Abstract
Biological membranes encompass and compartmentalize cells and organelles and are a prerequisite to life as we know it. One defining feature of membranes is an astonishing diversity of building blocks. The mechanisms and principles organizing the thousands of proteins and lipids that make up membrane bilayers in cells are still under debate. Many terms and mechanisms have been introduced over the years to account for certain phenomena and aspects of membrane organization and function. Recently, the different viewpoints – focusing on lipids vs. proteins or physical vs. molecular driving forces for membrane organization – are increasingly converging. Here we review the basic properties of biological membranes and the most common theories for lateral segregation of membrane components before discussing an emerging model of a self-organized, multi-domain membrane or ‘patchwork membrane'.
Introduction
Biological membranes surround organelles and cells, providing selective barriers to the cytosol or extracellular environment, respectively. The plasma membrane (PM) plays an essential role in nutrient uptake and as a signal-processing hub, making it one of the most important targets for pharmaceutical drugs. Perturbations in PM organization and/or function frequently result in cell death or contribute to the development of diseases, including stroke (Arinaminpathy et al. Citation2009) and cancer (Fiegl et al. Citation2010, Mollinedo and Gajate Citation2010, Gajate and Mollinedo Citation2011). A detailed understanding of the mechanisms for biological membrane organization is therefore not only crucial for basic biology but also for the development of novel disease treatments.
Although lateral compartmentalization of the PM has become a widely accepted fact (Sharma et al. Citation2004, Berchtold and Walther Citation2009, Hundt et al. Citation2009, Rocks et al. Citation2010), the underlying mechanisms and the functional implications of protein segregation are still subject to active debates. Arguments often arise from a difference in thematic focus or methodology, rather than from mutually exclusive theories. Biophysical approaches have traditionally had a lipid-centric view and focused on the energetic and thermodynamic aspects of lipid-mixing or phase separation in model membranes. In contrast, biologists have stressed the role of specific proteins and lipids or protein-lipid interactions in the formation of membrane domains in cells. These different approaches have co-existed for several decades. But only recently, with the development of new techniques that facilitate systematic analysis of the components in biological membranes and their molecular structure, the different aspects and viewpoints have begun to converge towards a unified model for membrane organization.
In this review, we first describe the fundamental properties of membranes and their constituents before discussing the most important biophysical and molecular models of membrane-domain formation, including hydrophobic matching, lipid rafts, molecular scaffolds and picket fences. We will discuss differences and similarities of various models and then propose a unified model of a ‘patchwork membrane'. We exemplify the properties of such a membrane with results from a recent systematic study of PM organization in budding yeast.
Membrane components: Lipids and proteins
Biological membranes are lipid bilayers that are assembled from a large number of different lipid species. Due to the hydrophobic effect, amphipathic lipids tend to self-assemble into structures that minimize exposure of the apolar regions to water. In cells, the most common assembly resulting from this entropic force is the lipid bilayer.
The diversity of cellular lipids is astonishingly large, with several hundreds of lipid species present in simple eukaryotes such as budding yeast (Ejsing et al. Citation2009, Guan et al. Citation2010) and likely thousands in mammalian cells (Sampaio et al. Citation2011). Appreciation of this enormous diversity has been dramatically increased by recent advances in mass spectrometry that allow the characterization of whole lipidomes (Wenk Citation2010, Han et al. Citation2012). It is currently not clear, why cells require a large lipid diversity, but it is worth noting that in contrast to proteins, DNA and carbohydrates, lipids do not form polymers. Some of their diverse functions therefore likely result from the properties and structures of individual lipids. Examples of highly specific lipid interactions can be found in several structural studies of membrane proteins (Lee Citation2011).
The lipids present in cellular membranes can be divided into three classes: Glycerophospholipids, sphingolipids and sterols. Glycerophospholipids can be further categorized with respect to their head groups, with the main groups being phosphatidyl-serine (PS), -inositol (PI), -ethanolamine (PE) and -choline (PC). Head groups vary in charge (PS and PI are negatively charged) or shape (PE has a small head group and is cone-shaped, PC has a bulky head group and is cylindrical), while the two hydrophobic acyl chains vary in length and degree of saturation. Sphingolipids are based on ceramides, with sphingosine backbone and a single acyl chain that is mostly saturated and 16–24 carbon atoms in length. The combination of variable acyl chains, ceramide backbones and hundreds of different carbohydrate head groups in glycosphingolipids can potentially give rise to tens of thousands of distinct sphingolipid species (Yetukuri et al. Citation2008).
Abundance of various lipid species differs strongly between the leaflets of biological membranes, with negatively charged PI and PS as well as PE enriched in the inner leaflet of the PM, while sphingolipids and PC are enriched in the outer leaflet. This is partly due to asymmetric synthesis of those lipids but also to active maintenance of asymmetry through flippases (from outside to inside) or floppases (inside to outside) (van Meer Citation2011). In addition, lipid composition varies between organelles, with, e.g., cholesterol-content increasing along the secretory pathway in mammalian cells (van Meer et al. Citation2008).
Lipids in cells are nearly exclusively found in lipid bilayers or the lamellar phase. However, many lipid species can also adopt more curved topological conformations, such as cubic or hexagonal phases (Frolov et al. Citation2011), which can be biologically highly relevant during processes such as membrane-fusion, virus-entry or pore-formation. They have also found practical use in expression and crystallization procedures for membrane proteins (Cherezov Citation2011). In addition, even within the bilayer, propensity of some lipids to adopt curved conformations could contribute to local membrane deformations for example during endocytosis (Mouritsen Citation2011b).
The importance of cellular membranes is reflected by the fact that approximately 30% of all proteins are integral membrane proteins (Almen et al. Citation2009) and many others are recruited to membranes. Interestingly, the fraction of integral membrane proteins remains relatively stable from bacteria to man (Wallin and von Heijne Citation1998). Hence, even a relatively simple eukaryote, such as the budding yeast Saccharomyces cerevisiae, contains more than 300 predicted PM proteins, of which around 150 are likely expressed at any given time. While little more than 300 unique structures of integral membrane proteins have been solved to date (White Citation2012), this number is expected to rapidly grow with recent technological advances (Bill et al. Citation2011).
Integral membrane proteins can be divided into transmembrane, intrinsic or polytopic proteins that span the whole bilayer once or multiple times, and monotopic proteins that are anchored to the bilayer via covalent lipid modifications from one side only (myristoyl, prenyl and palmitoyl groups on inner leaflet, GPI anchors on outer leaflet). Transmembrane sequences differ in length between different organelles with proteins in the ER and Golgi having shorter hydrophobic stretches compared to PM (Sharpe et al. Citation2010). Specific amino acids within the transmembrane region were also shown to be important for targeting (Scheiffele et al. Citation1997, Thomas et al. Citation2008, Sharpe et al. Citation2010). Finally, peripheral or extrinsic membrane proteins reversibly bind either to integral membrane proteins or to the head groups of the lipid bilayer via specialized lipid-binding domains.
Protein-lipid interactions
Annular lipids represent the shell of lipids surrounding the membrane-permeating part of an integral membrane protein. These lipids can adapt to the shape of the bound protein as recently shown in a crystal structure for aquaporin (Gonen et al. Citation2004, Yeung and Grinstein Citation2007, Goldenberg and Steinberg Citation2010). However annular lipids only transiently interact with protein surfaces and can still rapidly exchange with bulk lipids (East et al. Citation1985, Lee Citation2011).
Structural or buried lipids are able to penetrate deeper into the structure of proteins and fill holes in the protein-lipid interface (Hite et al. Citation2010). One example is the phosphatidylglycerol of nitrate reductase A (Bertero et al. Citation2003), where the lipid head group lies in a pocket formed by all three subunits and probably plays an important role in keeping the subunits together.
Electrostatic interactions between positively-charged protein patches or lipid-binding domains of peripheral membrane proteins and negatively charged lipid head groups of PI and PS play an important role in many signaling pathways (Hurley and Meyer Citation2001, Lemmon Citation2008, Goldenberg and Steinberg Citation2010). A well characterized example is the interaction of pleckstrin homology (PH) domains with phosphoinositides (Shewan et al. Citation2011). In addition, electrostatic interactions are also used by integral membrane proteins. In one recent study, the interaction of the C-terminal tail of the sodium-proton pump Nhe3 with anionic membranes was shown to be required for exchange activity (Alexander et al. Citation2011).
A systematic screen for protein-lipid interactions in yeast (Gallego et al. Citation2010) showed that proteins can often interact with several distinct lipid species, which would provide the capacity for combinatorial interactions with nearly limitless variations. Prominent examples of such combinatorial interactions are small GTPases, which often contain two separate localization domains, such as prenyl and palmitoyl modifications or polybasic patches (Heo et al. Citation2006).
Proteins are also capable of shaping or bending the membrane using several different mechanisms (Prinz and Hinshaw Citation2009). Some proteins bend membranes by inserting amphipathic domains into one of the leaflets of the bilayer. Others, notably proteins containing the Bin-Amphiphysin-Rvs167 (Bar) domain, form a rigid scaffold that deforms the underlying membrane (Suetsugu Citation2010). Finally, proteins can deform membranes indirectly by clustering lipids.
In a complex multi-particle system such as the biological membrane countless options exist for interactions between individual components. These interactions can occur directly or indirectly and vary greatly in strength and time scale while the sum of all interactions ultimately determines the organization of membranes.
Lipid-based membrane organization
In the fluid mosaic model (Singer and Nicolson Citation1972) the bilayer is depicted as a many-component but homogenous system with lipids as simple solvent that encompass the functional proteins. Since then many new insights have added significant modifications to this simple model. Most importantly it has been widely recognized that most membranes are laterally organized into domains of varying size and stability. We will discuss some of the most common concepts behind this lateral compartmentalization.
Phase transition and criticality
Because of their cooperative behavior, even simple lipid mixtures have a strong tendency to phase separate or form lateral domains (Bagatolli et al. Citation2010). The range of this co-operativity is reflected by the coherence length and depends on the composition and properties of the constituent lipids such as orientation, chain length and chain order. All these factors influence the lateral mobility and density fluctuations of lipids and hence the size of the domains formed. The most important phase transition with respect to lateral domain formation is the ‘main transition' from the solid-ordered to the liquid-disordered phase.
Another important concept is the description of lipid phase transitions as a ‘critical phenomenon', which describes the behavior under conditions (temperature, pressure etc.) close to a critical point (Honerkamp-Smith et al. Citation2009), where density fluctuations are predicted to be large. Interestingly, plasma membranes have been shown to exist close to a critical point (Veatch et al. Citation2008), which provides an attractive explanation formation of lateral domains (Honerkamp-Smith et al. Citation2009).
Hydrophobic matching
Cooperative effects and long-range interactions within lipid bilayers not only lead to lateral separation but also impose considerable forces on the actual bilayer structure that can be described in a lateral pressure profile (Mouritsen Citation2011b). This profile is a result of counteracting forces within the two-dimensional plane of the bilayer. Driven by the hydrophobic effect, the interfacial surface between aliphatic lipid chains and the surrounding water molecules has to be minimized, resulting in negative curvature and a tight alignment of the lipid head groups. This force is counteracted by steric hindrance of the head groups and interactions of the acyl chains of the lipids (Cantor Citation1999). Due to local inhomogeneities and fluctuations the lateral pressure can sum to hundreds of atmospheric pressures (Bagatolli et al. Citation2010) and can either support lateral segregation of lipids or be released in membrane curvature. Most importantly, if such a system is disturbed, e.g., through incorporation of integral membrane proteins, either the lipids or the proteins will have to adapt their conformation (Jensen and Mouritsen Citation2004). The concept of hydrophobic matching was first formulated in 1977 (Israelachvili Citation1977) and was later refined in the mattress model (Mouritsen and Bloom Citation1984). It assumes that membrane-spanning hydrophobic regions, such as the transmembrane segments of a protein, have to match the local thickness of the bilayer. In a heterogeneous lipid environment the protein will therefore be driven into a region with matching lipid lengths. Alternatively, membrane curvature can be introduced to match proteins and lipids. It has indeed been shown in early experiments that the bilayer close to a protein differs from distant regions (Pink et al. Citation1984) and that proteins can induce local lipid heterogeneities (Gawrisch et al. Citation1995). More recently, hydrophobic mismatch was demonstrated to drive phase separation and domain formation in artificial membranes (McIntosh et al. Citation2003, Vidal and McIntosh Citation2005, Kaiser et al. Citation2011). From these experiments the coherence length for a typical protein perturbation was estimated to be in the order of several lipids (Mouritsen Citation2010).
In principle, larger proteins or protein complexes are expected to affect more lipids and therefore induce formation of larger domains. One additional consequence of the hydrophobic matching model is that diffusion coefficients of transmembrane peptides depend on the extent of mismatch as shown in artificial membranes (Gambin et al. Citation2010). Due to hydrophobic mismatch, peptides containing interfacially-localized tryptophans – such as Gramicidin – can alter thickness of the bilayer and even induce transition into a non-lamellar phase (Morein et al. Citation2000). Conversely, the energy required for hydrophobic matching of the soft bilayer can also impact on the structure of the much harder protein, although this generally leads to small conformational rearrangements rather than large changes in protein folding (Jensen and Mouritsen Citation2004, Andersen and Koeppe Citation2007).
Lipid rafts
When model membranes are assembled from a ternary mix of saturated and unsaturated lipids with cholesterol, a unique liquid-ordered or liquid-crystalline phase is formed (Ipsen et al. Citation1987). This is due to the preferential association of the flat and rigid cholesterol with saturated acyl chains, which leads to straightening of the associated lipids and therefore to a more ordered state and thicker bilayer. The liquid-ordered phase has also been proposed to exist in biological membranes, where in the outer leaflet of the PM cholesterol segregates together with sphingolipids into distinct domains termed lipid rafts (van Meer et al. Citation1987, Simons and van Meer Citation1988). Importantly it has so far not been unambiguously demonstrated that liquid-ordered domains occur in cells and even the exact characteristics of the liquid-ordered phase are still under debate (Fidorra et al. Citation2006, Subczynski et al. Citation2007). However, it has become widely accepted that sterols and sphingolipids indeed associate with defined membrane regions. Historically, proteins associated with lipid rafts were biochemically defined by their resistance to cold detergent extraction (Brown and Rose Citation1992). This method clearly does not reflect physical domain-formation within membranes (Munro Citation2003, Simons and Gerl Citation2010, Tanner et al. Citation2011). Currently, lipid rafts are defined as a diverse population of nanometer-scale (Sharma et al. Citation2004, Eggeling et al. Citation2009) assemblies of varying lipid composition and the unifying fact of an enrichment in sterols and sphingolipids (Mishra and Joshi Citation2007). In addition, rafts are dynamic entities that exist only on a sub-second timescale, but that are able to coalesce into larger and more stable platforms upon specific signals (Lingwood et al. Citation2009). In one study, membrane tension was shown to be sufficient to drive domain coalescence at physiological temperatures (Ayuyan and Cohen Citation2008).
To explain the ability of rafts to include specific transmembrane proteins – something never seen in liquid-ordered phases of model membranes – formation of rafts is not longer explained by pure lipid-phase separation. Instead it is proposed to involve manifold interactions between proteins and lipids that ultimately result in laterally segregated domains (Kaiser et al. Citation2009). In this scenario raft formation is simply a sterol/sphingolipid-specific variant of the more general hydrophobic matching model (Kaiser et al. Citation2011). The long-range, cooperative forces derived from hydrophobic mismatch have also been described as ‘wetting' of raft-protein aggregates by a lipid film (Akimov et al. Citation2008, Coskun and Simons Citation2011).
Another model for raft formation is provided by the lipid shell hypothesis (Anderson and Jacobson Citation2002). In this model cholesterol and sphingolipid molecules surrounding a protein form a relatively static shell (in contrast to the dynamic annular lipids introduced above) that acts as address for assembly into the larger rafts. Such lipid shells are estimated to be small (7 nm for the GPI-anchored Thy-1 (Anderson and Jacobson Citation2002)) and are not expected to be in a separate phase. Experimental evidence for the existence of lipid shells is currently lacking.
Of scaffolds and fences
In addition to the lipid-driven mechanisms of lateral membrane organization we have discussed so far, several processes have been described where proteins drive domain formation and membrane compartmentalization by either acting as scaffolds or as diffusion barriers.
Several large and temporally stable domains in the PM of eukaryotic cells are known to be based on interactions between membrane proteins and scaffold structures. One prevalent example is interaction of membrane proteins with the extracellular matrix (ECM) in animal cells or the equivalent cell wall of fungal cells. Upon activation of PM-spanning integrin heterodimers by their ECM-bound ligands, a large number of cytosolic and peripheral membrane proteins are recruited, that assemble into focal adhesions that mediate cell adhesion (Kanchanawong et al. Citation2010). These structures are stable over minutes and connect the extracellular interaction site to the actin cytoskeleton.
In the PM of budding yeast cells, protein-cell wall interaction has been shown for the integral membrane protein Sur7, which localizes to small patches that are stable over hours and require the cell wall for their stability (Young et al. Citation2002). Sur7 also associates with eisosomes (Walther et al. Citation2006). These self-assembled structures form around the BAR-domain-containing core proteins Pil1 and Lsp1 (Karotki et al. Citation2011, Olivera-Couto et al. Citation2011), which bind preferentially to PI(4,5)P2 and distort the PM into elongated furrows (Strádalová et al. Citation2009, Ziolkowska et al. Citation2011). The function of eisosomes is still not entirely understood (Douglas et al. Citation2011, Ziolkowska et al. Citation2012) and roles have been proposed in sphingolipid signaling (Frohlich et al. Citation2009), nutrient uptake (Malinska et al. Citation2004), membrane protein turnover (Walther et al. Citation2006, Grossmann et al. Citation2008) as well as cell wall biosynthesis (Alvarez et al. Citation2009, Wang et al. Citation2011).
Caveolae are domains in mammalian PMs that are also built around self assembled protein cores (Parton and Simons Citation2007). These cup-shaped PM invaginations of 60–80 nm diameter again exhibit remarkable temporal stability and are built around the polymer-forming integral membrane protein caveolin. Caveolae have been reported to be enriched in sphingolipids (Ortegren et al. Citation2004), cholesterol (Fujimoto et al. Citation1997) and PI(4,5)P2 (Fujita et al. Citation2009) and they are connected to both microtubules and the actin cytoskeleton (Mundy et al. Citation2002). Some types of caveolae have been clearly shown to be involved in clathrin-independent endocytosis (Anderson Citation1993, Kirkham and Parton Citation2005). Additional functions include mechanosensation and lipid regulation (Bastiani and Parton Citation2010).
The superfamily of tetraspanin proteins was proposed to function as organizers of membrane domains. These four transmembrane-containing proteins homo- or hetero-dimerize and provide binding sites for many other membrane proteins (Charrin et al. Citation2009). These tetraspanin ‘webs' have been proposed to contribute to many processes including membrane transport and cell fusion (Rubinstein Citation2011). Interestingly, tetraspanins were shown to interact with cholesterol and this interaction was dependent on a palmitoyl modification (Yang et al. Citation2004). This modification has been recently suggested to act as a general recruitment signal for rafts (Levental et al. Citation2010).
One important factor organizing the PM is the cortical cytoskeleton, which in animal cells mostly consists of actin filaments and its associated proteins. Besides its role during intracellular trafficking, actin is closely associated with the PM and plays a major role in restricting mobility of membrane components that reach out of the cytosolic face of the membrane. High-speed tracking of gold-labeled lipids at 40,000 frames/sec revealed a confined movement of the lipids, while movement became freely diffusive after depolymerization of the actin cytoskeleton (Fujiwara et al. Citation2002). This observation led to the formulation of the picket-fence model, in which actin creates fences across the PM through association with transmembrane protein pickets (Kusumi et al. Citation1993). In addition, proteins that sterically penetrate from the inner leaflet into the actin fence region will become confined within a compartment for a certain time, until the actin reorganizes or the proteins pass (hop) through the fence (Kusumi et al. Citation1993, Sako and Kusumi Citation1994). Such actin-dependent membrane corrals can therefore confine diffusion of lipids and integral membrane proteins and thereby lead to formation of mesoscale membrane domains on the order of several 100 nm (Kusumi et al. Citation2011).
Next to the picket fence model, several alternative scenarios for actin-mediated formation of membrane domains have been proposed. Nanoclusters of GPI-linked proteins have been shown to depend on cortical actin (Goswami et al. Citation2008) and formation of clusters has been proposed to act via transient interactions with remodeling actin filaments (Chaudhuri et al. Citation2011). Along a similar line, the actin cytoskeleton was proposed to organize membrane proteins by providing anchor points for proteins along the filaments, which in turn results in formation of nanoscale domains (Machta et al. Citation2011).
Towards a patchwork membrane
With so many possible mechanisms for the formation of lateral domains and inhomogeneities in membranes, which of the proposed models is now more correct, and what does a biological membrane actually look like? The most obvious, but obviously unsatisfactory, answer is that all models are correct to some extent and can contribute to the explanation of membrane organization – albeit on different length and time scales. Recent technological advances and discoveries have led to a remarkable consensus among researchers in the field. A large number of recent reviews beautifully summarize the various aspects of membrane organization and reach fairly consistent conclusions (Coskun and Simons Citation2011, Kusumi et al. Citation2011, Lee Citation2011, Mouritsen Citation2011a).
The emerging picture is that of a membrane where segregation and clustering of lipids and proteins occurs via interactions on two levels. First, self-organization of membranes occurs through coordinated, weak interactions between proteins and lipids. This level of organization can be understood by mathematical approximations and theories and is largely driven by lipids (providing cooperativity) as described by the hydrophobic matching model. Lipid rafts can be considered as a subgroup of such domains with sterols providing a specialized but non-exclusive ordering function. Specificity in domain formation is provided by general properties of the interaction partners, such as size, rigidity and charge. Self-organized domains are highly dynamic and vary from ten to hundreds of nm in size. Secondly, stable or meso-stable assemblies are generated by specific and higher-affinity molecular interactions between lipids, proteins and carbohydrates of the ECM. The resulting domains are generally larger, longer-lived and include static scaffold-based assemblies as well as larger macro-domains defined by polymeric fences such as cortical actin filaments or septin polymers (Caudron and Barral Citation2009). Both levels of organization are interdependent and cooperate in patterning biological membranes.
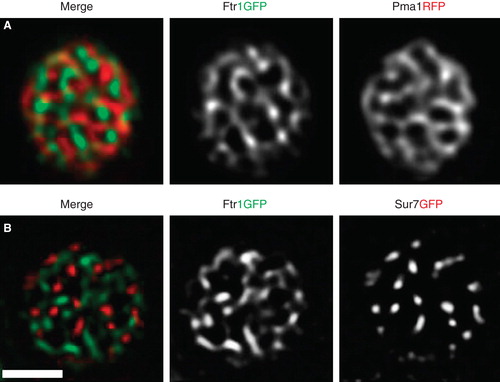
One prediction that arises from all currently discussed membrane models is that an environment with hundreds of different protein and lipid species should generate a multitude of defined lateral domains. Nevertheless, nearly all studies to date have focused on the segregation of individual proteins or proteins classes – with a few exceptions: Three distinct sphingomyelin-containing domains were reported in the PM of erythrocytes and CHO cells (Tyteca et al. Citation2010, D'Auria et al. Citation2011). Four different membrane regions have been implicated by super resolution microscopy in COS cells (Sengupta et al. Citation2011). Finally, three distinct PM domains, termed MCC, MCP and MCT (Membrane Compartments occupied by Can1, Pma1 and TORC2, respectively) were reported in budding yeast (Malinska et al. Citation2003, Berchtold and Walther Citation2009). In this well-studied fungal system the identified domains are clearly formed by several distinct mechanisms. MCC represent static patches that associate with eisosomal scaffolds (Ziolkowska et al. Citation2012). These structures that are conserved in various fungi (Alvarez et al. Citation2009, Vangelatos et al. Citation2010, Reijnst et al. Citation2011) are stable over hours and show no lateral mobility. MCT patches are defined by components of the TORC2 complex (Berchtold and Walther Citation2009), which only transiently interacts with the PM. It is therefore a subject for debate whether the MCT can be considered as bona fide lateral membrane domain or rather represents large soluble complexes that transiently interact with a particular receptor on the PM. A hybrid situation can be considered for endocytic actin patches that also assemble transiently, but – like eisosomes – form around distinct membrane invaginations (Kaksonen et al. Citation2005). Finally, the MCP domain so far solely contains the ATPase Pma1 and was reported to cover the whole PM excepting MCC regions (Malinska et al. Citation2003). It could therefore simply be a result of exclusion from the static MCC regions. In conclusion, no conclusive evidence exists for lipid-based lateral segregation of proteins in yeast.
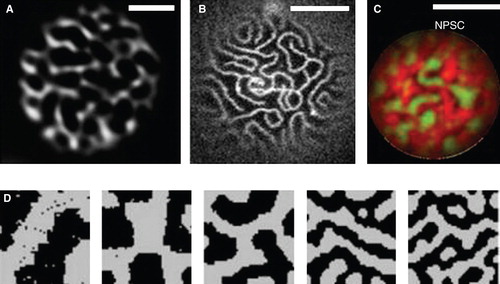
Recently we conducted a systematic study on a large number of PM proteins in yeast and found that many integral proteins localized in cell-spanning network-like patterns of elongated tracks around larger gaps (Spira et al. Citation2012). These patterns could be clearly distinguished by total internal reflection microscopy (). Similar network-like patterns have been typically described for lipid-driven domain formation in model membranes () and in simulations of segregation due to hydrophobic mismatch (). Most strikingly, we found that nearly all of the identified protein-domains were clearly separated from each other (Spira et al. Citation2012). The iron transporter Ftr1, for example, occupied a domain distinct from those formed by Pma1 or Sur7 (). The surprisingly large size of network-domains in the yeast PM stands in contrast to the mostly nanometer-sized rafts and clusters found in mammalian cells. Interestingly, transmembrane (Valdez-Taubas and Pelham Citation2003, Ries et al. Citation2008, Spira et al. Citation2012), lipid-modified proteins (Marco et al. Citation2007) and lipids (Greenberg and Axelrod Citation1993) in the yeast PM exhibit much slower dynamics than in mammalian systems. According to the hydrophobic matching theory, such slow diffusion rates are expected to correlate with long coherence lengths and could therefore lead to formation of the observed large domains. In conclusion, our systematic study of protein distribution in the yeast PM indicates the existence of a multitude of overlapping macroscopic domains. Such a patchwork membrane likely arises from a combination of weak and strong interactions between the manifold lipid and protein constituents of biological membranes, using the established principles of hydrophobic matching and scaffolding. Ultimately, segregation of proteins into numerous distinct domains is expected to facilitate efficient regulation of the myriad functions that are simultaneously occurring at cellular membranes.
Figure 1. Network-like domains of proteins and lipids. (A) Pattern of the ATPase Pma1 in the budding yeast PM imaged by TIRFM. (B) Domains of FITC-LB21 peptide in a GUV made of PC and cholesterol. Reproduced from Kaiser et al. (Citation2011). (C) Network pattern in a GUV made from native pulmonary surfactant (NPS), with DiIC18 (red) and Bodipy-PC (green). Reproduced from Bernardino De La Serna et al. (Citation2009). (D) Patterns formed in simulations of lipid segregation by hydrophobic mismatch. Reproduced from Wallace et al. (Citation2006). Scale bars: 2 μm (A) and 10 μm (B, C). This Figure is reproduced in color in the online version of Molecular Membrane Biology.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Akimov SA, Frolov VA, Kuzmin PI, Zimmerberg J, Chizmadzhev YA, Cohen FS. 2008. Domain formation in membranes caused by lipid wetting of protein. Phys Rev E, Statist, nonlinear soft matter phys 77:051901.
- Alexander RT, Jaumouille V, Yeung T, Furuya W, Peltekova I, Boucher A, 2011. Membrane surface charge dictates the structure and function of the epithelial Na+/H+ exchanger. EMBO J 30:679–691.
- Almen MS, Nordstrom KJ, Fredriksson R, Schioth HB. 2009. Mapping the human membrane proteome: A majority of the human membrane proteins can be classified according to function and evolutionary origin. BMC Biol 7:50.
- Alvarez FJ, Douglas LM, Konopka JB. 2009. The Sur7 protein resides in punctate membrane subdomains and mediates spatial regulation of cell wall synthesis in Candida albicans.. Communicat Integrat Biol 2:76–77.
- Andersen OS, Koeppe RE II. 2007. Bilayer thickness and membrane protein function: An energetic perspective. Annual Rev Biophys Biomolec Struct 36:107–130.
- Anderson RG. 1993. Caveolae: Where incoming and outgoing messengers meet. Proc Natl Acad Sci USA 90:10909–10913.
- Anderson RG, Jacobson K. 2002. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 296:1821–1825.
- Arinaminpathy Y, Khurana E, Engelman DM, Gerstein MB. 2009. Computational analysis of membrane proteins: The largest class of drug targets. Drug Discovery Today 14:1130–1135.
- Ayuyan AG, Cohen FS. 2008. Raft composition at physiological temperature and pH in the absence of detergents. Biophys J 94:2654–2666.
- Bagatolli LA, Ipsen JH, Simonsen AC, Mouritsen OG. 2010. An outlook on organization of lipids in membranes: Searching for a realistic connection with the organization of biological membranes. Progr Lipid Res 49:378–389.
- Bastiani M, Parton RG. 2010. Caveolae at a glance. J Cell Sci 123:3831–3836.
- Berchtold D, Walther TC. 2009. TORC2 plasma membrane localization is essential for cell viability and restricted to a distinct domain. Molec Biol Cell 20:1565–1575.
- Bernardino De La Serna J, Oradd G, Bagatolli LA, Simonsen AC, Marsh D, Lindblom G, 2009. Segregated phases in pulmonary surfactant membranes do not show coexistence of lipid populations with differentiated dynamic properties. Biophys J 97:1381–1389.
- Bertero MG, Rothery RA, Palak M, Hou C, Lim D, Blasco F, 2003. Insights into the respiratory electron transfer pathway from the structure of nitrate reductase A. Nature Struct Biol 10:681–687.
- Bill RM, Henderson PJ, Iwata S, Kunji ER, Michel H, Neutze R, 2011. Overcoming barriers to membrane protein structure determination. Nature Biotechnol 29:335–340.
- Brown DA, Rose JK. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533–544.
- Cantor RS. 1999. Lipid composition and the lateral pressure profile in bilayers. Biophys J 76:2625–2639.
- Caudron F, Barral Y. 2009. Septins and the lateral compartmentalization of eukaryotic membranes. Develop Cell 16:493–506.
- Charrin S, Le Naour F, Silvie O, Milhiet PE, Boucheix C, Rubinstein E. 2009. Lateral organization of membrane proteins: Tetraspanins spin their web. Biochem J 420:133–154.
- Chaudhuri A, Bhattacharya B, Gowrishankar K, Mayor S, Rao M. 2011. Spatiotemporal regulation of chemical reactions by active cytoskeletal remodeling. Proc Natl Acad Sci USA 108:14825–14830.
- Cherezov V. 2011. Lipidic cubic phase technologies for membrane protein structural studies. Current Opin Struct Biol 21:559–566.
- Coskun U, Simons K. 2011. Cell membranes: The lipid perspective. Structure 19:1543–1548.
- D'Auria L, Van Der Smissen P, Bruyneel F, Courtoy PJ, Tyteca D. 2011. Segregation of fluorescent membrane lipids into distinct micrometric domains: Evidence for phase compartmentation of natural lipids? PloS one 6:e17021.
- Douglas LM, Wang HX, Li L, Konopka JB. 2011. Membrane compartment occupied by Can1 (MCC) and eisosome subdomains of the fungal plasma membrane. Membranes 1:394–411.
- East JM, Melville D, Lee AG. 1985. Exchange rates and numbers of annular lipids for the calcium and magnesium ion dependent adenosinetriphosphatase. Biochemistry 24:2615–2623.
- Eggeling C, Ringemann C, Medda R, Schwarzmann G, Sandhoff K, Polyakova S, 2009. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature 457:1159–1162.
- Ejsing CS, Sampaio JL, Surendranath V, Duchoslav E, Ekroos K, Klemm RW, 2009. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc Natl Acad Sci USA 106:2136–2141.
- Fidorra M, Duelund L, Leidy C, Simonsen AC, Bagatolli LA. 2006. Absence of fluid-ordered/fluid-disordered phase coexistence in ceramide/POPC mixtures containing cholesterol. Biophys J 90:4437–4451.
- Fiegl M, Samudio I, Mnjoyan Z, Korchin B, Fritsche H, Andreeff M. 2010. Physiological hypoxia promotes lipid raft and PI3K-dependent activation of MAPK 42/44 in leukemia cells. Leukemia: Off J Leukemia Soc Am, Leukemia Research Fund, UK 24:1364–1367.
- Frohlich F, Moreira K, Aguilar PS, Hubner NC, Mann M, Walter P, 2009. A genome-wide screen for genes affecting eisosomes reveals Nce102 function in sphingolipid signaling. J Cell Biol 185:1227–1242.
- Frolov VA, Shnyrova AV, Zimmerberg J. 2011. Lipid polymorphisms and membrane shape. Cold Spring Harbor Perspect Biol 3:a004747.
- Fujimoto T, Hayashi M, Iwamoto M, Ohno-Iwashita Y. 1997. Crosslinked plasmalemmal cholesterol is sequestered to caveolae: Analysis with a new cytochemical probe. J Histochem Cytochem: Off J Histochem Soc 45:1197–1205.
- Fujita A, Cheng J, Tauchi-Sato K, Takenawa T, Fujimoto T. 2009. A distinct pool of phosphatidylinositol 4,5-bisphosphate in caveolae revealed by a nanoscale labeling technique. Proc Natl Acad Sci USA 106:9256–9261.
- Fujiwara T, Ritchie K, Murakoshi H, Jacobson K, Kusumi A. 2002. Phospholipids undergo hop diffusion in compartmentalized cell membrane. J Cell Biol 157:1071–1081.
- Gajate C, Mollinedo F. 2011. Lipid rafts and Fas/CD95 signaling in cancer chemotherapy. Recent Patents on Anti-Cancer Drug Discov 6:274–283.
- Gallego O, Betts MJ, Gvozdenovic-Jeremic J, Maeda K, Matetzki C, Aguilar-Gurrieri C, 2010. A systematic screen for protein-lipid interactions in Saccharomyces cerevisiae. Molec Syst Biol 6:430.
- Gambin Y, Reffay M, Sierecki E, Homble F, Hodges RS, Gov NS, 2010. Variation of the lateral mobility of transmembrane peptides with hydrophobic mismatch. J Phys Chem B 114:3559–3566.
- Gawrisch K, Barry JA, Holte LL, Sinnwell T, Bergelson LD, Ferretti JA. 1995. Role of interactions at the lipid-water interface for domain formation. Molec Memb Biol 12:83–88.
- Goldenberg NM, Steinberg BE. 2010. Surface charge: A key determinant of protein localization and function. Cancer Res 70:1277–1280.
- Gonen T, Sliz P, Kistler J, Cheng Y, Walz T. 2004. Aquaporin-0 membrane junctions reveal the structure of a closed water pore. Nature 429:193–197.
- Goswami D, Gowrishankar K, Bilgrami S, Ghosh S, Raghupathy R, Chadda R, 2008. Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell 135:1085–1097.
- Greenberg ML, Axelrod D. 1993. Anomalously slow mobility of fluorescent lipid probes in the plasma membrane of the yeast Saccharomyces cerevisiae. J Membrane Biol 131:115–127.
- Grossmann G, Malinsky J, Stahlschmidt W, Loibl M, Weig-Meckl I, Frommer WB, 2008. Plasma membrane microdomains regulate turnover of transport proteins in yeast. J Cell Biol 183:1075–1088.
- Guan XL, Riezman I, Wenk MR, Riezman H. 2010. Yeast lipid analysis and quantification by mass spectrometry. Meth Enzymol 470:369–391.
- Han X, Yang K, Gross RW. 2012. Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass Spectrom Rev 31:134–178.
- Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, 2006. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science 314:1458–1461.
- Hite RK, Li Z, Walz T. 2010. Principles of membrane protein interactions with annular lipids deduced from aquaporin-0 2D crystals. EMBO J 29:1652–1658.
- Honerkamp-Smith AR, Veatch SL, Keller SL. 2009. An introduction to critical points for biophysicists; observations of compositional heterogeneity in lipid membranes. Biochim Biophysica Acta 1788:53–63.
- Hundt M, Harada Y, De Giorgio L, Tanimura N, Zhang W, Altman A. 2009. Palmitoylation-dependent plasma membrane transport but lipid raft-independent signaling by linker for activation of T cells. J Immunol 183:1685–1694.
- Hurley JH, Meyer T. 2001. Subcellular targeting by membrane lipids. Curr Opin Cell Biol 13:146–152.
- Ipsen JH, Karlstrom G, Mouritsen OG, Wennerstrom H, Zuckermann MJ. 1987. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim Biophys Acta 905:162–172.
- Israelachvili JN. 1977. Refinement of the fluid-mosaic model of membrane structure. Biochim Biophys Acta 469:221–225.
- Jensen MO, Mouritsen OG. 2004. Lipids do influence protein function-the hydrophobic matching hypothesis revisited. Biochim Biophys Acta 1666:205–226.
- Kaiser HJ, Lingwood D, Levental I, Sampaio JL, Kalvodova L, Rajendran L, 2009. Order of lipid phases in model and plasma membranes. Proc Natl Acad Sci USA 106:16645–16650.
- Kaiser HJ, Orlowski A, Rog T, Nyholm TK, Chai W, Feizi T, 2011. Lateral sorting in model membranes by cholesterol-mediated hydrophobic matching. Proc Natl Acad Sci USA 108:16628–16633.
- Kaksonen M, Toret CP, Drubin DG. 2005. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell 123:305–320.
- Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, 2010. Nanoscale architecture of integrin-based cell adhesions. Nature 468:580–584.
- Karotki L, Huiskonen JT, Stefan CJ, Ziolkowska NE, Roth R, Surma MA, 2011. Eisosome proteins assemble into a membrane scaffold. J Cell Biol 195:889–902.
- Kirkham M, Parton RG. 2005. Clathrin-independent endocytosis: New insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta 1746:349–363.
- Kusumi A, Sako Y, Yamamoto M. 1993. Confined lateral diffusion of membrane receptors as studied by single particle tracking (nanovid microscopy). Effects of calcium-induced differentiation in cultured epithelial cells. Biophys J 65:2021–2040.
- Kusumi A, Suzuki KG, Kasai RS, Ritchie K, Fujiwara TK. 2011. Hierarchical mesoscale domain organization of the plasma membrane. Trends Biochem Sci 36:604–615.
- Lee AG. 2011. Biological membranes: The importance of molecular detail. Trends Biochem Sci 36:493–500.
- Lemmon MA. 2008. Membrane recognition by phospholipid-binding domains. Nat Rev Molec Cell Biol 9:99–111.
- Levental I, Lingwood D, Grzybek M, Coskun U, Simons K. 2010. Palmitoylation regulates raft affinity for the majority of integral raft proteins. Proc Natl Acad Sci USA 107:22050–22054.
- Lingwood D, Kaiser HJ, Levental I, Simons K. 2009. Lipid rafts as functional heterogeneity in cell membranes. Biochem Soc Transact 37:955–960.
- Machta BB, Papanikolaou S, Sethna JP, Veatch SL. 2011. Minimal model of plasma membrane heterogeneity requires coupling cortical actin to criticality. Biophys J 100:1668–1677.
- Malinska K, Malinsky J, Opekarova M, Tanner W. 2003. Visualization of protein compartmentation within the plasma membrane of living yeast cells. Molec Biol Cell 14:4427–4436.
- Malinska K, Malinsky J, Opekarova M, Tanner W. 2004. Distribution of Can1p into stable domains reflects lateral protein segregation within the plasma membrane of living S. cerevisiae cells. J Cell Sci 117:6031–6041.
- Marco E, Wedlich-Söldner R, Li R, Altschuler SJ, Wu LF. 2007. Endocytosis optimizes the dynamic localization of membrane proteins that regulate cortical polarity. Cell 129:411–422.
- Mcintosh TJ, Vidal A, Simon SA. 2003. Sorting of lipids and transmembrane peptides between detergent-soluble bilayers and detergent-resistant rafts. Biophys J 85:1656–1666.
- Mishra S, Joshi PG. 2007. Lipid raft heterogeneity: An enigma. J Neurochem 103(Suppl 1):135–142.
- Mollinedo F, Gajate C. 2010. Lipid rafts, death receptors and CASMERs: New insights for cancer therapy. Future Oncol 6:491–494.
- Morein S, Koeppe IR, Lindblom G, De Kruijff B, Killian JA. 2000. The effect of peptide/lipid hydrophobic mismatch on the phase behavior of model membranes mimicking the lipid composition in Escherichia coli membranes. Biophys J 78:2475–2485.
- Mouritsen OG. 2010. The liquid-ordered state comes of age. Biochim Biophys Acta 1798:1286–1288.
- Mouritsen OG. 2011a. Lipidology and lipidomics – quo vadis? A new era for the physical chemistry of lipids. Phys Chem Chemical Phys 13:19195–19205.
- Mouritsen OG. 2011b. Lipids, curvature, and nano-medicine. Eur J Lipid Sci Technol 113:1174–1187.
- Mouritsen OG, Bloom M. 1984. Mattress model of lipid-protein interactions in membranes. Biophys J 46:141–153.
- Mundy DI, Machleidt T, Ying YS, Anderson RG, Bloom GS. 2002. Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J Cell Sci 115:4327–4339.
- Munro S. 2003. Lipid rafts: Elusive or illusive? Cell 115:377–388.
- Olivera-Couto A, Grana M, Harispe L, Aguilar PS. 2011. The eisosome core is composed of BAR domain proteins. Molec Biol Cell 22:2360–2372.
- Ortegren U, Karlsson M, Blazic N, Blomqvist M, Nystrom FH, Gustavsson J, 2004. Lipids and glycosphingolipids in caveolae and surrounding plasma membrane of primary rat adipocytes. Eur J Biochem/FEBS 271:2028–2036.
- Parton RG, Simons K. 2007. The multiple faces of caveolae. Nat Rev Molec Cell Biol 8:185–194.
- Pink DA, Chapman D, Laidlaw DJ, Wiedmer T. 1984. Electron spin resonance and steady-state fluorescence polarization studies of lipid bilayers containing integral proteins. Biochemistry 23:4051–4058.
- Prinz WA, Hinshaw JE. 2009. Membrane-bending proteins. Critical Rev Biochem Molec Biol 44:278–291.
- Reijnst P, Walther A, Wendland J. 2011. Dual-colour fluorescence microscopy using yEmCherry-/GFP-tagging of eisosome components Pil1 and Lsp1 in Candida albicans. Yeast 28:331–338.
- Ries J, Klose C, Walch-Solimena C, Schwille P. 2008. How to measure slow diffusion in yeast cell membranes. In: Popp J, editor. Biophotonics: Photonics solutions for better health care. Strasbourg: Proceedings of SPIE 6991, 8.
- Rocks O, Gerauer M, Vartak N, Koch S, Huang ZP, Pechlivanis M, 2010. The palmitoylation machinery is a spatially organizing system for peripheral membrane proteins. Cell 141:458–471.
- Rubinstein E. 2011. The complexity of tetraspanins. Biochem Soc Transact 39:501–505.
- Sako Y, Kusumi A. 1994. Compartmentalized structure of the plasma membrane for receptor movements as revealed by a nanometer-level motion analysis. J Cell Biol 125:1251–1264.
- Sampaio JL, Gerl MJ, Klose C, Ejsing CS, Beug H, Simons K, 2011. Membrane lipidome of an epithelial cell line. Proc Natl Acad Sci USA 108:1903–1907.
- Scheiffele P, Roth MG, Simons K. 1997. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J 16:5501–5508.
- Sengupta P, Jovanovic-Talisman T, Skoko D, Renz M, Veatch SL, Lippincott-Schwartz J. 2011. Probing protein heterogeneity in the plasma membrane using PALM and pair correlation analysis. Nature Meth 8:969–975.
- Sharma P, Varma R, Sarasij RC, Ira Gousset K, Krishnamoorthy G, Rao M, 2004. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell 116:577–589.
- Sharpe HJ, Stevens TJ, Munro S. 2010. A comprehensive comparison of transmembrane domains reveals organelle-specific properties. Cell 142:158–169.
- Shewan A, Eastburn DJ, Mostov K. 2011. Phosphoinositides in cell architecture. Cold Spring Harbor Perspect Biol 3:a004796.
- Simons K, Gerl MJ. 2010. Revitalizing membrane rafts: New tools and insights. Nat Rev Molec Cell Biol 11:688–699.
- Simons K, Van Meer G. 1988. Lipid sorting in epithelial cells. Biochemistry 27:6197–6202.
- Singer SJ, Nicolson GL. 1972. The fluid mosaic model of the structure of cell membranes. Science 175:720–731.
- Spira F, Mueller NS, Beck G, Von Olshausen P, Beig J, Wedlich-Söldner R. 2012. Patchwork organization of the yeast plasma membrane into numerous coexisting domains. Nature Cell Biol DOI: 10.1038/ncb2487.
- Strádalová V, Stahlschmidt W, Grossmann G, Blazikova M, Rachel R, Tanner W, 2009. Furrow-like invaginations of the yeast plasma membrane correspond to membrane compartment of Can1. J Cell Sci.122:2887–2894.
- Subczynski WK, Wisniewska A, Hyde JS, Kusumi A. 2007. Three-dimensional dynamic structure of the liquid-ordered domain in lipid membranes as examined by pulse-EPR oxygen probing. Biophys J 92:1573–1584.
- Suetsugu S. 2010. The proposed functions of membrane curvatures mediated by the BAR domain superfamily proteins. J Biochem 148:1–12.
- Tanner W, Malinsky J, Opekarova M. 2011. In plant and animal cells, detergent-resistant membranes do not define functional membrane rafts. Plant Cell 23:1191–1193.
- Thomas CL, Bayer EM, Ritzenthaler C, Fernandez-Calvino L, Maule AJ. 2008. Specific targeting of a plasmodesmal protein affecting cell-to-cell communication. PLoS Biol 6:e7.
- Tyteca D, D'auria L, Der Smissen PV, Medts T, Carpentier S, Monbaliu JC, 2010. Three unrelated sphingomyelin analogs spontaneously cluster into plasma membrane micrometric domains. Biochim Biophys Acta 1798:909–927.
- Valdez-Taubas J, Pelham HR. 2003. Slow diffusion of proteins in the yeast plasma membrane allows polarity to be maintained by endocytic cycling. Curr Biol 13:1636–1640.
- Van Meer G. 2011. Dynamic transbilayer lipid asymmetry. Cold Spring Harbor Perspect Biol 3.
- Van Meer G, Stelzer EH, Wijnaendts-Van-Resandt RW, Simons K. 1987. Sorting of sphingolipids in epithelial (Madin-Darby canine kidney) cells. J Cell Biol 105:1623–1635.
- Van Meer G, Voelker DR, Feigenson GW. 2008. Membrane lipids: Where they are and how they behave. Nat Rev Molec Cell Biol 9:112–124.
- Vangelatos I, Roumelioti K, Gournas C, Suarez T, Scazzocchio C, Sophianopoulou V. 2010. Eisosome organization in the filamentous ascomycete Aspergillus nidulans.. Eukaryotic Cell 9:1441–1454.
- Veatch SL, Cicuta P, Sengupta P, Honerkamp-Smith A, Holowka D, Baird B. 2008. Critical fluctuations in plasma membrane vesicles. ACS Chem Biol 3:287–293.
- Vidal A, Mcintosh TJ. 2005. Transbilayer peptide sorting between raft and nonraft bilayers: Comparisons of detergent extraction and confocal microscopy. Biophys J 89:1102–1108.
- Wallace EJ, Hooper NM, Olmsted PD. 2006. Effect of hydrophobic mismatch on phase behavior of lipid membranes. Biophys J 90:4104–4118.
- Wallin E, Von Heijne G. 1998. Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci: A public Protein Soc 7:1029–1038.
- Walther TC, Brickner JH, Aguilar PS, Bernales SN, Pantoja C, Walter P. 2006. Eisosomes mark static sites of endocytosis. Nature 439:998–1003.
- Wang HX, Douglas LM, Aimanianda V, Latge JP, Konopka JB. 2011. The Candida albicans Sur7 protein is needed for proper synthesis of the fibrillar component of the cell wall that confers strength. Eukaryotic Cell 10:72–80.
- Wenk MR. 2010. Lipidomics: New tools and applications. Cell 143:888–895.
- White S. 2012. http://blanco.biomol.uci.edu/index.shtml; Online.
- Yang X, Kovalenko OV, Tang W, Claas C, Stipp CS, Hemler ME. 2004. Palmitoylation supports assembly and function of integrin-tetraspanin complexes. J Cell Biol 167:1231–1240.
- Yetukuri L, Ekroos K, Vidal-Puig A, Oresic M. 2008. Informatics and computational strategies for the study of lipids. Molec BioSyst 4:121–127.
- Yeung T, Grinstein S. 2007. Lipid signaling and the modulation of surface charge during phagocytosis. Immunolog Rev 219:17–36.
- Young ME, Karpova TS, Brugger B, Moschenross DM, Wang GK, Schneiter R, 2002. The Sur7p family defines novel cortical domains in Saccharomyces cerevisiae, affects sphingolipid metabolism, and is involved in sporulation. Molec Cellular Biol 22:927–934.
- Ziolkowska NE, Christiano R, Walther TC. 2012. Organized living: formation mechanisms and functions of plasma membrane domains in yeast. Trends Cell Biol 3:151–158.
- Ziolkowska NE, Karotki L, Rehman M, Huiskonen JT, Walther TC. 2011. Eisosome-driven plasma membrane organization is mediated by BAR domains. Nature Struct Molec Biol 18:854–856.