adaptors termed GGAs [Golgi-localized, gamma-ear containing, ARF (ADP ribosylation factor)-binding proteins], including GGA1, GGA2 and GGA3, with similar sorting functions, bind acidic cluster-dileucine motifs through a VHS domain (Bonifacino Citation2004). Although AP-1A and GGAs cooperate in cargo selection (Doray et al. Citation2002), their relative functions are not yet fully understood. GGAs have been detected both on the same or different clathrin/AP-1-coated domains of the TGN but have not been detected in purified clathrin-coated carriers, possibly due to their unstable association with TGN membranes (Hirst et al. Citation2001), (Citation2009), (Doray et al. Citation2002), (Mardones et al. Citation2007). Thus, it has been suggested that GGAs function prior to AP-1 in protein sorting (Hirst et al. Citation2001), (Doray et al. Citation2002) or that they function in parallel pathways (Mardones et al. Citation2007), (Hirst et al. Citation2009). The VHS and GAT domains of at least GGA3 bind ubiquitin (Puertollano and Bonifacino Citation2004), (Scott et al. Citation2004). Therefore, GGAs may have a more specific role in sorting ubiquitinated cargos at the TGN for subsequent transport to endosomes, where the endosomal sorting complexes required for transport (ESCRT complexes), which also bind ubiquitinated cargos, could sort them into nascent multivesicular bodies for subsequent degradation (David Citation2007).
A second coat mediating transport of cargos to lysosome and lysosome-related organelles (e.g., melanosomes in pigmented cells) is AP-3 (Le Borgne and Hoflack Citation1998), (Bonifacino and Traub Citation2003), which, like AP-1A, binds tyrosine motifs via μ3 and acidic di-leucine motifs via δ/σ3 (Dell'Angelica et al. Citation1997), (Janvier et al. Citation2003). It is not yet clear where AP-3 functions. Although most of AP-3 is found on endosomal tubular profiles (Peden et al. Citation2004), it cannot be excluded that AP-3 also functions at the TGN or that AP-1 and AP-3 function sequentially. In yeast, AP-3 does not appear to require clathrin association for its function (Vowels and Payne Citation1998), (Peden et al. Citation2002). In mammals however, AP-3 can bind the clathrin heavy chain via its β3 subunit (Dell'Angelica et al. Citation1998) and ultrastructural analyses have shown that AP-3 co-localizes with clathrin, but to a much lower extent than AP-1 (Peden et al. Citation2004), thereby suggesting that AP-3 binds the clathrin heavy chains with a lower affinity. Therefore, AP-3 would not drastically compete with the ESCRT component hepatocyte growth-factor-regulated tyrosine kinase substrate Hrs, which binds clathrin efficiently (Raiborg et al. Citation2001) and could more easily segregate cargos into endosomal membrane domains distinct from those containing ubiquitinated proteins destined to be degraded in multivesicular bodies (Raiborg et al. Citation2002) ().
Figure 1. Model of (A) anterograde and (B) retrograde trafficking pathways between the secretory and endocytic pathway. Clathrin/AP-2-coated endocytic vesicles traffick between the plasma membrane and early endosomes (EE). At the trans-Golgi network (TGN), proteins are incorporated into distinct carriers coated with either clathrin/AP-1A or clathrin/GGAs or AP-3 to be transported to the endosomal system. In polarized epithelial cells, clathrin/AP-1B-coated carriers forming at the TGN and/or recycling endosomes (RE) transport their cargos to the basolateral plasma membrane. On early endosomes, AP-3 coats select their cargos for subsequent transport to late endosomes (LE), a step of membrane traffic, which involve a maturation process including the formation of multivesicular bodies. On early endosomes, the retromer complexes transport proteins back to the TGN. On late endosomes, some proteins are retrieved to the TGN via Rab9 and TIP47 positive carriers.
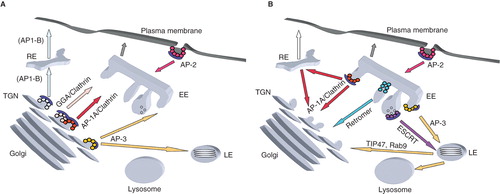
In specialized cells, AP-1 and AP-3 isoforms have slightly different functions. In epithelial cells, the AP-1B complex (containing a μ1B subunit) targets specific cargos from recycling endosomes to the basolateral plasma membrane (Folsch et al. Citation2003), as does AP-4 (Bonifacino and Traub Citation2003). Neuronal AP-3 isoforms and AP-1 σ1B regulate the synaptic vesicle cycle (Newell-Litwa et al. Citation2007), (Glyvuk et al. Citation2010).
Interaction of coat components with membrane microdomains
The accuracy of membrane traffic depends upon organelles being correctly recognized by coat components. The recruitment of adaptor proteins onto specific membrane microdomains relies on the combinatorial use of multiple low-affinity, sometimes short-lived, membrane components, which comprise an active, GTP-bound ARF-1 GTPase, phosphatidylinositide (PIs) and sorting motifs in cargo tails (Baust et al. Citation2006).
ARF-1, which cycles between an inactive, GDP-bound, cytosolic and an active, GTP-bound, membrane-associated form, functions as a general regulator of AP-1, GGAs and AP-3 coat association (D'Souza-Schorey and Chavrier Citation2006). Therefore, pathway-specific local nucleotide exchange and hydrolysis on ARF-1 must rely on the recruitment of specific guanine nucleotide exchange factors (GEFs) (Casanova Citation2007) and GTPase-activating proteins (GAPs) to these membrane subdomains (Donaldson and Honda Citation2005), (Spang et al. Citation2010). Thus, an ARF-1 GEF, BIG2, is implicated in AP-1 and GGA1 membrane association (Shinotsuka et al. Citation2002), (Baust et al. Citation2006), (Ishizaki et al. Citation2008), whereas BIG1 is linked to AP-3-dependent transport (Baust et al. Citation2008). AGAP1 and AGAP2 associate with AP-3 and AP-1, respectively (Nie et al. Citation2003), and the clathrin heavy chain (CHC)-interacting SMAP-type GAP (SMAP2) with AP-1 (Natsume et al. Citation2006). ARF GAPs may exhibit a dual function; they favour vesicle uncoating via ARF-1 hydrolysis in a manner analogous to Sec23 in COP-II vesicle-mediated ER-to-Golgi transport (Bi et al. Citation2002) or, alternatively, control ARF-1 activation during coat binding and carrier biogenesis (Kliouchnikov et al. Citation2009).
PIs are critical determinants of membrane domain identity (De Matteis et al. Citation2005), (Di Paolo and De Camilli Citation2006). They contribute to the recruitment of coat components and PI-binding domain (e.g., ENTH, FYVE, PH, PX) containing proteins functioning in various aspects of carrier biogenesis (Vicinanza et al. Citation2008). PI4P promotes adaptor recruitment on the TGN, while PI3P promotes that of AP-3 on early endosomes. At the TGN, AP-1γ (via a positively charged sequence), GGAs (via a phospho-tyrosine binding domain) and Epsin R (via ENTH domain) can all bind to PI4P (Hirst et al. Citation2003), (Wang et al. Citation2003), (Citation2007). Some ARFGAPs involved in endo-lysosomal trafficking have PH domains, as do certain ARFGEFs (Vicinanza et al. Citation2008) and RhoGEFs such as the Rac1 GEF RhoGEF7 (also known as β-PIX) (InterProScan, IPR001849 domain, http://www.ebi.ac.uk/interpro/) and the Golgi-localized Cdc42 GEF FGD1 (PH and FYVE domains) (Egorov et al. Citation2009). Three PI4 kinases, PI4KIIα, PI4KIIβ and PI4KIIIβ, and a clathrin-associated PI4,5P2 phosphatase, OCRL1 (oculocerebrorenal syndrome of Lowe) (Choudhury et al. Citation2005), can regulate PI4P levels on Golgi membranes. PI4KIIα regulates AP-1 binding (Wang et al. Citation2003). However, it also interacts with AP-3 (Craige et al. Citation2008), and its knock-down affects both AP-1-dependent MPR and AP-3-dependent LAMP trafficking (Baust et al. Citation2008). PI4KIIIβ has also been detected on AP-1 coated membranes (Baust et al. Citation2006), but its precise role remains to be clarified. PI3P levels on endosomes depend on class III PI3 kinase (yeast Vps34) and phosphatases such as myotubularin (MTM1) and myotubularin related proteins (De Matteis et al. Citation2005). The recruitment and the activity of these PI-modifying enzymes are regulated by ARF-1 and by their interactions with coat components (Godi et al. Citation1999).
Cargo content is important for stabilizing coats on membranes. Together with ARF-1 and PIs, cargos are essential for promoting the high affinity interaction of AP-1 and AP-3 (Baust et al. Citation2006), (Citation2008), GGA (Hirst et al. Citation2007), as well as that of AP-2 (Ehrlich et al. Citation2004) with membranes. The binding of cargo to AP-1 induces a conformational change in the AP-1 core domain enhancing its interaction with active ARF-1 (Lee et al. Citation2008). Recently, time-lapse imaging of live cells has illustrated a cargo-dependent regulation of both the size and the dynamics of clathrin- and AP-2 coated endocytic carriers (Mettlen et al. Citation2010).
Carrier biogenesis during post-Golgi transport: Early stages
The biogenesis of clathrin-, AP-1- and GGA-coated carriers starts to be understood. In contrast, that of AP-3-coated carriers, which may involve septins (Baust et al. Citation2008), still remains elusive. AP-1- and GGA-dependent transport relies on the formation of pleomorphic tubulo-vesicular carriers (Puertollano et al. Citation2003), (Waguri et al. Citation2003). This involves different protein networks that must be spatially and temporally coordinated in order to induce membrane curvature, tubule formation, elongation and fission ().
Figure 2. Model of clathrin/AP-1-coated carrier formation on TGN membranes. ARF1, PI-4-P and sorting motifs in cargo tails recruit AP-1 and clathrin to specific membrane subdomains inducing membrane curvature. Actin nucleation complexes associate with CHC at the edges of the clathrin coats. These complexes activate the Arp2/3 complex and trigger actin polymerization, thereby providing the force necessary to initiate carrier tubulation. Tubulated membranes may then recruit BAR domain-containing proteins that interact with N-WASP to sustain further actin polymerization via Arp2/3. Inhibitors of actin polymerization (HIP1R) which bind to clathrin light chains may prevent actin polymerization on the surface of the clathrin coats. Gadkin links AP-1 coat with kinesins and with microtubules. Carrier fission is induced by dynamin linked to the actin cytoskeleton. Thus, actin and microtubules may provide the forces necessary during the late stages of tubule formation, fission and subsequent microtubule based transport.
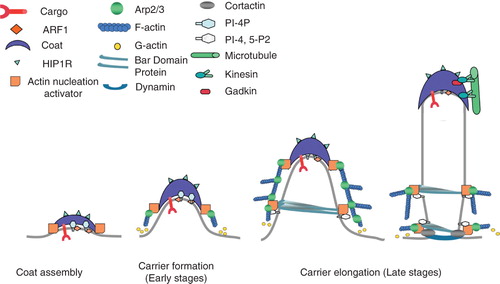
The first step in carrier biogenesis is the assembly of a protein network able to bend a lipid bilayer in order to generate a bud. Several proteins or complexes have been implicated in this process. A first complex is the clathrin triskelion made of three clathrin heavy and light chains that interact with the AP-1β1 and GGAs via clathrin-box motives present in clathrin heavy chains (Gallusser and Kirchhausen Citation1993), (Puertollano et al. Citation2001). Clathrin triskelions can assemble into flat lattices or bended structures (Kirchhausen et al. Citation1997). In addition to clathrin triskelions, adaptor proteins also interact, via their terminal ear domains, with accessory proteins that can induce membrane curvature via PI4P and clathrin-binding epsin N-terminal homology (ENTH) or AP180 N-terminal homology (ANTH) domains (Legendre-Guillemin et al. Citation2004). Competition between different accessory proteins for binding to adaptor proteins and/or clathrin may occur during various steps of carrier formation. As an example, the ENTH-containing Epsin R, found on TGN and endosomes binds AP-1, GGAs (Kalthoff et al. Citation2002), (Mills et al. Citation2003), (Chidambaram et al. Citation2004) and the SNARE Vt1b thus mediating fusion of post-Golgi derived carriers with their target compartment (Hirst et al. 2004). Eps15 (EGFR pathway substrate clone 15) that contains, an Epsin 15 homology (EH) domain also identified in γ-synergin (Page et al. Citation1999), is associated with AP-1 on TGN (Chi et al. Citation2008).
The second step in clathrin/AP-1 carrier biogenesis is the elongation of membrane buds enriched in ARF-1, PI4P, coat and ENTH/EH domain-containing proteins (Puertollano et al. Citation2003), (Waguri et al. Citation2003), (Polishchuk et al. Citation2006). These tubules display distinct regions (tip, surface and neck region, probably with different lipid and protein compositions) (Idrissi et al. Citation2008), (Liu et al. Citation2009). Several studies have illustrated the role of the actin cytoskeleton and Rho GTPases in controlling the dynamics of post-Golgi traffic (Merrifield et al. Citation2002), (Kaksonen et al. Citation2003), (Carreno et al. Citation2004) (). For example, Rac1 controls MPR trafficking by activating OCRL1 (Faucherre et al. Citation2005) and the post-TGN trafficking of E-cadherin to the plasma membrane (Wang et al. Citation2005). Cdc42 together with its effectors ARHGAP10 and FGD1 regulates the exit of exocytic post-TGN carriers via Arp2/3-dependent actin nucleation (Musch et al. Citation2001), (Dubois et al. Citation2005), (Egorov et al. Citation2009). A more recent study has illustrated how complexes made of CYFIP/Sra/Pir121, Abi1 and Nap1, which activate the Arp2/3 complex via WAVE/WASP (Neural Wiskott-Aldrich Syndrome Protein) family members (Schenck et al. Citation2003), (Innocenti et al. Citation2005), (Takenawa and Suetsugu Citation2007) promote the elongation of clathrin/AP-1 carriers from the TGN (Anitei et al. Citation2010). These complexes are recruited onto membranes via interactions between CYFIP and the N-terminal domain of CHC accessible at the edges of coated buds. CYFIP also binds Rac1 (Schenck et al. Citation2003), which is activated by the GEF β-PIX, known to form PAK-regulated complexes with the ARF-1 GAP GITs (Zhao et al. Citation2000). Thus, the CYFIP-containing complexes coordinate ARF-1-dependent clathrin/AP-1 coat assembly and Rac1-dependent actin polymerization necessary for tubule elongation. At the same time, the huntingtin-interacting protein 1-related (HIP1R), which binds clathrin light chains accessible at the surface of clathrin coats can inhibit actin polymerization at the surface of coated buds (Carreno et al. Citation2004), (Poupon et al. Citation2008) ().
During carrier formation, membrane curvature must be induced and stabilized, a process also relying on lipid modifying enzymes (De Matteis and Godi Citation2004). For example, phospholipase D (PLD), activated by ARF-1 and PI4,5P2, controls the synthesis of phosphatidic acid (PtdOH) and diacylglycerol (DAG), which induce asymmetry in the lipid bilayer and thus sustain carrier formation (Roth Citation2008). In turn, membrane curvature may control the availability of active ARF-1 as seen for ArfGAP1, which interacts preferentially with positively curved membranes through its amphipathic lipid packing sensor (ALPS) motifs and regulates the formation of an ARF-1 gradient along tubules during COPI-dependent transport (Ambroggio et al. Citation2010).
Carrier biogenesis during post-Golgi transport: Late stages
Curved membranes are enriched in proteins with BAR/Bin/Amphiphysin/Rvs or LPS/Lipid Packing Sensor domains. These proteins can sense increased membrane curvature and also bend the lipid bilayer in vitro (Itoh and De Camilli Citation2006). The recruitment of BAR domain proteins depends on their ability to bind, possibly via additional domains, either to PI-containing membranes (Itoh et al. Citation2005), or to small GTPases, like the Golgi-localized arfaptin, which binds both ARF-1 and Rac1 (Tarricone et al. Citation2001). The precise mechanisms regulating the recruitment of BAR domain proteins on the TGN membranes remain however to be understood. At the TGN, BAR domain proteins could be recruited via interactions with PI4P, as shown for the yeast Rho GTPase-activating protein Rgd1p (Prouzet-Mauleon et al. Citation2008), or with PI4,5P2 whose presence at the TGN remains however to be established, even though a PI4P 5 kinase (Godi et al. Citation1999), (Jones et al. Citation2000) and the OCRL PI4,5P2 phosphatase (Choudhury et al. Citation2005) are detected on these membranes. While stabilizing nascent tubules, BAR domain containing proteins could also sustain actin polymerization due to their ability to bind N-WASP (Takenawa and Suetsugu Citation2007) ().
The last step in the biogenesis of clathrin/AP-1-coated carriers is their fission from TGN membranes. This requires dynamin, a large GTPase recruited around the neck of the elongated carriers where it provides the force necessary for constriction of membranes (Mettlen et al. Citation2009) that are under a tension likely provided by actin polymerization (Merrifield et al. Citation2005). Dynamin itself, via cortactin acting either directly or via N-WASP, may control actin polymerization (Uruno et al. Citation2001), (Schafer et al. Citation2002), (Weaver et al. Citation2002). In addition, the BAR domain-containing syndapin 2, which binds dynamin 2, could be involved in such processes (Kessels et al. Citation2006). However, the order in which these molecules are engaged is still unclear, since actin may be necessary for the dynamin 2 recruitment (Cao et al. Citation2005). Newly formed Post-TGN carriers are transported towards endosomes via microtubule tracks. Early work identified the kinesin KIF13A as an AP-1 β1 interactor (Nakagawa et al. Citation2000), (Delevoye et al. Citation2009). Recently, an interaction between Gadkin/γ-BAR, an AP-1-associated protein, and KIF5 was identified (Neubrand et al. Citation2005), (Schmidt et al. Citation2009), (Maritzen et al. Citation2010). Membrane Rab GTPases may connect carriers with the molecular motors that mediate trafficking along actin filaments or microtubules (Stenmark Citation2009). Microtubule and associated motors may thus provide, together with actin, the forces necessary for carrier elongation, fission and transport.
Sorting complexes organizing endosome to TGN retrieval
Multiple routes can be used for the retrieval of components from endosomes back to the trans-Golgi network and retrograde transport may occur from both early and late endosomes (Pfeffer Citation2009). These multiple retrograde transport routes can be distinguished by their molecular requirements, in particular their sorting machineries (Bonifacino and Rojas Citation2006), (Johannes and Popoff Citation2008) ().
Figure 3. Model for retromer mediated cargo selection and carrier formation. (A) The GTPase Rab 7 recruits the cargo selective subcomplex of the retromer formed by VPS26, VPS29 and VPS35 onto the endosomal membrane where it can engage with its cargo. The membrane interacting subcomplex formed by combinations of SNX1/SNX2 and SNX5/SNX6 is associated via its PI3P binding PX domains with the endosomal membrane. Association of the cargo selective with the membrane interacting subcomplex and possibly multimerisation of the two complexes could lead to the formation of a tubular subdomain of the endosomal membrane, for which the curvature sensing/inducing bar domains of the SNXes are thought to be crucial. Formation of a tubular domain is likely to be assisted by force development achieved through the coupling of the minus end directed dynein/dynactin motor complex to the retromer via SNX5/SNX6. (B) The WASH/FAM21 complex is recruited by the retromer followed by actin polymerisation and Arp2/3 complex mediated actin filament branching which could assist force development. Currently it is unclear how recruitment of dynamin or a dynamin like factor to accomplish membrane fission of the formed carrier is regulated (C).
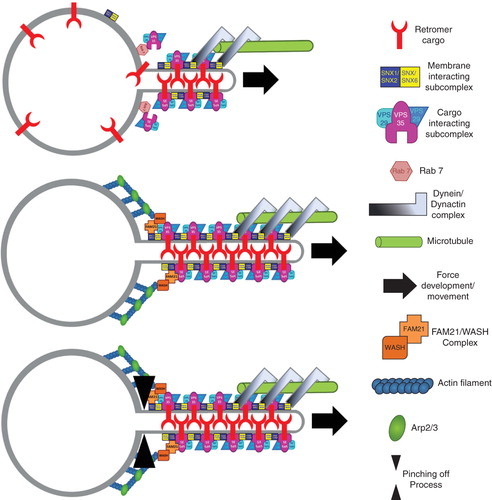
The retromer complex sorts a large variety of cargos (MPR/VPS10-like- and signalling receptors) in early endosomes, and organizes their subsequent transport to the TGN most likely through recycling endosomes (Bonifacino and Hurley Citation2008), (Seaman Citation2008), (Attar and Cullen Citation2009). Originally identified in yeast where it consists of five subunits (Vps5, Vps17, Vps26, Vps29 and Vps35) (Seaman et al. Citation1998), it has become clear that the retromer composition is more complex in mammals, where seven subunits support its function (SNX1, SNX2, SNX5, SNX6, Vps26, Vps29 and Vps35) (Attar and Cullen Citation2009), (Wassmer et al. Citation2009). The functional retromer consists of two sub-complexes, a cargo-binding sub-complex consisting of Vps26, Vps29 and Vps35 and a membrane interacting one comprising Vps5 and Vps17 in yeast (Seaman et al. Citation1998), and combinations of SNX1, SNX2, SNX5 and SNX6 in mammals (Wassmer et al. Citation2007), (Citation2009).
The cargo-selective sub-complex (primarily via Vps35), binds the cytosolic tails of numerous trans-membrane receptors. Well-characterized cargos of the retromer are CD- and CI-MPR, sortilin, wntless and SorLa (Attar and Cullen Citation2009). The membrane-binding sub-complex (Vps5, Vps17, SNX1/2, SNX5/6) may be recruited onto endosomal membranes by a combination of PX-domains binding to PI3P (and possibly PI3, 5P2) and BAR-domains sensing membrane curvature of SNX1 (Carlton et al. Citation2004). Multimerization of cargo-binding and membrane interacting subcomplexes could thus achieve efficient cargo concentration and membrane deformation, a prerequisite for the formation of retromer-positive tubular carriers (Carlton et al. Citation2004), (Wassmer et al. Citation2007) (). Recent studies have revealed that the retromer can regulate clathrin dynamics on endosomes: SNX-4, as well as SNX-1, SNX-2 and SNX-3 interact with clathrin heavy chain via a conserved motif present in PX-domain proteins (Skanland et al. Citation2009). SNX-1 associates with the endosomal receptor-mediated endocytosis 8 (RME-8) protein which activates the clathrin uncoating Hsc70 to inhibit clathrin recruitment on endosomes; in the absence of RME-8 and Hsp70, retrograde cargo transport from endosomes to the TGN is impaired (Popoff et al. Citation2009), (Shi et al. Citation2009).
Both the microtubule and the actin cytoskeletons support the retromer sorting function. The membrane interacting sub-complex can bind the p150glued/dynactin 1 subunit of dynein/dynactin via SNX5 and SNX6, an interaction relevant for efficient endosome to TGN transport (Wassmer et al. Citation2009). This link provides some mechanistic insight into how cargo binding and concentration can be coupled to membrane deformation, carrier formation and ultimately carrier motility. The actin nucleation factor Wash coordinates Fam21 and Arp2/3 recruitment at the base of endosomal retromer decorated tubules (Gomez and Billadeau Citation2009). This complex is important for efficient retromer mediated recycling of the CI-MPR and may provide the necessary shearing forces at the interface between retromer-coated tubules and the limiting endosomal membrane to assist fission (Gomez and Billadeau Citation2009). As a Wash protein containing complex may be of general relevance in catalyzing endosomal membrane fission (Derivery et al. Citation2009), the following scenario seems to emerge (): Rab7 recruits cargo selective sub-complexes on the endosomal membrane (Rojas et al. Citation2008), where they recognize and bind cytoplasmic receptor tails. Membrane-interacting subcomplexes already present on PI3P-rich endosomal membranes are recruited, followed by force generation by the dynein/dynactin motor complex through interaction with SNX5/SNX6. The pulling force could lead to formation of a tubular sub-domain of the endosome that is stabilized by multimerization of cargo- and membrane-interacting subcomplexes. Recruitment of Wash/Fam21, Arp2/3 and associated factors at the base of these tubules leads to the formation of a branched actin network and development of shearing force. Scission factors like dynamin or EHD family proteins (Daumke et al. Citation2007), (Gokool et al. Citation2007) could then separate tubular membrane from the limiting endosomal membrane, followed by rapid, minus-end directed transport mediated by dynein/dynactin.
In addition to the retromer complex, it has been proposed that clathrin, epsinR (which binds AP-1γ and the Vit1b SNARE), and dynamin (Lauvrak et al. Citation2004), (Saint-Pol et al. Citation2004), as well as AP-1 (Robinson et al. Citation2010) mediate protein retrieval from the early endosomes to the TGN. However, it is still difficult to understand how the clathrin/AP-1 coat could function both in retrograde and anterograde directions i.e. in two distinct membrane environments (reviewed in (Hinners and Tooze Citation2003), (Johannes and Popoff Citation2008)) and why retrograde transport from early endosomes would require two different sorting machineries. It is possible that clathrin-AP-1 coat and the retromer complex select cargos destined to two different destinations, TGN and recycling endosomes, respectively.
TIP47 and the late endosomal Rab 9 GTPase were the first components described to mediate the retrieval of the MPR from the late endosomes to the TGN (Diaz and Pfeffer Citation1998) (Carroll et al. Citation2001), (Pfeffer Citation2009). TIP47 binds both MPR sorting signals and Rab9. In addition Rab9 binds RhoBTB3, a protein with an ATPase activity (Espinosa et al. Citation2009). Although clearly important for MPR retrieval, the precise function of RhoBTB3 is still unknown. In contrast to the retromer complex, TIP47 and Rab9 were acquired late during evolution. This would suggest that TIP47 and Rab9 define a salvage pathway to ensure the quantitative recovery of the MPRs from the late endosomal system. More recently, TIP47 has also been involved in lipid droplet biogenesis (Bulankina et al. Citation2009). Therefore, it remains to understand how TIP47 could fulfill these two different functions.
TGN-endosome trafficking, signalling and development
Many examples illustrate the relevance of trafficking between the TGN and endosomes in key developmental processes, as did the embryonic lethality of mice lacking either the AP-1γ or μ1a subunits (Zizioli et al. Citation1999), (Meyer et al. Citation2000). A huge advance in the understanding of the mechanisms involved was the discovery of morphogens such as Wnt, released by certain cell types to form long range gradients in a developing organism, eliciting a reaction of morphogen receiving cells in a concentration dependent manner. In screens performed in Caenorhabditis elegans, retromer subunits were identified as key components required for the efficient release of wnt (Coudreuse et al. Citation2006), (Prasad and Clark Citation2006). Another important step in the understanding of wnt release was the discovery that the wnt-binding, seven-pass transmembrane receptor wntless/evi is necessary for secretion of wnt (Banziger et al. Citation2006), (Bartscherer et al. Citation2006). Quickly it became clear that wntless/evi recycling by the retromer is necessary for wnt release. In the absence of functional retromer, wntless is lysosomally degraded and wnt release is thus abolished (Belenkaya et al. Citation2008), (Franch-Marro et al. Citation2008), (Pan et al. Citation2008), (Port et al. Citation2008), (Yang et al. Citation2008). One possible interpretation of this data could be that wntless associates with wnt at the level of the Golgi and mediates its transport to the plasma membrane where wnt is released. Wntless undergoes endocytosis mediated by AP-2 (Pan et al. Citation2008) and is then recycled to the TGN by retromer, ready for another round of wnt delivery (Lorenowicz and Korswagen Citation2009).
Another type of signalling pathway that regulates development involves the proper intracellular trafficking of the Notch receptor and its ligand Delta. Plasma membrane Notch receptors are endocytosed and then sorted in early endosomes, at least partially via the ESCRT complex (Vaccari and Bilder Citation2005): Activated receptors are directed towards lysosomes, whereas unactivated receptors are transported to recycling endosomes from where they can be again recruited to the plasma membrane (Fortini and Bilder Citation2009). In Drosophila melanogaster, the transport of the Delta/Serrate/Lag2 (DSL) Notch ligand, depends on a homolog of Epsin R, Liquid facets (Wang and Struhl Citation2004) essential for cellular proliferation and differentiation (Lee et al. Citation2009). Moreover, the activation of the Notch receptor may also occur on intracellular membranes, mediated, for example, by binding to DLL3, a Golgi-associated Notch ligand (Geffers et al. Citation2007).
These are just two of the numerous examples that illustrate the impact of trafficking on signalling processes that shape development. These developmental processes, as well as cellular homeostasis and functionality, depend on a plethora of signalling pathways that need to be integrated. The dynamics of membrane traffic is also dependent on extracellular signals transmitted via plasma membrane receptors and relayed by the corresponding signalling cascades. This notion comes from comprehensive analyses based on high throughput screening combining RNA interference and automated, quantitative multiparametric image analysis revealing the importance of signalling pathways such as Wnt, integrin/cell adhesion, transforming growth factor (TGF)-beta and Notch for the endocytosis of transferrin and EGF (Collinet et al. Citation2010). Little is actually known about the substrates of the kinases underlying these signalling pathways and the information remains fragmentary. For example, EGF-EGFR signalling modulates GGA3 phosphorylation (Kametaka et al. Citation2005). Wnt3a signalling via Frizzled and Dishevelled receptors may control PI4P levels by activating PI4KIIα (Qin et al. Citation2009). A major challenge will be to identify the substrates of key kinases to obtain a comprehensive understanding of how signalling cascades regulate membrane traffic.
TGN-endosome trafficking and human diseases
The characterization of the cellular machineries mediating the bidirectional transport between the TGN and the endosomal system and the dissection of their means of interaction and regulation are now shedding light onto the molecular bases of some human diseases. Improper clathrin/AP-1 dependent sorting appears to be associated with mental retardation in humans. Somatic mutations in AP-1σ2 (Tarpey et al. Citation2006) as well as in clathrin/AP-1 coat associated CYFIP (Schenck et al. Citation2001) or p21-activated kinase 3 (PAK3) (Allen et al. Citation1998), (Baust et al. Citation2006) have been associated with X-linked mental retardation. Furthermore, CYFIP, which coordinates ARF-1 and Rac1 signalling for generating clathrin/AP-1 transport carriers, provides a direct link between this pathway and the fragile-X mental retardation protein (FMRP), the most commonly affected molecule in this mental retardation syndrome (Schenck et al. Citation2003), (Anitei et al. Citation2010). This suggests that membrane transport steps regulated by these molecules found in the same protein network (Baust et al. Citation2006) contribute, if only partially, to neuronal differentiation and functionality. Recent data points towards specific neuronal trafficking steps affected in the absence of these molecules. The synaptic vesicle cycle is impaired in AP-1σ1B-/- mice (Glyvuk et al. Citation2010). In the post-synaptic compartment, functional FMRP is necessary for the trafficking of the AMPA glutamate receptors 1 and 5 (Nakamoto et al. Citation2007) that are recruited to the cell surface from recycling endosomes, in an AP-1μ and Rab11-dependent manner (Correia et al. Citation2008), (Margeta et al. Citation2009).
A proper bidirectional transport between the TGN and the endosomal system is also relevant to Alzheimer's disease. In affected patients, neurological dysfunctions occur due to amyloid plaque accumulation. A central component of these plaques is amyloid β (Aβ), a product of amyloid precursor protein (APP) β-cleavage by β-site APP-cleaving enzyme (BACE). Both APP and BACE are transmembrane proteins trafficking between the TGN, endosomes and the plasma membrane; thus, the correct sorting of both the enzyme and its substrate are important for β-processing (Small and Gandy Citation2006). FRET studies indicate that intracellular APP and BACE interact mostly in early endosomes (Kinoshita et al. Citation2003), favoring this compartment as the principal candidate for APP β-cleavage. Similar to depletion of GGA1, GGA2 or GGA3, the knock-down of the Vps26 subunit of the retromer resulted in BACE accumulation in early endosomes (He et al. Citation2005). This is supported by recent data indicating that, in mice, the absence of retromer correlates with synaptic dysfunction and increased levels of Aβ (Muhammad et al. Citation2008). However, it cannot be excluded that β-processing also occurs in the TGN, especially since APP binds not only AP1-B μ1B, but also the AP-4 μ4 subunit and μ4 depletion results in APP accumulation in the TGN with a simultaneous increase in Aβ secretion (Icking et al. Citation2007), (Burgos et al. Citation2010). In conclusion, the full understanding of the way APP and BACE cycle between TGN, endosomes and the plasma membrane remains a challenge for the future.
Concluding remarks
The last two decades brought significant advances in identifying components of the sorting machineries active on TGN and endosomal membranes. It has also become clear that these complexes function in a coordinated manner, regulating membrane curvature, fission and fusion, together with the actin cytoskeleton and microtubules. While the key players and many of their accessory proteins have been identified, a large number of important questions remain unanswered. For many transport steps the precise sequence of molecular events remains ill defined, thus seriously limiting our possibilities of fully understanding the process. In vitro reconstitution approaches using giant unilamellar synthetic membranes and measuring protein interactions in time and space may help in addressing these questions. Another important question is how antero- and retrograde transport steps are regulated and what differential regulation mechanisms are used in different tissues and cell types to ‘tweak’ the transport system to serve a specialized, tissue-specific function. However, besides these questions much more fundamental issues persist. How many different retrograde routes from endosomes to the TGN actually exist? How can directionality in transport from the TGN to endosomes be achieved when AP-1 can form carriers on both compartments? Do AP-1 and AP-3 function in mammalian cells in a parallel or a serial manner? Addressing these questions will be essential for understanding the principles that guide transport between the biosynthetic and the endocytic pathways. Furthermore, it will be essential to comprehensively understand if and how the dynamics of the proteins networks involved in membrane trafficking is regulated during, i.e., changes in environmental conditions as seen during development or in certain pathological conditions.
Acknowledgements
We thank the different lab members for their helpful discussions and critical comments. We apologize for not being able to quote the work of many groups that contributed to our current understanding of the trafficking pathways described in this review. This work was supported in part by grants from DFG (TRR 83/1-2010, HO 2584/1-1, HO 2584/2-1, HO 2584/6-1, HO 2584/8-1, HO 2584/9-1), and TU-Dresden.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Allen KM, Gleeson JG, Bagrodia S, Partington MW, Macmillan JC, Cerione RA, Mulley JC, Walsh CA. 1998. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat Genet 20:25–30.
- Ambroggio E, Sorre B, Bassereau P, Goud B, Manneville JB, Antonny B. 2010. ArfGAP1 generates an Arf1 gradient on continuous lipid membranes displaying flat and curved regions. Embo J 29:292–303.
- Anitei M, Stange C, Parshina I, Baust T, Schenck A, Raposo G, Kirchhausen T, Hoflack B. 2010. Protein complexes containing CYFIP/Sra/PIR121 coordinate Arf1 and Rac1 signalling during clathrin-AP-1-coated carrier biogenesis at the TGN. Nat Cell Biol 12:330–340.
- Attar N, Cullen PJ. 2009. The retromer complex. Adv Enzyme Regul.
- Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. 2006. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125:509–522.
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M. 2006. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 125:523–533.
- Baust T, Anitei M, Czupalla C, Parshyna I, Bourel L, Thiele C, Krause E, Hoflack B. 2008. Protein networks supporting AP-3 function in targeting lysosomal membrane proteins. Mol Biol Cell 19:1942–1951.
- Baust T, Czupalla C, Krause E, Bourel-Bonnet L, Hoflack B. 2006. Proteomic analysis of adaptor protein 1A coats selectively assembled on liposomes. Proc Natl Acad Sci USA 103:3159–3164.
- Belenkaya TY, Wu Y, Tang X, Zhou B, Cheng L, Sharma YV, Yan D, Selva EM, Lin X. 2008. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell 14:120–131.
- Bi X, Corpina RA, Goldberg J. 2002. Structure of the Sec23/24-Sar1 pre-budding complex of the COPII vesicle coat. Nature 419:271–277.
- Bonifacino JS. 2004. The Gga proteins: Adaptors on the move. Nat Rev Mol Cell Biol 5:23–32.
- Bonifacino JS, Hurley JH. 2008. Retromer. Curr Opin Cell Biol 20:427–436.
- Bonifacino JS, Rojas R. 2006. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol, 7, 568–579.
- Bonifacino JS, Traub LM. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem 72:395–447.
- Braulke T, Bonifacino JS. 2009. Sorting of lysosomal proteins. Biochim Biophys Acta 1793:605–614.
- Bulankina AV, Deggerich A, Wenzel D, Mutenda K, Wittmann JG, Rudolph MG, Burger KN, Honing S. 2009. TIP47 functions in the biogenesis of lipid droplets. J Cell Biol 185:641–655.
- Burgos PV, Mardones GA, Rojas AL, Dasilva LL, Prabhu Y, Hurley JH, Bonifacino JS. 2010. Sorting of the Alzheimer's disease amyloid precursor protein mediated by the AP-4 complex. Dev Cell 18:425–436.
- Cao H, Weller S, Orth JD, Chen J, Huang B, Chen JL, Stamnes M, McNiven MA. 2005. Actin and Arf1-dependent recruitment of a cortactin-dynamin complex to the Golgi regulates post-Golgi transport. Nat Cell Biol 7:483–492.
- Carlton J, Bujny M, Peter BJ, Oorschot VM, Rutherford A, Mellor H, Klumperman J, Mcmahon HT, Cullen PJ. 2004. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high-curvature membranes and 3-phosphoinositides. Curr Biol 14:1791–1800.
- Carreno S, Engqvist-Goldstein AE, Zhang CX, McDonald KL, Drubin DG. 2004. Actin dynamics coupled to clathrin-coated vesicle formation at the trans-Golgi network. J Cell Biol 165:781–788.
- Carroll KS, Hanna J, Simon I, Krise J, Barbero P, Pfeffer SR. 2001. Role of Rab9 GTPase in facilitating receptor recruitment by TIP47. Science 292:1373–1376.
- Casanova JE. 2007. Regulation of Arf activation: The Sec7 family of guanine nucleotide exchange factors. Traffic 8:1476–1485.
- Chi S, Cao H, Chen J, McNiven MA. 2008. Eps15 mediates vesicle trafficking from the trans-Golgi network via an interaction with the clathrin adaptor AP-1. Mol Biol Cell 19:3564–3575.
- Chidambaram S, Mullers N, Wiederhold K, Haucke V, Von Mollard GF. 2004. Specific interaction between SNAREs and epsin N-terminal homology (ENTH) domains of epsin-related proteins in trans-Golgi network to endosome transport. J Biol Chem 279:4175–4179.
- Choudhury R, Diao A, Zhang F, Eisenberg E, Saint-Pol A, Williams C, Konstantakopoulos A, Lucocq J, Johannes L, Rabouille C, Greene LE, Lowe M. 2005. Lowe syndrome protein OCRL1 interacts with clathrin and regulates protein trafficking between endosomes and the trans-Golgi network. Mol Biol Cell 16:3467–3479.
- Collinet C, Stoter M, Bradshaw CR, Samusik N, Rink JC, Kenski D, Habermann B, Buchholz F, Henschel R, Mueller MS, Nagel WE, Fava E, Kalaidzidis Y, Zerial M. 2010. Systems survey of endocytosis by multiparametric image analysis. Nature 464:243–249.
- Correia SS, Bassani S, Brown TC, Lise MF, Backos DS, El-Husseini A, Passafaro M, Esteban JA. 2008. Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nat Neurosci 11:457–466.
- Coudreuse DY, Roel G, Betist MC, Destree O, Korswagen HC. 2006. Wnt gradient formation requires retromer function in Wnt-producing cells. Science 312:921–924.
- Craige B, Salazar G, Faundez V. 2008. Phosphatidylinositol-4-kinase type II alpha contains an AP-3-sorting motif and a kinase domain that are both required for endosome traffic. Mol Biol Cell 19:1415–1426.
- D'souza-Schorey C, Chavrier P. 2006. ARF proteins: Roles in membrane traffic and beyond. Nat Rev Mol Cell Biol 7:347–358.
- Daumke O, Lundmark R, Vallis Y, Martens S, Butler PJ, Mcmahon HT. 2007. Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature 449:923–927.
- David R. 2007. Endocytosis: Division of labour between ESCRTs. Nat Rev Mol Cell Biol 11:314.
- De Matteis MA, Di Campli A, Godi A. 2005. The role of the phosphoinositides at the Golgi complex. Biochim Biophys Acta 1744:396–405.
- De Matteis MA, Godi A. 2004. PI-loting membrane traffic. Nat Cell Biol 6:487–492.
- Delevoye C, Hurbain I, Tenza D, Sibarita JB, Uzan-Gafsou S, Ohno H, Geerts WJ, Verkleij AJ, Salamero J, Marks MS, Raposo G. 2009. AP-1 and KIF13A coordinate endosomal sorting and positioning during melanosome biogenesis. J Cell Biol 187:247–264.
- Dell'Angelica EC, Klumperman J, Stoorvogel W, Bonifacino JS. 1998. Association of the AP-3 adaptor complex with clathrin. Science 280:431–434.
- Dell'Angelica EC, Ohno H, Ooi CE, Rabinovich E, Roche KW, Bonifacino JS. 1997. AP-3: An adaptor-like protein complex with ubiquitous expression. Embo J 16:917–928.
- Derivery E, Sousa C, Gautier JJ, Lombard B, Loew D, Gautreau A. 2009. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev Cell 17:712–723.
- Di Paolo G, De Camilli P. 2006. Phosphoinositides in cell regulation and membrane dynamics. Nature 443:651–657.
- Diaz E, Pfeffer SR. 1998. TIP47: A cargo selection device for mannose 6-phosphate receptor trafficking. Cell 93:433–443.
- Donaldson JG, Honda A. 2005. Localization and function of Arf family GTPases. Biochem Soc Trans 33:639–642.
- Doray B, Ghosh P, Griffith J, Geuze HJ, Kornfeld S. 2002. Cooperation of GGAs and AP-1 in packaging MPRs at the trans-Golgi network. Science 297:1700–1703.
- Dubois T, Paleotti O, Mironov AA, Fraisier V, Stradal TE, De Matteis MA, Franco M, Chavrier P. 2005. Golgi-localized GAP for Cdc42 functions downstream of ARF1 to control Arp2/3 complex and F-actin dynamics. Nat Cell Biol 7:353–364.
- Egorov MV, Capestrano M, Vorontsova OA, Di Pentima A, Egorova AV, Mariggio S, Ayala MI, Tete S, Gorski JL, Luini A, Buccione R, Polishchuk RS. 2009. Faciogenital dysplasia protein (FGD1) regulates export of cargo proteins from the golgi complex via Cdc42 activation. Mol Biol Cell 20:2413–2427.
- Ehrlich M, Boll W, Van Oijen A, Hariharan R, Chandran K, Nibert ML, Kirchhausen T. 2004. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118:591–605.
- Espinosa EJ, Calero M, Sridevi K, Pfeffer SR. 2009. RhoBTB3: A Rho GTPase-family ATPase required for endosome to Golgi transport. Cell 137:938–948.
- Faucherre A, Desbois P, Nagano F, Satre V, Lunardi J, Gacon G, Dorseuil O. 2005. Lowe syndrome protein Ocrl1 is translocated to membrane ruffles upon Rac GTPase activation: A new perspective on Lowe syndrome pathophysiology. Hum Mol Genet 14:1441–1448.
- Folsch H, Pypaert M, Maday S, Pelletier L, Mellman I. 2003. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J Cell Biol 163:351–362.
- Fortini ME, Bilder D. 2009. Endocytic regulation of Notch signaling. Curr Opin Genet Dev 19:323–328.
- Franch-Marro X, Wendler F, Guidato S, Griffith J, Baena-Lopez A, Itasaki N, Maurice MM, Vincent JP. 2008. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol 10:170–177.
- Gallusser A, Kirchhausen T. 1993. The beta 1 and beta 2 subunits of the AP complexes are the clathrin coat assembly components. Embo J 12:5237–5244.
- Geffers I, Serth K, Chapman G, Jaekel R, Schuster-Gossler K, Cordes R, Sparrow DB, Kremmer E, Dunwoodie SL, Klein T, Gossler A. 2007. Divergent functions and distinct localization of the Notch ligands DLL1 and DLL3 in vivo. J Cell Biol 178:465–476.
- Ghosh P, Dahms NM, Kornfeld S. 2003. Mannose 6-phosphate receptors: New twists in the tale. Nat Rev Mol Cell Biol 4:202–212.
- Glyvuk N, Tsytsyura Y, Geumann C, D'Hooge R, Huve J, Kratzke M, Baltes J, Boning D, Klingauf J, Schu P. 2010. AP-1/sigma1B-adaptin mediates endosomal synaptic vesicle recycling, learning and memory. Embo J.
- Godi A, Pertile P, Meyers R, Marra P, Di Tullio G, Iurisci C, Luini A, Corda D, De Matteis MA. 1999. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol 1:280–287.
- Gokool S, Tattersall D, Seaman MN. 2007. EHD1 interacts with retromer to stabilize SNX1 tubules and facilitate endosome-to-Golgi retrieval. Traffic 8:1873–1886.
- Gomez TS, Billadeau DD. 2009. A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev Cell 17:699–711.
- He X, Li F, Chang WP, Tang J. 2005. GGA proteins mediate the recycling pathway of memapsin 2 (BACE). J Biol Chem 280:11696–11703.
- Hinners I, Tooze SA. 2003. Changing directions: Clathrin-mediated transport between the Golgi and endosomes. J Cell Sci 116:763–771.
- Hirst J, Lindsay MR, Robinson MS. 2001. GGAs: Roles of the different domains and comparison with AP-1 and clathrin. Mol Biol Cell 12:3573–3588.
- Hirst J, Motley A, Harasaki K, Peak Chew SY, Robinson MS. 2003. EpsinR: An ENTH domain-containing protein that interacts with AP-1. Mol Biol Cell 14:625–641.
- Hirst J, Sahlender DA, Choma M, Sinka R, Harbour ME, Parkinson M, Robinson MS. 2009. Spatial and functional relationship of GGAs and AP-1 in Drosophila and HeLa cells. Traffic 10:1696–1710.
- Hirst J, Seaman MN, Buschow SI, Robinson MS. 2007. The role of cargo proteins in GGA recruitment. Traffic 8:594–604.
- Icking A, Amaddii M, Ruonala M, Honing S, Tikkanen R. 2007. Polarized transport of Alzheimer amyloid precursor protein is mediated by adaptor protein complex AP1-1B. Traffic 8:285–296.
- Idrissi FZ, Grotsch H, Fernandez-Golbano IM, Presciatto-Baschong C, Riezman H, Geli MI. 2008. Distinct acto/myosin-I structures associate with endocytic profiles at the plasma membrane. J Cell Biol 180:1219–1232.
- Innocenti M, Gerboth S, Rottner K, Lai FP, Hertzog M, Stradal TE, Frittoli E, Didry D, Polo S, Disanza A, Benesch S, Di Fiore PP, Carlier MF, Scita G. 2005. Abi1 regulates the activity of N-WASP and WAVE in distinct actin-based processes. Nat Cell Biol 7:969–976.
- Ishizaki R, Shin HW, Mitsuhashi H, Nakayama K. 2008. Redundant roles of BIG2 and BIG1, guanine-nucleotide exchange factors for ADP-ribosylation factors in membrane traffic between the trans-Golgi network and endosomes. Mol Biol Cell 19:2650–2660.
- Itoh T, De Camilli P. 2006. BAR, F-BAR (EFC) and ENTH/ANTH domains in the regulation of membrane-cytosol interfaces and membrane curvature. Biochim Biophys Acta 1761:897–912.
- Itoh T, Erdmann KS, Roux A, Habermann B, Werner H, De Camilli P. 2005. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev Cell 9:791–804.
- Janvier K, Kato Y, Boehm M, Rose JR, Martina JA, Kim BY, Venkatesan S, Bonifacino JS. 2003. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes. J Cell Biol 163:1281–1290.
- Johannes L, Popoff V. 2008. Tracing the retrograde route in protein trafficking. Cell 135:1175–1187.
- Jones DH, Morris JB, Morgan CP, Kondo H, Irvine RF, Cockcroft S. 2000. Type I phosphatidylinositol 4-phosphate 5-kinase directly interacts with ADP-ribosylation factor 1 and is responsible for phosphatidylinositol 4,5-bisphosphate synthesis in the golgi compartment. J Biol Chem 275:13962–13966.
- Kaksonen M, Sun Y, Drubin DG. 2003. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell 115:475–487.
- Kalthoff C, Groos S, Kohl R, Mahrhold S, Ungewickell EJ. 2002. Clint: A novel clathrin-binding ENTH-domain protein at the Golgi. Mol Biol Cell 13:4060–4073.
- Kametaka S, Mattera R, Bonifacino JS. 2005. Epidermal growth factor-dependent phosphorylation of the GGA3 adaptor protein regulates its recruitment to membranes. Mol Cell Biol 25:7988–8000.
- Kessels MM, Dong J, Leibig W, Westermann P, Qualmann B. 2006. Complexes of syndapin II with dynamin II promote vesicle formation at the trans-Golgi network. J Cell Sci 119:1504–1516.
- Kinoshita A, Fukumoto H, Shah T, Whelan CM, Irizarry MC, Hyman BT. 2003. Demonstration by FRET of BACE interaction with the amyloid precursor protein at the cell surface and in early endosomes. J Cell Sci 116:3339–3346.
- Kirchhausen T, Bonifacino JS, Riezman H. 1997. Linking cargo to vesicle formation: Receptor tail interactions with coat proteins. Curr Opin Cell Biol 9:488–495.
- Kliouchnikov L, Bigay J, Mesmin B, Parnis A, Rawet M, Goldfeder N, Antonny B, Cassel D. 2009. Discrete determinants in ArfGAP2/3 conferring Golgi localization and regulation by the COPI coat. Mol Biol Cell 20:859–869.
- Lauvrak SU, Torgersen ML, Sandvig K. 2004. Efficient endosome-to-Golgi transport of Shiga toxin is dependent on dynamin and clathrin. J Cell Sci 117:2321–2331.
- Le Borgne R, Hoflack B. 1998. Mechanisms of protein sorting and coat assembly: Insights from the clathrin-coated vesicle pathway. Curr Opin Cell Biol 10:499–503.
- Lee I, Doray B, Govero J, Kornfeld S. 2008. Binding of cargo sorting signals to AP-1 enhances its association with ADP ribosylation factor 1-GTP. J Cell Biol 180:467–472.
- Lee JH, Overstreet E, Fitch E, Fleenor S, Fischer JA. 2009. Drosophila liquid facets-Related encodes Golgi epsin and is an essential gene required for cell proliferation, growth, and patterning. Dev Biol 331:1–13.
- Legendre-Guillemin V, Wasiak S, Hussain NK, Angers A, McPherson PS. 2004. ENTH/ANTH proteins and clathrin-mediated membrane budding. J Cell Sci 117:9–18.
- Liu J, Sun Y, Drubin DG, Oster GF. 2009. The mechanochemistry of endocytosis. PLoS Biol 7:e1000204.
- Lorenowicz MJ, Korswagen HC. 2009. Sailing with the Wnt: Charting the Wnt processing and secretion route. Exp Cell Res 315:2683–2689.
- Mardones GA, Burgos PV, Brooks DA, Parkinson-Lawrence E, Mattera R, Bonifacino JS. 2007. The trans-Golgi network accessory protein p56 promotes long-range movement of GGA/clathrin-containing transport carriers and lysosomal enzyme sorting. Mol Biol Cell 18:3486–3501.
- Margeta MA, Wang GJ, Shen K. 2009. Clathrin adaptor AP-1 complex excludes multiple postsynaptic receptors from axons in C. elegans. Proc Natl Acad Sci USA 106:1632–1637.
- Maritzen T, Schmidt MR, Kukhtina V, Higman VA, Strauss H, Volkmer R, Oschkinat H, Dotti CG, Haucke V. 2010. A novel subtype of AP-1-binding motif within the palmitoylated trans-Golgi network/endosomal accessory protein Gadkin/gamma-BAR. J Biol Chem 285:4074–4086.
- Merrifield CJ, Feldman ME, Wan L, Almers W. 2002. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat Cell Biol 4:691–698.
- Merrifield CJ, Perrais D, Zenisek D. 2005. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell 121:593–606.
- Mettlen M, Loerke D, Yarar D, Danuser G, Schmid SL. 2010. Cargo- and adaptor-specific mechanisms regulate clathrin-mediated endocytosis. J Cell Biol 188:919–933.
- Mettlen M, Pucadyil T, Ramachandran R, Schmid SL. 2009. Dissecting dynamin's role in clathrin-mediated endocytosis. Biochem Soc Trans 37:1022–1026.
- Meyer C, Zizioli D, Lausmann S, Eskelinen EL, Hamann J, Saftig P, Von Figura K, Schu P. 2000. mu1A-adaptin-deficient mice: Lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. Embo J 19:2193–2203.
- Mills IG, Praefcke GJ, Vallis Y, Peter BJ, Olesen LE, Gallop JL, Butler PJ, Evans PR, Mcmahon HT. 2003. EpsinR: An AP1/clathrin interacting protein involved in vesicle trafficking. J Cell Biol 160:213–222.
- Muhammad A, Flores I, Zhang H, Yu R, Staniszewski A, Planel E, Herman M, Ho L, Kreber R, Honig LS, Ganetzky B, Duff K, Arancio O, Small SA. 2008. Retromer deficiency observed in Alzheimer's disease causes hippocampal dysfunction, neurodegeneration, and Abeta accumulation. Proc Natl Acad Sci USA 105:7327–7332.
- Munier-Lehmann H, Mauxion F, Hoflack B. 1996. Function of the two mannose 6-phosphate receptors in lysosomal enzyme transport. Biochem Soc Trans 24:133–136.
- Musch A, Cohen D, Kreitzer G, Rodriguez-Boulan E. 2001. cdc42 regulates the exit of apical and basolateral proteins from the trans-Golgi network. Embo J 20:2171–2179.
- Nakagawa T, Setou M, Seog D, Ogasawara K, Dohmae N, Takio K, Hirokawa N. 2000. A novel motor, KIF13A, transports mannose-6-phosphate receptor to plasma membrane through direct interaction with AP-1 complex. Cell 103:569–581.
- Nakamoto M, Nalavadi V, Epstein MP, Narayanan U, Bassell GJ, Warren ST. 2007. Fragile X mental retardation protein deficiency leads to excessive mGluR5-dependent internalization of AMPA receptors. Proc Natl Acad Sci USA 104:15537–15542.
- Natsume W, Tanabe K, Kon S, Yoshida N, Watanabe T, Torii T, Satake M. 2006. SMAP2, a novel ARF GTPase-activating protein, interacts with clathrin and clathrin assembly protein and functions on the AP-1-positive early endosome/trans-Golgi network. Mol Biol Cell 17:2592–2603.
- Neubrand VE, Will RD, Mobius W, Poustka A, Wiemann S, Schu P, Dotti CG, Pepperkok R, Simpson JC. 2005. Gamma-BAR, a novel AP-1-interacting protein involved in post-Golgi trafficking. Embo J 24:1122–1133.
- Newell-Litwa K, Seong E, Burmeister M, Faundez V. 2007. Neuronal and non-neuronal functions of the AP-3 sorting machinery. J Cell Sci 120:531–541.
- Nie Z, Boehm M, Boja ES, Vass WC, Bonifacino JS, Fales HM, Randazzo PA. 2003. Specific regulation of the adaptor protein complex AP-3 by the Arf GAP AGAP1. Dev Cell 5:513–521.
- Page LJ, Sowerby PJ, Lui WW, Robinson MS. 1999. Gamma-synergin: An EH domain-containing protein that interacts with gamma-adaptin. J Cell Biol 146:993–1004.
- Pan CL, Baum PD, Gu M, Jorgensen EM, Clark SG, Garriga G. 2008. C. elegans AP-2 and retromer control Wnt signaling by regulating mig-14/Wntless. Dev Cell 14:132–139.
- Peden AA, Oorschot V, Hesser BA, Austin CD, Scheller RH, Klumperman J. 2004. Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J Cell Biol 164:1065–1076.
- Peden AA, Rudge RE, Lui WW, Robinson MS. 2002. Assembly and function of AP-3 complexes in cells expressing mutant subunits. J Cell Biol 156:327–336.
- Pfeffer SR. 2009. Multiple routes of protein transport from endosomes to the trans Golgi network. FEBS Lett 583:3811–3816.
- Polishchuk RS, San Pietro E, Di Pentima A, Tete S, Bonifacino JS. 2006. Ultrastructure of long-range transport carriers moving from the trans Golgi network to peripheral endosomes. Traffic 7:1092–1103.
- Popoff V, Mardones GA, Bai SK, Chambon V, Tenza D, Burgos PV, Shi A, Benaroch P, Urbe S, Lamaze C, Grant BD, Raposo G, Johannes L. 2009. Analysis of articulation between clathrin and retromer in retrograde sorting on early endosomes. Traffic 10:1868–1880.
- Port F, Kuster M, Herr P, Furger E, Banziger, C, Hausmann G, Basler K. 2008. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol 10:178–185.
- Poupon V, Girard M, Legendre-Guillemin V, Thomas S, Bourbonniere L, Philie J, Bright NA, McPherson PS. 2008. Clathrin light chains function in mannose phosphate receptor trafficking via regulation of actin assembly. Proc Natl Acad Sci USA 105:168–173.
- Prasad BC, Clark SG. 2006. Wnt signaling establishes anteroposterior neuronal polarity and requires retromer in C. elegans. Development 133:1757–1766.
- Prouzet-Mauleon V, Lefebvre F, Thoraval D, Crouzet M, Doignon F. 2008. Phosphoinositides affect both the cellular distribution and activity of the F-BAR-containing RhoGAP Rgd1p in yeast. J Biol Chem 283:33249–33257.
- Puertollano R, Bonifacino JS. 2004. Interactions of GGA3 with the ubiquitin sorting machinery. Nat Cell Biol 6:244–251.
- Puertollano R, Randazzo PA, Presley JF, Hartnell LM, Bonifacino JS. 2001. The GGAs promote ARF-dependent recruitment of clathrin to the TGN. Cell 105:93–102.
- Puertollano R, Van Der Wel NN, Greene LE, Eisenberg E, Peters PJ, Bonifacino JS. 2003. Morphology and dynamics of clathrin/GGA1-coated carriers budding from the trans-Golgi network. Mol Biol Cell 14:1545–1557.
- Qin Y, Li L, Pan W, Wu D. 2009. Regulation of phosphatidylinositol kinases and metabolism by Wnt3a and Dvl. J Biol Chem 284:22544–22548.
- Raiborg C, Bache KG, Gillooly DJ, Madshus IH, Stang E, Stenmark H. 2002. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat Cell Biol 4:394–398.
- Raiborg C, Bache KG, Mehlum A, Stang E, Stenmark H. 2001. Hrs recruits clathrin to early endosomes. Embo J 20:5008–5021.
- Reczek D, Schwake M, Schroder J, Hughes H, Blanz J, Jin X, Brondyk W, Van Patten S, Edmunds T, Saftig P. 2007. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell 131:770–783.
- Robinson MS, Sahlender DA, Foster SD. 2010. Rapid inactivation of proteins by rapamycin-induced rerouting to mitochondria. Dev Cell 18:324–331.
- Rojas R, Van Vlijmen T, Mardones GA, Prabhu Y, Rojas AL, Mohammed S, Heck AJ, Raposo G, Van Der Sluijs P, Bonifacino JS. 2008. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol 183:513–526.
- Roth MG. 2008. Molecular mechanisms of PLD function in membrane traffic. Traffic 9:1233–1239.
- Saint-Pol A, Yelamos B, Amessou M, Mills IG, Dugast M, Tenza D, Schu P, Antony C, Mcmahon HT, Lamaze C, Johannes L. 2004. Clathrin adaptor epsinR is required for retrograde sorting on early endosomal membranes. Dev Cell 6:525–538.
- Schafer DA, Weed SA, Binns D, Karginov AV, Parsons JT, Cooper JA. 2002. Dynamin2 and cortactin regulate actin assembly and filament organization. Curr Biol 12:1852–1857.
- Schenck A, Bardoni B, Langmann C, Harden N, Mandel JL, Giangrande A. 2003. CYFIP/Sra-1 controls neuronal connectivity in Drosophila and links the Rac1 GTPase pathway to the fragile X protein. Neuron 38:887–898.
- Schenck A, Bardoni B, Moro A, Bagni C, Mandel JL. 2001. A highly conserved protein family interacting with the fragile X mental retardation protein (FMRP) and displaying selective interactions with FMRP-related proteins FXR1P and FXR2P. Proc Natl Acad Sci USA 98:8844–8849.
- Schmidt MR, Maritzen T, Kukhtina V, Higman VA, Doglio L, Barak NN, Strauss H, Oschkinat H, Dotti CG, Haucke V. 2009. Regulation of endosomal membrane traffic by a Gadkin/AP-1/kinesin KIF5 complex. Proc Natl Acad Sci USA 106:15344–15349.
- Scott PM, Bilodeau PS, Zhdankina O, Winistorfer SC, Hauglund MJ, Allaman MM, Kearney WR, Robertson AD, Boman AL, Piper RC. 2004. GGA proteins bind ubiquitin to facilitate sorting at the trans-Golgi network. Nat Cell Biol 6:252–259.
- Seaman MN. 2008. Endosome protein sorting: Motifs and machinery. Cell Mol Life Sci 65:2842–2858.
- Seaman MN, Mccaffery JM, Emr SD. 1998. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol 142:665–681.
- Shi A, Sun L, Banerjee R, Tobin M, Zhang Y, Grant BD. 2009. Regulation of endosomal clathrin and retromer-mediated endosome to Golgi retrograde transport by the J-domain protein RME-8. Embo J 28:3290–3302.
- Shinotsuka C, Waguri S, Wakasugi M, Uchiyama Y, Nakayama K. 2002. Dominant-negative mutant of BIG2, an ARF-guanine nucleotide exchange factor, specifically affects membrane trafficking from the trans-Golgi network through inhibiting membrane association of AP-1 and GGA coat proteins. Biochem Biophys Res Commun 294:254–260.
- Skanland SS, Walchli S, Brech A, Sandvig K. 2009. SNX4 in complex with clathrin and dynein: Implications for endosome movement. PLoS One 4:e5935.
- Small SA, Gandy S. 2006. Sorting through the cell biology of Alzheimer's disease: Intracellular pathways to pathogenesis. Neuron 52:15–131.
- Spang A, Shiba Y, Randazzo PA. 2010. Arf GAPs: Gatekeepers of vesicle generation. FEBS Lett.
- Stenmark H. 2009. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10:513–525.
- Takenawa T, Suetsugu S. 2007. The WASP-WAVE protein network: Connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol 8:37–48.
- Tarpey PS, Stevens C, Teague J, Edkins S, O'Meara, S, Avis T, Barthorpe S, Buck G, Butler A, Cole J, Dicks E, Gray K, Halliday K, Harrison R, Hills K, Hinton J, Jones D, Menzies A, Mironenko T, Perry J, Raine K, Richardson D, Shepherd R, Small A, Tofts C, Varian J, West S, Widaa S, Yates A, Catford R, Butler J, Mallya U, Moon J, Luo Y, Dorkins H, Thompson D, Easton DF, Wooster R, Bobrow M, Carpenter N, Simensen RJ, Schwartz CE, Stevenson RE, Turner G, Partington M, Gecz J, Stratton MR, Futreal PA, Raymond FL. 2006. Mutations in the gene encoding the Sigma 2 subunit of the adaptor protein 1 complex, AP1S2, cause X-linked mental retardation. Am J Hum Genet 79:1119–1124.
- Tarricone C, Xiao B, Justin N, Walker PA, Rittinger K, Gamblin SJ, Smerdon SJ. 2001. The structural basis of Arfaptin-mediated cross-talk between Rac and Arf signalling pathways. Nature 411:215–219.
- Uruno T, Liu J, Zhang P, Fan Y, Egile C, Li R, Mueller SC, Zhan X. 2001. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat Cell Biol 3:259–266.
- Vaccari T, Bilder D. 2005. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev Cell 9:687–698.
- Vicinanza M, D'Angelo G, Di Campli A, De Matteis MA. 2008. Function and dysfunction of the PI system in membrane trafficking. Embo J 27:2457–2470.
- Vowels JJ, Payne GS. 1998. A dileucine-like sorting signal directs transport into an AP-3-dependent, clathrin-independent pathway to the yeast vacuole. Embo J 17:2482–2493.
- Waguri S, Dewitte F, Le Borgne R, Rouille Y, Uchiyama Y, Dubremetz JF, Hoflack B. 2003. Visualization of TGN to endosome trafficking through fluorescently labeled MPR and AP-1 in living cells. Mol Biol Cell 14:142–155.
- Wang B, Wylie FG, Teasdale RD, Stow JL. 2005. Polarized trafficking of E-cadherin is regulated by Rac1 and Cdc42 in Madin-Darby canine kidney cells. Am J Physiol Cell Physiol 288:C1411–1419.
- Wang J, Sun HQ, Macia E, Kirchhausen T, Watson H, Bonifacino JS, Yin HL. 2007. PI4P promotes the recruitment of the GGA adaptor proteins to the trans-Golgi network and regulates their recognition of the ubiquitin sorting signal. Mol Biol Cell 18:2646–2655.
- Wang W, Struhl G. 2004. Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development 131:5367–5380.
- Wang YJ, Wang J, Sun HQ, Martinez M, Sun YX, Macia E, Kirchhausen T, Albanesi JP, Roth MG, Yin HL. 2003. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell 114:299–310.
- Wassmer T, Attar N, Bujny MV, Oakley J, Traer CJ, Cullen PJ. 2007. A loss-of-function screen reveals SNX5 and SNX6 as potential components of the mammalian retromer. J Cell Sci 120:45–54.
- Wassmer T, Attar N, Harterink M, Van Weering JR, Traer CJ, Oakley J, Goud B, Stephens DJ, Verkade P, Korswagen HC, Cullen PJ. 2009. The retromer coat complex coordinates endosomal sorting and dynein-mediated transport, with carrier recognition by the trans-Golgi network. Dev Cell 17:110–122.
- Weaver AM, Heuser JE, Karginov AV, Lee WL, Parsons JT, Cooper JA. 2002. Interaction of cortactin and N-WASp with Arp2/3 complex. Curr Biol 12:1270–1278.
- Yang PT, Lorenowicz MJ, Silhankova M, Coudreuse DY, Betist MC, Korswagen HC. 2008. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell 14:140–147.
- Zhao ZS, Manser E, Loo TH, Lim L. 2000. Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol Cell Biol 20:6354–6363.
- Zizioli D, Meyer C, Guhde G, Saftig P, Von Figura K, Schu P. 1999. Early embryonic death of mice deficient in gamma-adaptin. J Biol Chem 274:5385–5390.