Abstract
The glucose transporter isoform 1 (GLUT1) is a key rate-limiting factor in the transport and metabolism of glucose in cancer cells. Recently, we found that GLUT1 expression is increased in hepatocellular carcinoma (HCC) and promotes tumorigenicity of HCC cells. Hypoxia further increased GLUT1 expression in HCC cells, and this induction was dependent on the activation of the transcription factor hypoxia-inducible factor (HIF)-1alpha. The promoter region of the GLUT1 gene harbors a single nucleotide polymorphism (SNP; Rs710218; A to T at -2841) closely positioned to a putative HIF-1alpha binding site, and recently, this SNP was found to be more frequent in patients with renal cell carcinoma. In the present study, the A-2841T genotype distribution did not differ significantly between HCC patients (n = 95; AA: 60%; AT 36% and TT: 4%) and healthy controls (n = 127; AA: 50%; AT 41% and TT: 9%). However and noteworthy, non-carriers of the T allele had higher GLUT1 expression levels in cancerous hepatic tissue, and tended to reveal a more aggressive tumour growth. These data indicate that the SNP Rs710218 is not associated with a higher risk for HCC but rather for HCC progression, potentially via HIF-1alpha mediated increased GLUT1 expression.
Keywords::
Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent tumour types, and both the incidence and mortality rates of HCC have increased in recent years (Jemal et al. Citation2008). The main risk factor for HCC development is liver cirrhosis, which develops as a result of chronic liver diseases leading to continuous hepatic inflammation and fibrogenesis. Despite surgical or loco-regional therapies, prognosis remains poor because of high tumour recurrence or tumour progression, and currently there are no well-established effective adjuvant therapies (Bruix et al. Citation2004, Farazi and DePinho Citation2006).
The glucose transporter isoform 1 (GLUT1, also known as SLC2A1; MIM#138140) is a key rate-limiting factor for transport and metabolism of glucose in cancer cells. GLUT1 expression is largely undetectable in normal epithelial tissues and benign epithelial tumours. However, GLUT1 is over expressed in a significant proportion of human carcinomas (Amann and Hellerbrand Citation2009). The apparent upregulation of a certain type of glucose transporter suggests that it plays an important role in tumour biology. Thus, it has been hypothesized that elevated GLUT1 expression by human carcinomas indicates an increased metabolic state, enhanced utilization of energy, and an associated increase in aggressive, metastatic behaviour. Actually, GLUT1 protein expression confers poor prognosis in a wide range of solid tumours (Cooper et al. Citation2003; Oliver et al. Citation2004).
We have recently shown that GLUT1 expression is increased in HCC, and noteworthy, high GLUT1 expression in HCC was associated with advanced tumour stages and poor differentiation. Hypoxia further increased GLUT1 expression in HCC cells, and this induction was dependent on the activation of the transcription factor hypoxia-inducible factor (HIF)-1alpha (Amann et al. Citation2009).
A recent study reported that a single nucleotide polymorphism (SNP) of the GLUT1 gene (Rs710218) was significantly more frequent in patients with clear-cell renal cell carcinoma than in a control population (Page et al. Citation2005). This SNP is localized in the promoter region at position 2841 bp upstream of the start of exon 1, consists of an A to T substitution and is closely positioned to a number of putative binding sites for transcription factors, including HIF-1alpha. Therefore, the aim of this study was to analyze the A-2841 SNP of the GLUT1 gene in patients with HCC.
Materials and methods
Patients and controls
GLUT1 genotype was studied retrospectively in 95 patients with hepatocellular carcinoma (HCC) (83 males and 12 females; mean age 60.7 years). Patients had been treated at the University Hospital of Regensburg, Germany, during the period 1996–2007. Inclusion criteria were histologically proven diagnosis of HCC and availability of genomic DNA for genotyping. Clinicopathological patient characteristics are summarized in . The control group included 127 healthy individuals. Patients and controls were Caucasians from Southern Germany.
Table I. -2841 GLUT1 haplotypes in HCC-tissue of 95 patients in relation to clinicopathological characteristics.
Human tissues and HCC tissue microarray (TMA)
HCC tissues and corresponding non-neoplastic liver tissues were obtained from HCC patients undergoing surgical resection at the University Hospital Regensburg (n = 55). A tissue microarray was constructed as described (Hellerbrand et al. Citation2008). Further, tissue samples of 24 patients were immediately snap frozen, stored at −80°C, and subsequently used for RNA isolation and analysis of mRNA expression. Informed consent was obtained from all patients and the study was approved by the local ethics committee.
Restriction fragment length polymorphism (RFLP) analysis
DNA isolation and GLUT1 genotyping were performed as described previously (Muhlbauer et al. Citation2003, Hodgkinson et al. Citation2005). Briefly, genomic DNA specimens were prepared from 200 μl blood or tissue using the QIAamp blood kit following the manufacturer's instructions (Qiagen, Hilden, Germany).
The A-2841T polymorphism, a thymine (T) to adenine (A) change was analyzed by performing PCR and subsequent restriction fragment length polymorphism (RFLP) analysis. PCR was performed under standard conditions (35 cycles, annealing temperature: 55°C) in a total reaction volume of 25 μl containing 2 μl of diluted genomic DNA, using the Taq PCR Master Kit (Qiagen), and the following pair of primers: for: 5′- GCT GAG AAT GGC CTT CCC TCA AT-3′ and rev: 5′- GTC TGC CTT ACT CAG CCC ATG GGT C-3′ (Sigma, Deisenhofen, Germany). PCR products were digested by HpyCH4V (NEB, Frankfurt, Germany), and the resulting fragments were separated by electrophoresis in a 2% agarose gel and visualized by ethidium bromide staining. If the HpyCH4V site is present, two fragments of 130 and 207 bp were generated; if the HpyCH4V is not present, a fragment of 339 bp in size was generated. To exclude incomplete digestion, all samples with genotype TT were sequenced, and sequencing confirmed RFLP results (data not shown).
GLUT1 expression analysis
Isolation of total cellular RNA and analysis of GLUT1 mRNA expression by quantitative real-time PCR were performed as described (Amann et al. Citation2009).
GLUT1 immunohistochemistry
Immunohistochemical analysis of MIB1 and GLUT1 expression on the HCC-TMA was performed as described (Amann et al. Citation2009).
Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM) or, when indicated as absolute number and percentage. Genotype frequencies are reported with their group percentages. Contingency table analysis and two-sided Chi-square test were used for comparison of qualitative variables. A p value < 0.05 was considered statistically significant.
Results
Frequency of the -2841 GLUT1 polymorphism in HCC patients and controls
The frequencies of the different GLUT1 genotypes A/A homozygotes, A/T heterozygotes, and T/T homozygotes are summarized in , revealing no significant differences between 95 patients with HCC and 127 healthy controls. Analysis of genomic DNA isolated from tumorous as well as non-tumorous hepatic tissue revealed identical results (data not shown). Frequencies of individual genotypes were similar to those previously reported in other control populations (Page et al. Citation2005). Together, these data indicate that the -2841 GLUT1 polymorphism has no impact on the development of HCC.
Table II. Frequency of the -2841 GLUT1 polymorphism in HCC patients and controls.
Because previous epidemiologic studies reported disease-related differences between -2841 GLUT1 genotypes AA and non-AA (i.e., AT or TT) (Hodgkinson et al. Citation2005), we continued this differentiation throughout the following analysis, and focused on the comparison of HCC patients with no T allele (AA homozygotes) and carriers of the T allele (genotypes AT or TT).
Correlation of -2841 GLUT1 haplotypes and GLUT1 expression in HCC
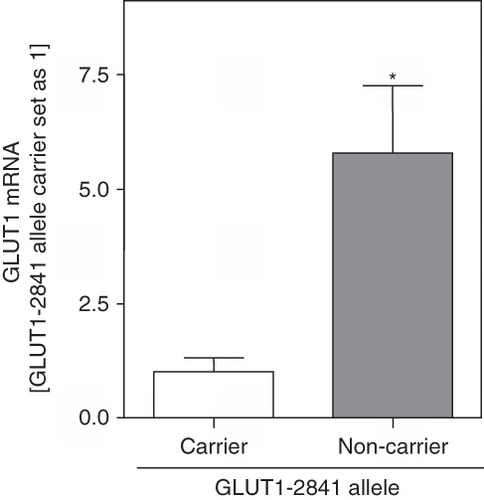
Since the -2841 GLUT1 polymorphism is located close to a HIF1alpha binding site, and we have recently shown that this transcription factor regulates GLUT1 expression in HCC (Amann et al. Citation2009), we analyzed GLUT1 mRNA expression in HCC patients with different GLUT1 haplotypes (). Mean GLUT1 mRNA expression in HCC tissues was significantly higher in non-carriers of the T-2841 allele (n = 17; 5.78 ± 1.48) than in carriers of the T-2841 allele (n = 10; 1.00 ± 0.32).
Figure 1. GLUT1 mRNA expression in HCC with different -2841 GLUT1 haplotypes. GLUT1 mRNA expression was analyzed by quantitative PCR in HCC tissues of patients with (carriers; n = 10) and without (non-carriers; n = 14) the T allele of the -2841 GLUT1 polymorphism. Bars indicate mean ± SEM; (*: p < 0.05).
Next, we compared the frequency of GLUT1 haplotypes in 55 HCC patients with (n = 10) and without (n = 45) positive GLUT1 immunosignal as assessed by immunohistochemistry applying tissue microarray (TMA) technology (Amann et al. Citation2009). Noteworthy, non-carriers of the T allele revealed significantly more often a positive GLUT1 immunosignal (9/34) than carriers of the T allele (1/21; p = 0.043).
Matched data of mRNA expression and semiquantitative protein expression analyzed on the TMA were available from 24 HCC patients. GLUT1 mRNA expression was significantly higher in HCC cases with positive GLUT1 immunosignal (n = 7) compared to cases, where no GLUT1 was detectable (n = 17; 3.9 ± 1.1-fold; p = 0.0003). Together, these findings indicate that increased GLUT1 expression is accurately detected by immunohistochemistry, and that the -2841 GLUT1 polymorphism affects GLUT1 expression in HCC.
Correlation of -2841 GLUT1 haplotypes and clinicopathological characteristics of HCC patients
Next, we investigated the correlation between the GLUT1 polymorphism and clinicopathological characteristics of HCC patients, since we have previously shown that GLUT1 expression affects tumorigenicity (Amann et al. Citation2009). Interestingly, the -2841 GLUT1 genotype AA (e.g., non-carrier of the T allele) was more frequent in patients with higher tumour staging (28/57 (49.1%) vs. 13/38 (34.2%) staging >2), and higher tumour grading (9/50 (18.0%) vs. 3/31 (9.7%) grading >2), but these correlations did not reach statistical significance (). Furthermore, non-carriers of the T allele tended to have a higher Ki67 labeling index in HCC-tissue (18.1 ± 4.5 vs. 10.3 ± 3.6; p = 0.230, data not shown). Distribution of age, gender and tumour size were similar in carriers and non-carriers of the -2841 GLUT1 T allele.
Discussion
Malignant cells have accelerated metabolism, high glucose requirements, and increased glucose uptake, respectively. Transport of glucose across the plasma membrane of mammalian cells is the first rate-limiting step for glucose metabolism, and accelerated glucose metabolism in cancerous cells has been associated with increased expression of the glucose transporter isoform 1 (GLUT1) in a wide range of solid tumours including HCC (Kim and Dang Citation2006, Brahimi-Horn et al. Citation2007, Amann et al. Citation2009).
Interestingly, a recent study reported an increased frequency of a polymorphism of the GLUT1 gene (Rs710218) in patients with clear-cell renal cell carcinoma (Page et al. Citation2005), but we observed similar distribution of GLUT1 genotypes in HCC patients and healthy controls. This indicates that in contrast to the kidney, this genetic polymorphism has no impact on the risk of tumour development in the liver. However, we found that Rs710218 affects GLUT1 expression in HCC tissue. Even more importantly, this genetic polymorphism tends to be associated with a more aggressive HCC growth.
As a potential explanation for this association appears that Rs710218 is located in the promoter region of the GLUT1 gene (-2841) closely positioned to a hypoxic response element (HRE) as putative HIF-1alpha binding site. Recently, we found that GLUT1 expression is increased in HCC and promotes tumorigenicity. Furthermore, hypoxia increased GLUT1 expression in HCC cells, and this induction was dependent on the activation of the transcription factor hypoxia-inducible factor (HIF)-1alpha (Amann et al. Citation2009). However and certainly, further studies are required to confirm that the genetic A-2841T polymorphisms functionally affects HIF-1alpha binding, and herewith, GLUT1 expression in HCC.
Furthermore, our results have to be confirmed in larger cohorts of HCC patients and in different ethnical groups, respectively. However, at present our data indicate the intriguing possibility that Rs710218 may serve as a genetic risk marker for a more aggressive HCC growth, which would have important clinical implications.
Acknowledgements
We would like to thank Monika Artinger for excellent technical assistance.
Declaration of interest: This work was supported by grants from the German Research Association (DFG) and the Medical Faculty of the University of Regensburg (ReForM) to A.K.B. and C.H. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Amann T, Hellerbrand C. 2009. GLUT1 as a therapeutic target in hepatocellular carcinoma. Expert Opin Ther Targets 13(12):1411–1427.
- Amann T, Maegdefrau U, Hartmann A, Agaimy A, Marienhagen J, Weiss TS, Stoeltzing O, Warnecke C, Scholmerich J, Oefner PJ, Kreutz M, Bosserhoff AK, Hellerbrand C. 2009. GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. Am J Pathol 174(4):1544–1552.
- Brahimi-Horn MC, Chiche J, Pouyssegur J. 2007. Hypoxia signalling controls metabolic demand. Curr Opin Cell Biol 19(2):223–229.
- Bruix J, Boix L, Sala M, Llovet JM. 2004. Focus on hepatocellular carcinoma. Cancer Cell 5(3):215–219.
- Cooper R, Sarioglu S, Sokmen S, Fuzun M, Kupelioglu A, Valentine H, Gorken IB, Airley R, West C. 2003. Glucose transporter-1 (GLUT-1): A potential marker of prognosis in rectal carcinoma? Br J Cancer 89(5):870–876.
- Farazi PA, DePinho RA. 2006. Hepatocellular carcinoma pathogenesis: From genes to environment. Nat Rev Cancer 6(9):674–687.
- Hellerbrand C, Amann T, Schlegel J, Wild P, Bataille F, Spruss T, Hartmann A, Bosserhoff AK. 2008. The novel gene MIA2 acts as a tumour suppressor in hepatocellular carcinoma. Gut 57(2):243–251.
- Hodgkinson AD, Page T, Millward BA, Demaine AG. 2005. A novel polymorphism in the 5′ flanking region of the glucose transporter (GLUT1) gene is strongly associated with diabetic nephropathy in patients with Type 1 diabetes mellitus. J Diabetes Complic 19(2):65–69a.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. 2008. Cancer statistics, 2008. CA Cancer J Clin 58(2):71–96.
- Kim JW, Dang CV. 2006. Cancer's molecular sweet tooth and the Warburg effect. Cancer Res 66(18):8927–8930.
- Muhlbauer M, Bosserhoff AK, Hartmann A, Thasler WE, Weiss TS, Herfarth H, Lock G, Scholmerich J, Hellerbrand C. 2003. A novel MCP-1 gene polymorphism is associated with hepatic MCP-1 expression and severity of HCV-related liver disease. Gastroenterology 125(4):1085–1093.
- Oliver RJ, Woodwards RT, Sloan P, Thakker NS, Stratford IJ, Airley RE. 2004. Prognostic value of facilitative glucose transporter Glut-1 in oral squamous cell carcinomas treated by surgical resection; results of EORTC Translational Research Fund studies. Eur J Cancer 40(4):503–507.
- Page T, Hodgkinson AD, Ollerenshaw M, Hammonds JC, Demaine AG. 2005. Glucose transporter polymorphisms are associated with clear-cell renal carcinoma. Cancer Genet Cytogenet 163(2):151–155.