Abstract
A group of prenyltransferases produce linear lipids by catalyzing consecutive condensation reactions of farnesyl diphosphate (FPP) with specific numbers of isopentenyl diphosphate (IPP), a common building block of isoprenoid compounds. Depending on the stereochemistry of the double bonds formed during IPP condensation, these prenyltransferases are categorized as cis- and trans-types. Undecaprenyl diphosphate synthase (UPPS) that catalyzes chain elongation of FPP by consecutive condensation reactions with eight IPP, to form C55 lipid carrier for bacterial cell wall biosynthesis, serves as a model for understanding cis-prenyltransferases. In this review, the current knowledge in UPPS kinetics, mechanisms, structures, and inhibitors is summarized.
Introduction
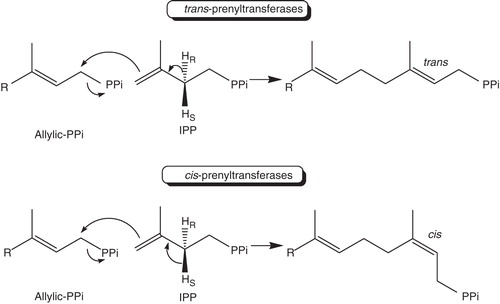
Isoprenoids are natural products derived from 5-carbon isopentenyl diphosphate (IPP) as the building block (Bloch et al. Citation1959). In addition to the mevalonate pathway for IPP biosynthesis, IPP can be also synthesized from the methylerythritol phosphate pathway in bacteria and plant plastids (Rohmer Citation1999). These compounds are ubiquitous in Eukarya, Bacteria and Archaea to serve a variety of different biological functions (Poulter and Rilling Citation1981). A group of prenyltransferases are responsible for making linear polyisoprenoid lipids, which are classified as cis- and trans-types according to the stereochemistry of the double bonds formed by condensation reactions of IPP with the allylic substrates (Liang et al. 2002, Liang Citation2009). As illustrated in , IPP's C4 approaches and nucleophilically attacks the allylic substrate's C1 that bears the phosphate leaving group, followed by removal of pro-R or pro-S proton from IPP to generate adduct with trans- or cis-double bond, respectively (Ogura and Koyama Citation1998). The IPP isomer dimethyallyl diphosphate (DMAPP) is the smallest allylic substrate, which is formed from IPP by isomerase (Durbecq et al. Citation2001), and C15 farnesyl diphosphate (FPP) catalyzed by FPP synthase (FPPS) serves as an outlet point leading to a variety of different isoprenoid compounds (Hosfield et al. Citation2004). As for product chain lengths and their functions, the trans-prenyltransferases identified so far catalyze products up to C50, including C15 FPP and C20 geranylgeranyl diphosphate (GGPP) used to modify signaling proteins for anchoring them into the cell membrane or as precursor of vitamins, pigments and many other natural isoprenopids, C25 farnesylgeranyl diphosphate (FGPP) as component of thermophilic Archaea membrane lipids, C30 ∼ C50 as side chains of ubiquinone and menaquinone in different species, with an exception of the presence of long-chain poly-trans prenols (>9 mers) in a plant, eucommia ulmoides oliver (Bamba et al. Citation2001). On the other hand, cis-prenyltranfserases make larger products, including C55 lipid carrier for bacterial cell wall biosynthesis, ∼C100-C120 dolichols for glycoprotein biosynthesis in ER, up to thousands of IPP for natural rubber. However, there is one exception that a cis-type FPPS identified in M. tuberculosis synthesizes C15 E,Z-FPP as a substrate for making C50 decaprenyl pyrophosphate lipid carrier (Schulbach et al. 2000).
The cis- and trans-prenyltransferases share no similarity on amino acid sequences and 3-D structures although both utilize the same substrates (Shimizu et al. Citation1998, Fujihashi et al. Citation2001). In addition to making various chain-lengths products with different stereochemistry on the double bonds, their catalytic mechanisms have been shown to be different as detailed below, explained by their different 3-D structures. Compared to the well-studied trans-type enzymes, particularly FPPS which serves as target of bisphosphonate drugs for treating osteoporosis, Paget's disease, hypercalcemia, and metastatic bone disease (Chen et al. 2008), cis-prenyltransferases in which the first enzyme, Undecaprenyl diphosphate Synthase (UPPS), was cloned about a decade ago are less understood. UPPS synthesizes UPP by catalyzing eight consecutive condensation reactions of IPP with FPP, which serves as the lipid carrier to mediate bacterial cell wall biosynthesis (Allen Citation1985). In this review, kinetics, mechanisms, structures and inhibitors of UPPS as a paradigm of cis-prenyltransferases are discussed.
Kinetics for IPP condensation and product release
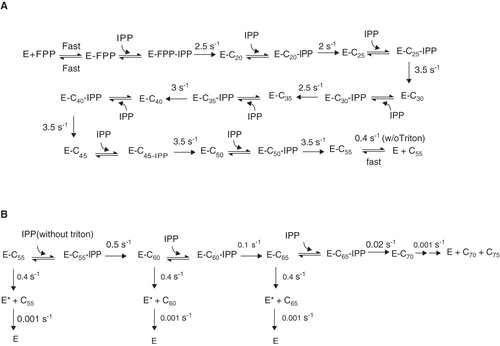
Substrate specificity examination on UPPS has proven FPP is the optimal allylic substrate (Chen et al. Citation2005). The complex kinetic scheme for the multiple IPP condensation steps from FPP catalyzed by UPPS () has been solved (Pan et al. Citation2000). Using rapid-quench instrument to stop the enzyme reaction in short periods of time ranging from 0.2–5 sec, the C20-C50 intermediates have been detected using radiolabeled [14C]IPP under UPPS single-turnover condition with excessive UPPS over FPP. Using KINSIM simulation program from KinTek Co. (www.kintek-corp.com) to analyze the time course data, the rate constants for all the IPP condensation steps were derived, which are approximately equal to 2.5 s-1 (). Moreover, the rate constants for product dissociation followed by conformational changes and slower condensation with extra IPP were obtained from the time courses of multiple-turnover reactions with [FPP] at 8-fold of [UPPS]. In conclusion, the product release is rate limiting in the absence of detergent, but with 0.1% Triton X-100, which stimulates product release, the IPP condensation becomes rate determining so that the steady-state k cat is increased by 190-fold from 0.013 s-1 to 2.5 s-1. The product release rate constants measured using stopped-flow instrument with a fluorescent substrate analog, 7-(2,6-dimethyl-8-pyrophospho-2,6-octadienyloxy)-8-methyl-4-trifluoromethyl-chromen-2-one (TFMC-GPP), as a competing agent to displace UPP from the active site, were determined to be 0.5 s-1, consistent with the prediction by KINSIM, in the absence of Triton (Chen, APC et al. Citation2002), and the product release rate is much faster (15.7 s-1) in the presence of 0.1% reduced-form Triton X-100 (modified to prevent fluorescence interference) (Lu et al. Citation2010).
Mechanisms for IPP condensation and product chain-length control
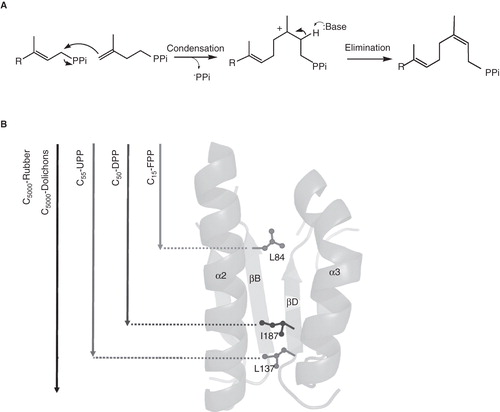
IPP condensation proceeds via either sequential or concerted mechanism. In sequential mechanism, pyrophosphate group from allylic substrate (e.g., FPP) is dissociated to form a carbocation that is attacked by IPP and the Pro-R proton in IPP is eliminated to form a new trans-double bond in adduct. Trans-type FPPS has been shown to utilize this ionization-condensation-elimination mechanism (Poulter and Satterwhite Citation1977, Poulter et al. 1977). In contrast, cis-type UPPS has been suggested to utilize concerted mechanism, where the release of pyrophosphate and attack of IPP occur simultaneously, followed by elimination of the pro-S proton in IPP to form a cis-double bond, by using the substrate analogs, 3-bromo-3-butenyl diphosphate (Br-IPP), 2-fluoro-FPP and [1,1-2H2]FPP (Lu et al. Citation2009, Citation2010). In these experiments, with electron-withdrawing Br replacing the methyl group on IPP to slow down the condensation step, no farnesyl cabocation was trapped as farnesol in UPPS reaction, but farnesol was detected for trans-type OPPS and GGPPS. Using electron-withdrawing fluoro to destabilize the carbocation if it can be formed, no reduction on IPP condensation rate was observed, indicating no carbocation is formed in UPPS reaction. The deuterium secondary kinetic isotope effect of 0.985 ± 0.022 using [1,1-2H2]FPP suggests associative transition state for UPPS reaction. The mechanism for IPP condensation in UPPS is illustrated by . This mechanism is also supported by the 3-D structures of UPPS as discussed later.
In general, cis-type prenyltransferases generate longer chain-length products with an exception of a cis-type FPPS identified in Mycobacterium tuberculosis (Schulbach et al. Citation2000). Based on site-directed mutagenesis studies, a molecular ruler mechanism for controlling the product chain lengths of cis-type prenyltransferases was concluded. In this mechanism as shown in , large amino acids in different cis-prenyltransferases block the further chain elongation and decide the chain lengths of products. In Escherichia coli UPPS, Leu137 at the bottom of active site was shown to play such a role in determining product chain length (Ko et al. Citation2001). The UPPS homologues in yeast, Rer2p and Srt1p, synthesize extended chain lengths peaking at C80 and C110, respectively, without a strict bacterial-like chain length control (Poznański and Szkopinska Citation2007). Based on sequence comparison, computer modeling suggests a significant increase in the size of the enzyme hydrophobic tunnel for Rer2p and Srt1p and predicts that rubber prenyltransferase that produces almost unlimited chain-length products has expanded tunnel volume and bottom opening due to insertion of a bottom loop between α3 helix and β-strand C (Poznański and Szkopinska Citation2007). Other protein factors may be involved in this elongating process (Cornish Citation1993).
3-D structures implicated in mechanisms and conformational changes
The above mechanisms for IPP condensation and final chain-length determination of UPPS can be supported by crystal structures of UPPS. As revealed by the structures, a Mg2+ is either associated with IPP substrate (in D26A mutant) or with FPP (wild type) (Guo et al. Citation2005), which support a concerted mechanism where IPP is bound as complex with Mg2+ and the Mg2+ is transferred to FPP (initial FPP binding to UPPS does not required Mg2+ as shown in a separate study (Chen, YH et al. Citation2002) to facilitate the pyrophosphate dissociation and IPP attacking. The C1 double bond of IPP is close to the pyrophosphate-bearing carbon on FPP (3.0–3.6 Å) for forming a covalent bond.
The crystal structures also revealed conformational changes during substrate binding, IPP condensation reactions and product release (Ko et al. Citation2001, Chang et al. Citation2003a, Citation2004), which have been probed using intrinsic Trp91 fluorescence in UPPS by stopped-flow fluorescence technology, showing three-phase trace (Chen, YH et al. 2002). The most variable regions are α3 and the loop (amino acids 72–83) linking α3 with βB. In the apo-form structure, the loop is mobile so that the electron density of the loop and some of α3 is missing in the solved crystal structure. As FPP is bound, the α3 is kinked towards the active-site tunnel to form a closed-form. On the other hand, when product is formed within the tunnel, it pushes the α3 to become an open-form for product release. These conformational changes as illustrated in allow the efficient IPP condensation reactions and finally the C55 lipid formed reaches the bottom of active site blocked by L137 and releases with the aid of detergent in vitro and probably bacterial cell membrane in vivo.
Figure 4. Conformational changes of UPPS during its reaction. Apo-UPPS has a flexible loop without visible electron density in crystal structure, and the binding of FPP and product-like Triton X-100 shift UPPS to closed form and open form, respectively.
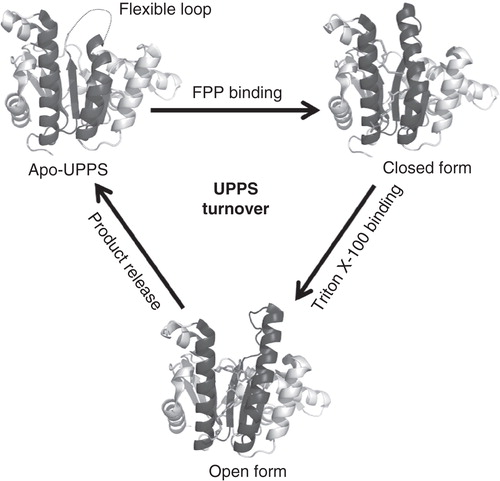
By increasing or decreasing length of the loop in UPPS mutants, which all yielded open-form UPPS with 104-fold lower k cat compared to the wild type, the closed form but not open form is catalytically active (Chang et al. Citation2003b).
Inhibitors and the new tool for screening inhibitors
Bisphosphonates mimicking allylic diphosphate substrate have been used as inhibitors of FPPS and GGPPS. For example, a bisphosphonate with bulky aromatic side chain inhibits UPPS by occupying the active-site tunnel with three molecules binding at the top (one at the same site of FPP substrate) and the other at the bottom (Guo et al. Citation2007). Tetramic, tetronic acids and dihydropyridin-2-ones have been identified through high throughput screening as inhibitors of Streptococcus pneumoniae UPPS (one example is shown in left panel of , with IC50 of 0.3 μM and MIC of 0.5 μg/ml against S. pneumoniae) without inhibiting human FPPS (Peukert et al. Citation2008). Its binding site was identified by using the inhibitor analog containing photoactivable dibenzophenone, to be at the location of Met49 in α3 helix of S. pneumoniae UPPS (Lee et al. Citation2010). The inhibitor is thus an allosteric inhibitor since it is bound close to FPP site and probably alters the active closed conformation to almost inactive open form of UPPS. Moreover, weak inhibitors from computer virtual screening (Kuo et al. Citation2008) and some inhibitors from high throughput screening without their structures being revealed have been obtained (Li et al. Citation2003). By applying molecular dynamics simulations, multiple ‘conformational states' of UPPS have been identified to be recognized by different classes of inhibitor molecules, rationalizing the difficulty in finding tight-binding inhibitors for UPPS (Sinko et al. Citation2011).
Figure 5. Fluorescent substrate analog MANT-O-GPP (A) for monitoring chain elongation by UPPS in real time (B).
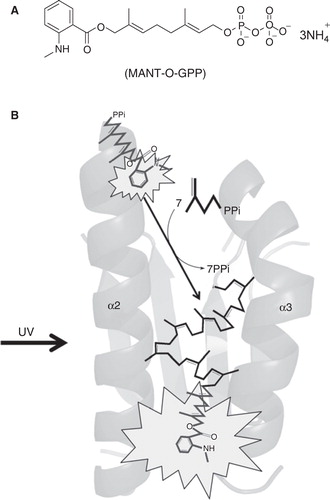
So far, only a limited number of inhibitors of UPPS are available. Radiolabeled [14C]IPP has been used for assay, but it is environmentally harmful due to the long decay half-life. EnzChek® Pyrophosphate Assay Kit (Invitrogen), a commercial coupling enzyme method, is available to detect released pyrophosphate through the formation of a chromophoric product for monitoring prenyltransferase reactions (Upson et al. Citation1996). Recently, a probe (2E,6E)-8-O-(N-Methyl-2-amin-obenzoyl)-3,7-dimethyl-2,6-octandien-1-pyrophosph-ate (MANT-O-GPP), with the smallest fluorescent MANT ester-linked to GPP to minimize the size, has been synthesized and proven to be successfully elongated by UPPS with concomitant fluorescence increase, which can be used to monitor UPPS activity in real time (see ) (Teng et al. Citation2011). Emission of MANT-O-GPP at 420 nm approaching visible light gives a suitable optical signal. This fluorogenic assay provides a convenient and environmentally friendly way for kinetics and inhibition studies on UPPS drug target and is suitable for high throughput screening, so that it should facilitate the inhibitor discovery.
Conclusions
In this review, the kinetics, mechanisms, structures and inhibitors of UPPS, a cis-prenyltransferase synthesizing lipid carrier for bacterial cell wall biosynthesis, have been summarized. The information as described here provides us more understanding regarding how a lipid is elongated with newly formed cis-double bonds. Compared to the well-studied trans-prenyltransferases, the cis-prenyltransferase shows different kinetic behavior, primary sequence, tertiary structures, and inhibitors. UPPS serves as a paradigm for understanding other cis-prenyltransferases, which are rich in nature, some with unknown functions.
Declaration of interest: We are grateful to the funds from National Science Council (grant number: 99-2113-M-001-014-MY3) and Academia Sinica of Taiwan ROC for supporting our research. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Allen CM. 1985. Purification and characterization of undecaprenyl pyrophosphate synthase. Methods Enzymol 110:281–299.
- Bamba T, Fukusaki E, Kajiyama S, Ute K, Kitayama T, Kobayashi A. 2001. The occurrence of geometric polyprenol isomers in the rubber-producing plant, Eucommia ulmoides Oliver. Lipids 36:727–736.
- Bloch K, Chaykin S, Phillips AH, de Waard A. 1959. Mevalonic acid pyrophosphate and isopentenyl pyrophosphate. J Biol Chem 234:2595–2608.
- Chang SY, Ko TP, Liang PH, Wang AHJ. 2003a. Catalytic mechanism revealed by the crystal structure of undecaprenyl pyrophosphate synthase in complex with sulfate, magnesium, and Triton. J Biol Chem 278:29298–29307.
- Chang SY, Chen YK, Wang AHJ, Liang PH. 2003b. Identification of the active conformation and the importance of length of the flexible loop in regulating conformational change in undecaprenyl pyrophosphate synthase. Biochemistry 42:14452–14459.
- Chang SY, Ko TP, Chen APC, Wang AHJ, Liang PH. 2004. Substrate binding mode and reaction mechanism of undecaprenyl pyrophosphate synthase deduced from crystallographic studies. Protein Sci 13:971–978.
- Chen APC, Chen YH, Liu HP, Li YC, Chen CT, Liang PH. 2002. Application of synthetic fluorescent substrate analogues to study ligand interactions for undecaprenyl pyrophosphate synthase. J Am Chem Soc 124:15217–15224.
- Chen APC, Chang SY, Lin YC, Sun YS, Chen CT, Wang AHJ, 2005. Substrate and product specificities of cis-type undecaprenyl pyrophosphate synthase. Biochem J 386:169–176.
- Chen CK, Hudock MP, Zhang Y, Kuo RT, Cao R, No JH, 2008. Inhibition of geranylgeranyl diphosphate synthase by bisphosphonates: A crystallographic and computational Investigation. J Med Chem 51:5594–5607.
- Chen YH, Chen APC, Chen CT, Wang AHJ, Liang PH. 2002. Probing the conformational change of E. coli undecaprenyl pyrophosphate synthase during catalysis using an inhibitor and tryptophan mutants. J Biol Chem 277:7369–7376.
- Cornish K. 1993. The separate roles of plant cis and trans prenyl transferases in cis-1,4-polyisoprene biosynthesis. Eur J Biochem 218:267–271.
- Durbecq V, Sainz S, Oudjama Y, Clantin B, Bompard-Gilles C, Tricot C, 2001. Crystal structure of isopentenyl diphosphate: dimethylally diphosphate isomerase. EMBO J 20:1530–1537.
- Fujihashi M, Zhang YW, Higuchi Y, Li XY, Koyama T, Miki K. 2001. Crystal structure of cis-prenyl chain elongating enzyme, undecaprenyl diphosphate synthase. Proc Natl Acad Sci USA 98:4337–4442.
- Guo RT, Ko TP, Chen APC, Kuo CJ, Wang AHJ, Liang PH. 2005. Crystal structures of undecaprenyl pyrophosphate synthase in complex with magnesium, isopentenyl pyrophosphate and farnesyl thiopyrophosphate: roles of the metal ion and conserved residues in catalysis. J Biol Chem 280:20762–20774.
- Guo RT, Cao R, Liang PH, Chang TH, Ko TP, Jeng WY, 2007. Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases. a. crystallographic investigation. Proc Natl Acad Sci USA 104:10022–10027.
- Hosfield DJ, Zhang Y, Dougan DR, Broun A, Tari LW, Swanson RV, 2004. Structural basis for bishosphontae-mediated inhibition of isoprenoid biosynthesis. J Biol Chem 279:8526–8529.
- Ko TP, Chen YK, Robinson H, Tsai PC, Gao YG, Chen APC, 2001. Mechanism of the product chain length determination and the role of a flexible loop in E. coli undecaprenyl pyrophosphate synthase catalysis. J Biol Chem 276:47474–47482.
- Kuo CJ, Guo RT, Lu IL, Liu HG, Wu SY, Ko TP, 2008. Structure-based inhibitors exhibited differential activities against H. pylori and E. coli undecaprenyl pyrophosphate synthases. J Biomed Biotechnol 841312.
- Lee LV, Granda B, Dean K, Tao J, Liu E, Zhang R, 2010. Biophysical investigation of the mode of inhibition of tetramic acids, the allosteric inhibiors of undecaprenyl pyrophosphate synthase. Biochemistry 49:5366–5376.
- Li H, Huang J, Jiang X, Seefeld M, McQuenery M, Macarron R. 2003. The effect of Triton concentration on the activity of undecaprenyl pyrophosphate synthase activity. J Biomol Screen 8:712–715.
- Liang PH, Ko TP, Wang AHJ. 2002. Structure, mechanism and function of prenyltransferases. Eur J Biochem 269:3339–3354.
- Liang PH. 2009. Reaction kinetics, catalytic mechanisms, conformational changes, and inhibitor design for prenyltransferases. Biochemistry 48:6562–6570.
- Lu YP, Liu HG, Liang PH. 2009. Different reaction mechanisms for cis- and trans-prenyltransferases. Biochem Biophys Res Commun 379:351–355.
- Lu YP, Liu HG, Teng KH, Liang PH. 2010. Mechanism of cis-prenyltransferase reaction probed by substrate analogues. Biophys Biochem Res Commun 400:758–762.
- Ogura K, Koyama T. 1998. Enzymatic aspects of isoprenoid chain elongation. Chem Rev 98:1263–1276.
- Pan JJ, Chiou ST, Liang PH. 2000. Product distribution and pre-steady-state kinetic analysis of Escherichia coli undecaprenyl pyrophosphate synthase reaction. Biochemistry 39:10936–10942.
- Peukert S, Sun Y, Zhang R, Hurley B, Sabio M, Shen X, 2008. Design and structure-activity relationships of potent and selective inhibitors of undecaprenyl pyrophosphate synthase (UPPS): Tetramic, tetronic acids and dihydropyridine-2-ones. Bioorg Med Chem Lett 18:1840–1844.
- Poulter CD, Argyle JC, Mash EA. 1977. Letter: Prenyltransferase. New evidence for an ionization-condensation-elimination mechanism with 2-fluorogeranyl pyrophosphate. J Am Chem Soc 99:957–959.
- Poulter CD, Satterwhite DM. 1977. Mechanism of the prenyl-transfer reaction: Studies with (E)- and (Z)-3-trifluoromethyl-2-buten-1-yl pyrophosphate. Biochemistry 16:5470–5478.
- Poulter CD, Rilling HC. 1981. Biosynthesis of isoprenoid compounds. (Porter, J. W., and Spurgeon, S. L., eds.), Vol. 1, pp 161–224, John Wiley & Sons, New York.
- Poznański J, Szkopinska A. 2007. Precise bacterial polyprenol length control fails in Saccharomyces cerevisiae. Biopolymers 86:155–164.
- Rohmer M. 1999. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae, and higher plants. Nat Prot Rep 16:565–674.
- Schulbach MC, Brennan PJ, Crick DC. 2000. Identification of a short (C15) chain Z-isoprenyl diphosphate synthase and a homologous long (C50) chain isoprenyl diphosphate synthase in Mycobacterium tuberculosis. J Biol Chem 275:22876–22881.
- Shimizu N, Koyama T, Ogura K. 1998. Molecular cloning, expression, and purification of undecaprenyl diphosphate synthase. J Biol Chem 273:19476–19481.
- Sinko W, de Oliveria C, Williams S, Wynsberghe AV, Durrant JD, Cao R, 2011. Applying molecular dynamics simulations to identify rarely sampled ligand-bound conformational states of undecaprenyl pyrophosphate synthase, an antibacterial target. Chem Biol Drug Des 7:412–420.
- Teng KH, Chen APC, Kuo CJ, Li YC, Liu HG, Chen CT, 2011. Fluorescent substrate analog for monitoring chain elongation by undecaprenyl pyrophosphate synthase in real time. Anal Biochem 417:136–141.
- Upson RH, Haugland RP, Malekzadeh MN. 1996. A spectrophotometric method to measure enzymatic activity in reactions that generate inorganic pyrophosphate. Anal Biochem 243:41–45.