Abstract
Lipid droplets are discrete organelles present in most cell types and organisms including bacteria, yeast, plants, insects and animals. Long considered as passive storage deposits, recent cell biology, proteomic and lipidomic analysis show that lipid droplets are dynamic organelles involved in multiple cellular functions. They have a central function in lipid distribution to different membrane-bound organelles and serve not only as main reservoirs of neutral lipids such as triglycerides and cholesterol but in addition, contain structural proteins, proteins involved in lipid synthesis and transmembrane proteins. A detailed model for how transmembrane proteins such as SNARE proteins can exist in lipid droplets is proposed.
Keywords::
Introduction
The lipid droplet is considered an ‘up and coming’ organelle with new properties ascribed to it repeatedly (Murphy Citation2001). These include lipid conversion, storage and mobilization, lipid synthesis, membrane protein trafficking and fusion as well as temporary storage of excess proteins and vitamin A (e.g., in hepatic stellate cells) (Athenstaedt et al. Citation1999, Tauchi-Sato et al. Citation2002, Brasaemle et al. Citation2004, Fujimoto et al. Citation2004, Liu et al. Citation2004, Cermelli et al. Citation2006, Katavic et al. Citation2006, Turro et al. Citation2006, Bostrom et al. Citation2007, Bartz et al. Citation2007a, Blaner et al. Citation2009, Zehmer et al. Citation2009). Although it is clear that lipid droplets are adaptable as well as central organelles, we know very little of their origin, i.e., how they form. There is also very little mechanistic insight into how lipid droplets contribute to disease. Ectopic lipid accumulation in liver and skeletal muscle is thought to impact the overall metabolism of the body with causal links to both diabetes mellitus 2 and atherosclerosis. In fatty liver disease, lipid droplets accumulate in hepatic cells and at a certain point, cause cell and tissue damage affecting, for example, the liver's capacity to adsorb insulin or fidelity to secrete intact lipoprotein particles (e.g., VLDL and LDL) potentially contributing to insulin insensitivity/diabetes mellitus 2 and atherosclerosis, respectively. By understanding the biogenesis of lipid droplets under normal and disease conditions, we will gain necessary insights into their nature and role in disease. Below, we summarize what is known about their formation and make suggestions for how new technology, proteomics, should help to fast track the biology of lipid droplets.
Biogenesis of lipid droplets – current models
Most organelles have as their demarcation at least one lipid bilayer surrounding one or more aqueous compartments. For example, the Golgi apparatus consists of multiple Golgi stacks with each stack made up of multiple cisternae, each functionally separated from the cytosol by a phospholipid bilayer surrounding its lumen. A COPI and COPII vesicle budding from a Golgi or ER cisterna, respectively, retains this topology having an inner luminal lipid leaflet and an outward cytosolic leaflet. In contrast, lipid droplets have only a single leaflet (a phospholipid monolayer) setting it apart from other organelles (Tauchi-Sato et al. Citation2002). The best analogy to describe a lipid droplet is to envisage a drop of mineral oil immersed in water. The shape of such an oil drop is always spherical as this maximizes content relative to surface area (Ohsaki et al. Citation2009). The size and number of droplets varies between cell types and within the same cell type. Lipid droplets have the ability to grow and shrink and do so in a controlled manner. In part, this appears to be regulated by lipid droplet associated proteins such as FSP27 (Nishino et al. Citation2008) and SNARE proteins (Bostrom et al. Citation2007). Regulating droplet size through, for example, FSP27 provides effective means of controlling exchange of lipids as exchange necessitates binding of peripheral cytosolic enzymes; for example, halving the diameter of a droplet results in a doubling of the surface area over volume ratio allowing for a more effective exchange of lipids. Conversely, doubling the diameter doubles the volume over surface area ratio offering effective means of decreasing the exchange of lipids. In this context, ablation of FSP27 in adipocytes results in fewer large lipid droplets but increased number of smaller lipid droplets favoring lipolysis over storage (Nishino et al. Citation2008). Indeed, knockout mice deficient in FSP27 become resistant to diet-induced obesity as a consequence of increased rate of lipolysis/mitochondrial activity over triglyceride storage.
The mechanisms of lipid droplet size regulation by FSP27, SNARE proteins or other factors are not well understood nor do we understand how lipid droplets form. By light microscopy, it appears that lipid droplets can form de novo (Thiele and Spandl Citation2008). Induction of droplet formation can be observed in multiple cell types including rat hepatocytes after partial hepatectomy, 3T3-L1, COS7, HeLa, McA-RH7777 cultured under de-lipidated condition followed by addition of fatty acids (Turro et al. Citation2006, Kuerschner et al. Citation2008). Conversely, lipid droplets plus their associated proteins disappear under metabolic conditions that favor consumption of lipids (McGookey and Anderson Citation1983, Xu et al. Citation2005, Masuda et al. Citation2006, Xu et al. Citation2006). Lipid droplets are also gregarious in nature in that they associate with multiple membrane bound organelles such as mitochondria, peroxisomes, the Golgi apparatus and the ER (Goodman Citation2008, Zehmer et al. Citation2009). Therefore, they offer an ideal platform for exchange of material (e.g., fatty acids for mitochondrial beta oxidation through lipolysis of triglycerides on the lipid droplet). Though gregarious, the origin of lipid droplets is not clear.
The most commonly held theory is that lipid droplets form via the ER, as the endoplasmic reticulum is the site of most phospholipid synthesis, and lipid droplets are often seen associated with parts of its cisternal membranes (Novikoff et al. Citation1980). This theory is supported by proteomics studies that identify a number of ER proteins associated with the lipid droplets including enzymes for lipid synthesis (Murphy Citation2001, Brasaemle et al. Citation2004, Cermelli et al. Citation2006, Turro et al. Citation2006, Cho et al. Citation2007, Wan et al. Citation2007, Bartz et al. Citation2007b). Also, the composition of lipid droplets is similar (but not identical, see below) to the ER suggesting a close relationship (Tauchi-Sato et al. Citation2002, Bartz et al. Citation2007a). Mechanistically, it is thought that lipid droplet formation is preceded by an increased accumulation of neutral lipids inside the lipid bilayer of ER membranes. Presumably, this would occur in specialized regions of the ER (Murphy Citation2001). Through expansion, the bilayer is gradually pushed apart allowing for the formation of a droplet.
The next step, pinching off from the ER, is not obvious as the luminal and cytosolic leaflet now surrounding the droplet must join, somehow. To explain this, a modified model was proposed where lipid droplet formation starts by accumulation of neutral lipids between the two ER leaflets and at the time of release from the ER, there is a formation of a transient bicelle (an oval shaped micelle with a planar region composed of long-chain phospholipid, surrounded by a rim of short-chain phospholipid) from both the luminal and the cytoplasmic leaflets of the ER that leaves a transient hole in the ER. This hole then seals such that the lipid droplet is now encircled by a phospholipid monolayer and additional ER bilayer parts that can accommodate membrane proteins (Ploegh Citation2007).
Alternative models predict that the lipid droplet remains an integral part of the ER, i.e., never pinches off (Goodman Citation2008). Lipid droplets would then be specialized regions of the ER that expand and shrink depending on the needs of the cell or the organism. A detailed version of this model predicts that lipid droplets bud out from the ER but remain physically attached through a continuous ER cytosolic leaflet (Zehmer et al. Citation2009). This ‘stalk’ model would allow peripheral and embedded proteins to move freely from the ER to the lipid droplet. Transmembrane proteins, on the other hand, would be prevented to enter such a structure. An ultra structural study supports this model showing continuity between the monolayer of the lipid droplet and the ER (Blanchette-Mackie et al. Citation1995). Lipidomics of the phospholipid composition of lipid droplets, however, suggest that it is somewhat dissimilar from that of the ER (Tauchi-Sato et al. Citation2002).
An alternative model builds on the interesting finding that caveolin-1, a cholesterol binding protein, is present in lipid droplets (Schlegel and Lisanti Citation2001, van Meer Citation2001). It has been assumed that caveolin-1 is transferred from the ER to the lipid droplet once the droplet has formed but it is equally plausible that caveolin-1 and other proteins are synthesized directly on the droplet (Robenek et al. Citation2004); proteomics and ultrastructural studies of lipid droplets find not only ER proteins but also RNA, ribosomes and extensive ER-like membranes within the lipid droplet (Dvorak et al. Citation2003). This may bear some resemblance to autophagosome formation (authors' note) but has led to the proposal that loops of ER integrate inside the emerging lipid droplet such that this enables local protein synthesis (Wan et al. Citation2007, Ohsaki et al. Citation2009). Comprehensive descriptions and illustrations of above models have been published (Ohsaki et al. Citation2009, Digel et al. Citation2010).
Our main problem with the above models is that it is difficult to comprehend how neutral lipids can be synthesized such that their presence pushes the bilayer of the ER apart, locally. The ER is a continuous organelle that not only envelops the nuclear content of the cell but also, pervades the entire cytosol. Although somewhat restricted through interactions with other components, membrane and luminal ER proteins are capable of diffusing throughout this vast membrane system (Presley et al. Citation1997, Storrie et al. Citation1998, Siggia et al. Citation2000). A neutral lipid, diffusing orders of magnitude faster than an average membrane protein would be expected to equilibrate rapidly. For local growth to occur such that this pushes apart the two leaflets of a part of an ER membrane neutral lipids would have to be restricted locally (e.g., lipid rafts) or their rate of synthesis is such that it overcomes lateral diffusion. Mitigating conditions might be envisaged where regions of ER membranes are locally destabilized through phospholipid synthesis such that transmembrane proteins are locally unfavoured and lipid leaflet wobbling is increased. All this is possible but in our opinion, requires firmer theoretical and experimental foundations.
Biogenesis of lipid droplets through vesicles
A drastically different model would be to first bud off a transport vesicle from the ER (Walther and Farese Citation2009) or other membrane systems (e.g., the Golgi). This will effectively prevent lateral diffusion of lipids. Walther and Farese suggest in their model a close interaction with the ER such that neutral lipids can be transferred from the ER to the vesicle allowing it to expand (its outer leaflet). Indeed, this is consistent with the cup-like incubator structures observed by EM where lipid droplets grow surrounded by ER membranes, presumably to enable transfer of material from and to the lipid droplet (Robenek et al. Citation2006, Citation2009).
Having a lipid droplet form through vesicle formation is an attractive model as this enables topology to be preserved whilst using existing vesicle coat proteins for vesicle formation. What would then be required to form a lipid droplet if starting from a transport vesicle such as a COPI or COPII vesicle? We know that the latter buds from ER membranes transporting proteins from the ER to the Golgi whilst the former transport resident proteins of the ER and the Golgi in the opposite (retrograde) direction returning proteins from distal compartment. In , we have outlined the birth of a lipid droplet using a COPI vesicle that has formed from the Golgi apparatus via the COPI coat machinery, then associates with the ER membrane and grows. We have here assumed that lipid synthesizing and modifying enzymes are present on the vesicle or delivered to the COPI vesicle through fusion with other transport vesicles (e.g., COPI and COPII vesicles).
Figure 1. Biogenesis of lipid droplet through COPI vesicles. In I, ER and Golgi trafficking through COPI and COPII vesicles. COPII vesicles bud from the ER and transport proteins and lipids from the ER to the Golgi. COPI vesicles mediate recycling of resident proteins from the Golgi back to the ER and from distal to proximal cisternae within the stack. In the proposed model, lipid droplets form in close association with ER membrane seeded from a COPI vesicle. As SNARE proteins and other trafficking machinery proteins will be present in the droplet, COPI and COPII can fuse with the growing droplet. Association with the ER persists throughout the life of the lipid droplet facilitating exchange of lipids and proteins. In II, COPI-derived lipid droplets form from Golgi membrane using the COPI coat machinery, and subsequently translocate to the ER. The ER holds the newly emerged lipid droplet in a cup-like incubator and feeding the droplet. In III, two lipid droplets fuse via the respective regions that provide opposing bilayers incorporating required transmembrane SNARE proteins such as Syntaxin 5.
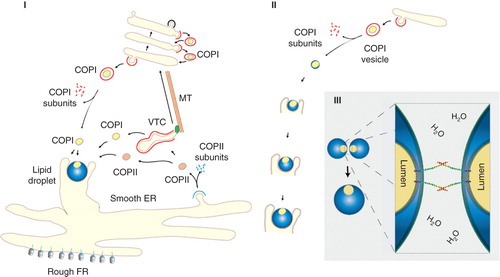
This model allows transmembrane proteins to exist in lipid droplets as true membrane spanning proteins as the lumen of the COPI vesicle and those of other vesicles fusing with the droplet is topologically preserved. The vesicle lumen (protected by the original luminal leaflet) would in a growing lipid droplet become minuscule (60 nm in diameter) by comparison to the size of the droplet (several micrometers). Nevertheless, the preserved bilayer part serves as ideal areas to incorporate, for example, SNARE proteins to enable fusion with other droplets, vesicles or membranes in a controlled manner ().
So is there support for COPI and COPII vesicles as potential seeding structures for lipid droplet formation? Indeed, recent studies show that both COPI and COPII components control the storage of neutral lipid in lipid droplets (Beller et al. Citation2008, Guo et al. Citation2008, Soni et al. Citation2009). Some lipid droplet-associated proteins are transported to the lipid droplet using components of ER to Golgi transportation machinery, including the small GTP-binding protein ARF1, its guanine-nucleotide exchange factor GBF1 and coatomer, a 7 subunit coat complex of COPI vesicles (Beller et al. Citation2008, Soni et al. Citation2009). Yeast two-hybrid screening has also identified ARF1 as a binding partner of the lipid droplet-associated protein ADRP (Nakamura et al. Citation2004) and its presence is thought to contribute to lipid droplet formation (Nakamura et al. Citation2005). Addition of the fungal metabolite, Brefeldin A, to inhibit COPI coat recruitment to membranes through GBF1, also suppresses lipid droplet formation (Nakamura et al. Citation2005) and knockdown of COPI vesicular transport components by RNA interference abrogates lipid droplet formation in Drosophila melanogaster (Guo et al. Citation2008). Finally, ARF1, ARFGAP1 and COPI have been identified through proteomics analysis on lipid droplets purified from CHO K2 cells (Bartz et al. Citation2007a). In fact, multiple trafficking proteins that operate in the ER-Golgi interface have been identified in a similar manner using proteomics (Brasaemle et al. Citation2004, Cermelli et al. Citation2006, Turro et al. Citation2006, Cho et al. Citation2007, Wan et al. Citation2007, Bartz et al. Citation2007b, Hodges and Wu Citation2010). Direct or indirect contributions of COPI and COPII components or trafficking proteins in lipid droplet biogenesis is, however, difficult to disentangle as COPI components and related trafficking proteins require COPII components to leave the ER. Likewise, COPI components are required to return trafficking proteins back to the ER through COPI-mediated recycling from the Golgi apparatus. To illustrate, a sterol dehydrogenase, NSDHL, is synthesized in the ER but requires transport to the Golgi apparatus and recycling via COPI vesicles through its K(X)KXX-like cytoplasmic C-terminal motif before it is incorporated into lipid droplets (Caldas and Herman Citation2003). Is this because NSDHL has to travel back to the ER where lipid droplets form or is it because NSDHL needs to travel to the lipid droplet via COPI vesicles or because the COPI vesicle containing NSDHL along with other components directly seed lipid droplets? Disentangling observed dependencies on COPI and COPII components and trafficking proteins is therefore difficult but if COPI or COPII vesicles seed a lipid droplet, then as a hypothesis, this is testable. For example, is it possible to grow a lipid droplet from isolated COPII or COPI vesicles, in vitro providing only cytosol and free fatty acids?
Lipid droplets in disease
Understanding the biogenesis of lipid droplets, how they form and function, automatically allows us to understand the cell biology of disease (Farese and Walther Citation2009). Of the many human diseases and disease states that exist today, the metabolic syndrome tops the list both in terms of mortality, shortened lifespan and decreased quality of life (Love and Oldford Citation2005). With an ageing population, the prediction is that the metabolic syndrome will outdo all other diseases and disease states combined. Included in the International Diabetes Federation definition is overweight/obesity over a certain BMI (>30 kg/m2) in combination with two the following: Raised triglycerides, reduced HDL cholesterol, raised blood pressure and/or raised fasting plasma glucose. What this definition does not explain is that the metabolic syndrome is often associated with an aggregation of co-morbidities including atherosclerosis, insulin resistance, type 2 diabetes mellitus, dyslipidemias, coagulation disorders, hypertension, and a pro-inflammatory state. More than 25% of all humans suffer from one or more of these states. Central to the metabolic syndrome is the effect of lipid storage in lipid droplets in cells such as adipocytes, hepatocytes and skeletal muscle (Le Lay and Dugail Citation2009). The white adipose tissue is thought of as being a specialized tissue for lipid storage whereas ectopic lipid storage in other tissues, primarily liver and skeletal muscle, is deemed potentially detrimental to that tissue. This is particularly true for the liver where lipid accumulation results in fatty liver (Lewis and Mohanty Citation2010). If unchecked, this develops into non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH) followed by fibrosis and cirrhosis. Apart from its many other functions (e.g., detoxification, gluconeogenesis, glycogenesis and ketone body formation) the liver is central for lipid trafficking in the body receiving digested lipids in the form of lipoproteins, repackaging them and then shipping them off to adipose tissues for storage. As noted above, fidelity and function is increasingly thought of as being potentially compromised during hepatic lipid accumulation such that it affects, for example, lipoprotein assembly that in turn, contribute to development of atherosclerosis. Likewise, increased levels of hepatic triglycerides may in turn adversely affect the function of pancreatic beta cells leading to pre-diabetes and diabetes.
The effect of lipid droplet formation in liver cells is, however, poorly understood but owing to its distinct nature, it is an ideal cellular structure to, visualize, isolate and characterize. Visualization is usually through Nile Red, Bodipy, direct/indirect immunofluorescence or through expression of a lipid droplet binding protein fused to a fluorescent protein (e.g., EGFP). Isolation is usually through sucrose gradient fractionation where lipid droplets accumulate on the top of the gradient. After a few rounds of centrifugation, lipids can be extracted and proteins precipitated using acetone. Proteins are then ready for analysis by Western blotting or proteomics and lipids by thin layer chromatography or mass spectrometry (MS). We favour MS for both protein and lipid analysis as this provides a more comprehensive snapshot of the structure analyzed compared to any other technique. When chartering disease progression such as NAFLD and NASH, it is here possible to compare lipid droplets quantitatively in a fully encompassing manner. To date, there are more than 200 mammalian proteins ascribed to the lipid droplet proteome. Of these, several proteins such as perilipin/ADRP/TIP47 are observed consistently whereas may others are seen occasionally (e.g., GBF1, ARF1, rab proteins) (Hodges and Wu Citation2010). Such variation can be due to inherent challenges with proteomics (Nilsson et al. Citation2010), cell/tissue type and/or the metabolic state of the cell/tissue type analyzed. Already, proteomics with other techniques show that lipid droplets play important roles in energy balance, lipid homeostasis, cell signaling, and vesicle trafficking. As an example, the PAT (perilipin/ADRP/TIP47) proteins of lipid droplets interact with lipases controlling the release of triglycerides as well as enzymes involved in conversion of lipids into triglycerides for storage. Mutations in PAT proteins lead to imbalance of neutral lipid homeostasis causing various lipid metabolic diseases (Meex et al. Citation2009). Studies on the PAT proteins in respect to disease will continue but as the proteome (and lipidome) of lipid droplets become more defined and insights into how they form and interact with other parts of the cell, so will also links to disease.
Acknowledgements
We wish to acknowledge Canadian Institute of Health Research, Canada Research Chairs and Canada Foundation for Innovation for their support.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Athenstaedt K, Zweytick D, Jandrositz A, Kohlwein SD, Daum G. 1999. Identification and characterization of major lipid particle proteins of the yeast Saccharomyces cerevisiae. J Bacteriol 181:6441–6448.
- Bartz R, Li WH, Venables B, Zehmer JK, Roth MR, Welti R, Anderson RG, Liu P, Chapman KD. 2007a. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J Lipid Res 48:837–847.
- Bartz R, Zehmer JK, Zhu M, Chen Y, Serrero G, Zhao Y, Liu P. 2007b. Dynamic activity of lipid droplets: Protein phosphorylation and GTP-mediated protein translocation. J Proteome Res 6:3256–3265.
- Beller M, Sztalryd C, Southall N, Bell M, Jackle H, Auld DS, Oliver B. 2008. COPI complex is a regulator of lipid homeostasis. PLoS Biol 6:e292.
- Blanchette-Mackie EJ, Dwyer NK, Barber T, Coxey RA, Takeda T, Rondinone CM, Theodorakis JL, Greenberg AS, Londos C. 1995. Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J Lipid Res 36:1211–1226.
- Blaner WS, O'Byrne SM, Wongsiriroj N, Kluwe J, D'Ambrosio DM, Jiang H, Schwabe RF, Hillman EM, Piantedosi R, Libien J. 2009. Hepatic stellate cell lipid droplets: A specialized lipid droplet for retinoid storage. Biochim Biophys Acta 1791:467–473.
- Bostrom P, Andersson L, Rutberg M, Perman J, Lidberg U, Johansson BR, Fernandez-Rodriguez J, Ericson J, Nilsson T, Boren J, Olofsson SO. 2007. SNARE proteins mediate fusion between cytosolic lipid droplets and are implicated in insulin sensitivity. Nat Cell Biol 9:1286–1293.
- Brasaemle DL, Dolios G, Shapiro L, Wang R. 2004. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem 279:46835–46842.
- Caldas H, Herman GE. 2003. NSDHL, an enzyme involved in cholesterol biosynthesis, traffics through the Golgi and accumulates on ER membranes and on the surface of lipid droplets. Human Molec Gen 12:2981–2991.
- Cermelli S, Guo Y, Gross SP, Welte MA. 2006. The lipid-droplet proteome reveals that droplets are a protein-storage depot. Curr Biol 16:1783–1795.
- Cho SY, Shin ES, Park PJ, Shin DW, Chang HK, Kim D, Lee HH, Lee JH, Kim SH, Song MJ, Chang IS, Lee OS, Lee TR. 2007. Identification of mouse Prp19p as a lipid droplet-associated protein and its possible involvement in the biogenesis of lipid droplets. J Biol Chem 282:2456–2465.
- Digel M, Ehehalt R, Fullekrug J. 2010. Lipid droplets lighting up: Insights from live microscopy. FEBS Lett 584:2168–2175.
- Dvorak AM, Morgan ES, Weller PF. 2003. RNA is closely associated with human mast cell lipid bodies. Histol Histopathol 18:943–968.
- Farese RV Jr, Walther TC. 2009. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 139:855–860.
- Fujimoto Y, Itabe H, Sakai J, Makita M, Noda J, Mori M, Higashi Y, Kojima S, Takano T. 2004. Identification of major proteins in the lipid droplet-enriched fraction isolated from the human hepatocyte cell line HuH7. Biochim Biophys Acta 1644:47–59.
- Goodman JM. 2008. The gregarious lipid droplet. J Biol Chem 283:28005–28009.
- Guo Y, Walther TC, Rao M, Stuurman N, Goshima G, Terayama K, Wong JS, Vale RD, Walter P, Farese RV. 2008. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature 453:657–661.
- Hodges BD, Wu CC. 2010. Proteomic insights into an expanded cellular role for cytoplasmic lipid droplets. J Lipid Res 51:262–273.
- Katavic V, Agrawal GK, Hajduch M, Harris SL, Thelen JJ. 2006. Protein and lipid composition analysis of oil bodies from two Brassica napus cultivars. Proteomics 6:4586–4598.
- Kuerschner L, Moessinger C, Thiele C. 2008. Imaging of lipid biosynthesis: How a neutral lipid enters lipid droplets. Traffic 9:338–352.
- Le Lay S, Dugail I. 2009. Connecting lipid droplet biology and the metabolic syndrome. Progress Lipid Res 48:191–195.
- Lewis JR, Mohanty SR. 2010. Nonalcoholic fatty liver disease: A review and update. Digestive Diseases Sci 55:560–578.
- Liu P, Ying Y, Zhao Y, Mundy DI, Zhu M, Anderson RG. 2004. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J Biol Chem 279:3787–3792.
- Love A, Oldford D. 2005. Metabolic syndrome. Canadian J Cardiovasc Nursing 15:6–8; quiz 9.
- Masuda Y, Itabe H, Odaki M, Hama K, Fujimoto Y, Mori M, Sasabe N, Aoki J, Arai H, Takano T. 2006. ADRP/adipophilin is degraded through the proteasome-dependent pathway during regression of lipid-storing cells. J Lipid Res 47:87–98.
- McGookey DJ, Anderson RG. 1983. Morphological characterization of the cholesteryl ester cycle in cultured mouse macrophage foam cells. J Cell Biol 97:1156–1168.
- Meex RC, Schrauwen P, Hesselink MK. 2009. Modulation of myocellular fat stores: Lipid droplet dynamics in health and disease. Am J Physiol Regul Integr Comp Physiol 297:R913–924.
- Murphy DJ. 2001. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res 40:325–438.
- Nakamura N, Akashi T, Taneda T, Kogo H, Kikuchi A, Fujimoto T. 2004. ADRP is dissociated from lipid droplets by ARF1-dependent mechanism. Biochem Biophys Res Commun 322:957–965.
- Nakamura N, Banno Y, Tamiya-Koizumi K. 2005. Arf1-dependent PLD1 is localized to oleic acid-induced lipid droplets in NIH3T3 cells. Biochem Biophys Res Commun 335:117–123.
- Nilsson T, Mann M, Aebersold R, Yates JR 3rd, Bairoch A, Bergeron JJ. 2010. Mass spectrometry in high-throughput proteomics: Ready for the big time. Nat Methods 7:681–685.
- Nishino N, Tamori Y, Tateya S, Kawaguchi T, Shibakusa T, Mizunoya W, Inoue K, Kitazawa R, Kitazawa S, Matsuki Y, Hiramatsu R, Masubuchi S, Omachi A, Kimura K, Saito M, Amo T, Ohta S, Yamaguchi T, Osumi T, Cheng J, Fujimoto T, Nakao H, Nakao K, Aiba A, Okamura H, Fushiki T, Kasuga M. 2008. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J Clin Invest 118:2808–2821.
- Novikoff AB, Novikoff PM, Rosen OM, Rubin CS. 1980. Organelle relationships in cultured 3T3-L1 preadipocytes. J Cell Biol 87:180–196.
- Ohsaki Y, Cheng J, Suzuki M, Shinohara Y, Fujita A, Fujimoto T. 2009. Biogenesis of cytoplasmic lipid droplets: From the lipid ester globule in the membrane to the visible structure. Biochim Biophys Acta 1791:399–407.
- Ploegh HL. 2007. A lipid-based model for the creation of an escape hatch from the endoplasmic reticulum. Nature 448:435–438.
- Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJ, Lippincott-Schwartz J. 1997. ER-to-Golgi transport visualized in living cells. Nature 389:81–85.
- Robenek H, Buers I, Hofnagel O, Robenek MJ, Troyer D, Severs NJ. 2009. Compartmentalization of proteins in lipid droplet biogenesis. Biochim Biophys Acta 1791:408–418.
- Robenek H, Hofnagel O, Buers I, Robenek MJ, Troyer D, Severs NJ. 2006. Adipophilin-enriched domains in the ER membrane are sites of lipid droplet biogenesis. J Cell Sci 119:4215–4224.
- Robenek MJ, Severs NJ, Schlattmann K, Plenz G, Zimmer KP, Troyer D, Robenek H. 2004. Lipids partition caveolin-1 from ER membranes into lipid droplets: Updating the model of lipid droplet biogenesis. Faseb J 18:866–868.
- Schlegel A, Lisanti MP. 2001. The caveolin triad: Caveolae biogenesis, cholesterol trafficking, and signal transduction. Cytokine Growth Factor Rev 12:41–51.
- Siggia ED, Lippincott-Schwartz J, Bekiranov S. 2000. Diffusion in inhomogeneous media: Theory and simulations applied to whole cell photobleach recovery. Biophys J 79:1761–1770.
- Soni KG, Mardones GA, Sougrat R, Smirnova E, Jackson CL, Bonifacino JS. 2009. Coatomer-dependent protein delivery to lipid droplets. J Cell Sci 122:1834–1841.
- Storrie B, White J, Rottger S, Stelzer EH, Suganuma T, Nilsson T. 1998. Recycling of golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J Cell Biol 143:1505–1521.
- Tauchi-Sato K, Ozeki S, Houjou T, Taguchi R, Fujimoto T. 2002. The surface of lipid droplets is a phospholipid monolayer with a unique Fatty Acid composition. J Biol Chem 277:44507–44512.
- Thiele C, Spandl J. 2008. Cell biology of lipid droplets. Curr Opin Cell Biol 20:378–385.
- Turro S, Ingelmo-Torres M, Estanyol JM, Tebar F, Fernandez MA, Albor CV, Gaus K, Grewal T, Enrich C, Pol A. 2006. Identification and characterization of associated with lipid droplet protein 1: A novel membrane-associated protein that resides on hepatic lipid droplets. Traffic 7:1254–1269.
- Van Meer G. 2001. Caveolin, cholesterol, and lipid droplets? J Cell Biol 152:F29–34.
- Walther TC, Farese RV Jr. 2009. The life of lipid droplets. Biochim Biophys Acta 1791:459–466.
- Wan HC, Melo RC, Jin Z, Dvorak AM, Weller PF. 2007. Roles and origins of leukocyte lipid bodies: Proteomic and ultrastructural studies. Faseb J 21:167–178.
- Xu G, Sztalryd C, Londos C. 2006. Degradation of perilipin is mediated through ubiquitination-proteasome pathway. Biochim Biophys Acta 1761:83–90.
- Xu G, Sztalryd C, Lu X, Tansey JT, Gan J, Dorward H, Kimmel AR, Londos C. 2005. Post-translational regulation of adipose differentiation-related protein by the ubiquitin/proteasome pathway. J Biol Chem 280:42841–42847.
- Zehmer JK, Huang Y, Peng G, Pu J, Anderson RG, Liu P. 2009. A role for lipid droplets in inter-membrane lipid traffic. Proteomics 9:914–921.