Abstract
Insolubility of membrane components in non-ionic detergents such as Triton X-100 at low temperature is a widely used biochemical criterion to identify, isolate and characterize membrane domains. In this work, we monitored the detergent insolubility of the serotonin1A receptor in CHO cell membranes and its modulation by membrane cholesterol. The serotonin1A receptor is an important member of the G-protein coupled receptor family. It is implicated in the generation and modulation of various cognitive, behavioral and developmental functions and serves as a drug target. Our results show that a significant fraction (∼ 28%) of the serotonin1A receptor resides in detergent-resistant membranes (DRMs). Interestingly, the fraction of the serotonin1A receptor in DRMs exhibits a reduction upon membrane cholesterol depletion. In addition, we show that contents of DRM markers such as flotillin-1, caveolin-1 and GM1 are altered in DRMs upon cholesterol depletion. These results assume significance since the function of the serotonin1A receptor has previously been shown to be affected by membrane lipids, specifically cholesterol. Our results are relevant in the context of membrane organization of the serotonin1A receptor in particular, and G-protein coupled receptors in general.
Introduction
The G-protein coupled receptor (GPCR) superfamily is the largest and most diverse protein family in mammals, involved in signal transduction across membranes (Pierce et al. Citation2002, Rosenbaum et al. Citation2009). GPCRs are prototypical members of the family of seven transmembrane domain proteins and include >800 members which together constitute ∼ 5% of the human genome (Zhang et al. Citation2006). GPCRs regulate physiological responses to a diverse array of stimuli, and mediate multiple physiological processes. GPCRs have therefore emerged as major targets for the development of novel drug candidates in all clinical areas (Heilker et al. Citation2009). It is estimated that ∼ 50% of clinically prescribed drugs act as either agonists or antagonists of GPCRs (Schlyer and Horuk Citation2006).
The major paradigm in GPCR signaling is that their stimulation leads to the recruitment and activation of heterotrimeric GTP-binding proteins (G-proteins). These initial events, fundamental in GPCR signaling, occur at the plasma membrane via protein-protein interactions and are controlled by lateral dynamics (diffusion) of the GPCR (Pucadyil et al. Citation2004). An important consequence is that the organization of molecules such as receptors and G-proteins in the membrane represents an important determinant in GPCR signaling (Ostrom and Insel Citation2004, Pucadyil and Chattopadhyay Citation2006). In this regard, the observation that GPCRs are not uniformly present on the plasma membrane, but are concentrated in specific membrane domains that are enriched in cholesterol assumes significance (Ostrom and Insel Citation2004). The role of membrane domains in GPCR function therefore represents a challenging aspect of GPCR signaling.
Current understanding of the organization of biological membranes involves the concept of lateral heterogeneities in the membrane, collectively termed membrane domains (Mukherjee and Maxfield Citation2004, Jacobson et al. Citation2007, Lingwood and Simons Citation2010). Many of these domains (sometimes termed as ‘lipid rafts’) are believed to be important for the maintenance of membrane structure and function, although characterizing the spatiotemporal resolution of these domains has proven to be challenging (Jacobson et al. Citation2007, Ganguly and Chattopadhyay Citation2010). These specialized regions are believed to be enriched in specific lipids and proteins, and facilitate processes such as trafficking, sorting, signal transduction and pathogen entry (Mukherjee and Maxfield Citation2004, Jacobson et al. Citation2007, Pucadyil and Chattopadhyay Citation2007a). Insolubility of membrane components in non-ionic detergents such as Triton X-100 is a widely used biochemical criterion to identify, isolate and characterize membrane domains (particularly ‘rafts’) (Brown and Rose Citation1992, Hooper Citation1999, Chamberlain Citation2004).
The serotonin1A receptor is an important member of the GPCR family and is implicated in the generation and modulation of various cognitive, behavioral and developmental functions (Pucadyil et al. Citation2005, Kalipatnapu and Chattopadhyay Citation2007b, Müller et al. Citation2007). The serotonin1A receptor agonists and antagonists have been shown to possess potential therapeutic effects in anxiety or stress-related disorders (Pucadyil et al. Citation2005). As a consequence, the serotonin1A receptor serves as an important target in the development of therapeutic agents for neuropsychiatric disorders such as anxiety and depression. Previous work from our laboratory comprehensively demonstrated the requirement of membrane cholesterol (Pucadyil and Chattopadhyay Citation2004, Paila et al. Citation2008, Shrivastava et al. Citation2010) and sphingolipids (Paila et al. Citation2010) in the function of the serotonin1A receptor (reviewed in Pucadyil and Chattopadhyay Citation2006, Paila and Chattopadhyay Citation2010).
In this work, we have explored detergent insolubility of the serotonin1A receptor and its modulation by membrane cholesterol. For this, we used DRM specific marker proteins (such as flotillin-1 and caveolin-1) and lipids (GM1 and cholesterol) to characterize DRM fractions isolated from CHO cell membranes and explored the effect of cholesterol depletion on the distribution of serotonin1A receptors in DRM fractions. Our results show that ∼ 28% of serotonin1A receptors are present in DRM fractions and cholesterol depletion leads to a decrease in DRM localization of serotonin1A receptors.
Methods
Materials
Gentamycin sulfate, BSA, penicillin, sodium bicarbonate, streptomycin, MβCD, Triton X-100 and DMPC were obtained from Sigma Chemical Co. (St Louis, MO, USA). Fetal calf serum, DMEM/F-12 (Dulbecco's modified Eagle's medium: nutrient mixture F-12 (Ham) (1:1) and geneticin (G 418) were from Invitrogen Life Technologies (Grand Island, NY, USA). Amplex Red cholesterol assay kit was from Molecular Probes (Eugene, OR, USA). BCA reagent for protein estimation was obtained from Pierce (Rockford, IL, USA). Protease inhibitor cocktail was from Roche Applied Science (Mannheim, Germany). All other chemicals used were of the highest purity available. Water was purified through a Millipore (Bedford, MA, USA) Milli-Q system and used throughout. Rabbit polyclonal antibodies raised against GFP and rabbit polyclonal antibody to transferrin receptor were from Abcam (Cambridge, MA, USA). Mouse anti-flotillin and rabbit anti-caveolin polyclonal antibodies were obtained from BD-Transduction Laboratories (Sparks, MD, USA).
Cells and cell culture
Chinese hamster ovary (CHO) cells stably expressing the serotonin1A receptor tagged to enhanced yellow fluorescent protein (referred to as CHO-5-HT1AR-EYFP) were used. Cells were grown in D-MEM/F-12 (1:1) supplemented with 2.4 g/l of sodium bicarbonate, 10% fetal calf serum, 60 μg/ml penicillin, 50 μg/ml streptomycin, and 50 μg/ml gentamycin sulfate in a humidified atmosphere with 5% CO2 at 37°C. Cells were maintained with 300 μg/ml geneticin and grown up to 90% confluency before being used for membrane preparation.
Cell membrane preparation
Membranes from CHO cells were prepared as described previously (Kalipatnapu et al. Citation2004). Total protein was determined using the BCA assay (Smith et al. Citation1985) and membranes were stored at −70°C until further use.
Cholesterol depletion from control membranes
Cholesterol from membranes prepared from CHO cells was depleted using 30 mM methyl-β-cyclodextrin (MβCD) as described previously (Pucadyil and Chattopadhyay Citation2007b). Membranes were then spun down at 40,000 g for 5 min at 4°C, washed and suspended in 50 mM Tris, pH 7.4 buffer.
Isolation of detergent resistant membrane fraction
Detergent resistant membranes were isolated from control or cholesterol-depleted membranes (CDMs), as described previously by Brown and Rose with modifications (Brown and Rose Citation1992). Cell membranes or CDMs were resuspended in 50 mM Tris, pH 7.4 buffer containing protease inhibitor cocktail and 1% (v/v) Triton X-100 at 2 mg/ml (referred to as solubilization mixture). The solubilization mixture was incubated at 4°C for 30 min with gentle mixing every 5 min. After incubation, the resulting mixture was mixed with an equal volume of 80% sucrose (prepared in 50 mM Tris, pH 7.4 buffer) to give 40% final sucrose concentration in the samples. The solubilization mixture containing 40% sucrose was layered successively with equal volumes of 30% and 5% sucrose in 50 mM Tris, pH 7.4 buffer to obtain a discontinuous sucrose density gradient. Gradients were centrifuged at 160,000 g for 22 h at 4°C in a Beckman SW41 rotor. Fractions (1 ml each) were harvested from the top of the gradient (12 fractions; see ) and proteins in each sample were precipitated by trichloroacetic acid (TCA). Fractions 4–7 were designated as DRM fractions based on their distinctively higher flotillin-1 and caveolin-1 contents. The rest of the fractions (8–12) were designated as DSM fractions. For lipid analysis, DRM (fractions 4–7) and DSM (fractions 8–12) fractions were pooled together, diluted 5 times and centrifuged at 250,000 g for 2 h at 4°C to get rid of sucrose. The membrane pellets were then resuspended in 50 mM Tris, pH 7.4 buffer and used for estimation of phospholipid, cholesterol and GM1.
Figure 1. Characterization of DRM and DSM fractions of membranes from CHO cells stably expressing the serotonin1A receptor tagged to EYFP (5-HT1AR-EYFP). (A) Typical pattern of cell membrane fractions on sucrose density gradient. In order to isolate DRM and DSM fractions, cell membranes were treated with 1% Triton X-100 at 4°C and fractionated on a discontinuous sucrose density gradient. (B) 1 ml fractions were collected from top of the tube (total 12 fractions) and immunoblotted for marker proteins of DRM (flotillin-1 and caveolin-1) and the serotonin1A receptor. No protein was detected in fractions 1–3 (not shown). Fractions 4–7 were designated as DRM due to the presence of distinctively higher contents of flotillin-1 and caveolin-1. The rest of the fractions (8–12) were termed as DSM. See Methods for other details.
TCA precipitation of proteins
Fractions collected from the sucrose density gradient were diluted 5 times with 50 mM Tris, pH 7.4 buffer to lower sucrose concentration in samples. Proteins in each fraction were precipitated with 10% TCA for 1 h at 4°C. The precipitate was pelleted at 20,000 g for 5 min, washed with ice-cold acetic acid, air dried and dissolved in 50 mM Tris, pH 7.4 buffer containing 2% SDS. Protein content of each fraction was determined by micro-BCA method (Smith et al. Citation1985) and proteins were probed by immunoblotting.
Immunoblotting and protein quantitation
Serotonin1A receptor, flotillin-1, caveolin-1 and transferrin receptor from control membranes, DRM, DSM and CDM were probed by immunoblotting. Total protein used for detection of flotillin-1, caveolin-1 and transferrin receptor was 20 μg while that for the serotonin1A receptor was 30 μg. Total protein from each fraction was mixed with electrophoresis sample buffer and incubated for 30 min at 37°C. Samples were loaded and separated on SDS-PAGE. Proteins were electrophoretically transferred to a nitrocellulose membrane (Hybond ECL, Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK) using a semidry transfer apparatus (Amersham). Non-specific binding sites were blocked with 3% BSA in TBST (137 mM NaCl, 20 mM Tris, 0.1% Tween-20, pH 7.6 buffer) for 2 h at room temperature (∼ 23°C). Serotonin1A receptors tagged to EYFP (5-HT1AR-EYFP) were probed with rabbit polyclonal antibodies raised against GFP (1:1000 dilution). Flotillin-1 and caveolin-1 were probed with mouse anti-flotillin and rabbit anti-caveolin polyclonal antibodies (1:1000 for flotillin-1 and 1: 4000 for caveolin-1). Transferrin receptor was probed with rabbit polyclonal antibody to transferrin receptor (1:1000). Binding of primary antibodies to respective proteins was carried out by incubating membranes overnight with their respective antibodies in TBST at 4°C. After incubation, membranes were washed with TBST three times. Membranes were then incubated with 1:4000 dilution of respective secondary antibodies (horseradish peroxidase [HRP]-conjugated anti-rabbit antibody for 5-HT1AR-EYFP, caveolin-1 and transferrin receptor and anti-mouse antibody for flotillin-1) in TBST for 45 min at room temperature (∼ 23°C). Membranes were then washed and developed using enhanced chemiluminesence detection reagents (Amersham Biosciences, Bucks, UK). Serotonin1A receptor, flotillin-1, caveolin-1 and transferrin receptor were detected using the chemiluminescence detection system (Chemi-Smart-5000, Vilber Loumat). Respective protein levels were estimated using Bio-2D+software (BioRad).
Estimation of membrane cholesterol and phospholipid contents
The cholesterol content in cell membranes was estimated using the Amplex Red cholesterol assay kit (Amundson and Zhou 1999). The phospholipid content in these membranes was determined after total digestion with perchloric acid as described previously (McClare Citation1971) using Na2HPO4 as a standard.
Dot blotting
To detect GM1 content in control membranes, CDMs and DRMs, 0.2 μg of protein from each membrane sample was dot blotted on nitrocellulose membrane, air dried and blocked for 2 h with 3% BSA in PBS (128 mM NaCl, 2 mM KCl, 8 mM Na2HPO4, 2 mM KH2PO4, pH 7.4 buffer) at room temperature. Membranes were incubated with HRP-conjugated cholera toxin β-subunit (1:5000) and detected with enhanced chemiluminesence detection reagents (Amersham Biosciences, Buckinghamshire, UK). GM1 in dot blot was detected using the chemiluminescence detection system (Chemi-Smart-5000, Vilber Loumat). Respective GM1levels were estimated using Bio-2D+software (BioRad).
Results
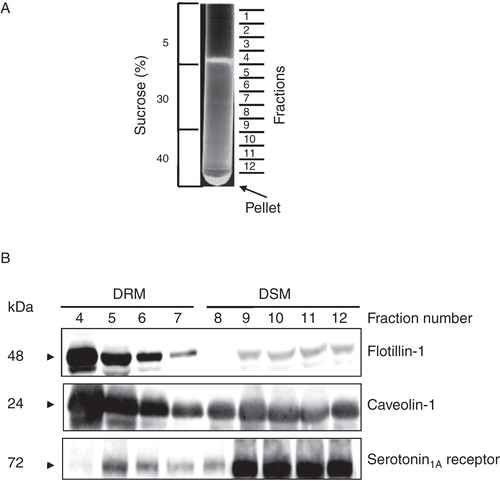
Membrane organization of the serotonin1A receptor, a pharmacologically important GPCR, represents an important determinant in its function (Kalipatnapu and Chattopadhyay Citation2007b). We previously reported the membrane organization of the serotonin1A receptor using both detergent insolubility and detergent-free approaches (Kalipatnapu and Chattopadhyay Citation2004, Citation2005, Citation2007a). However, domains monitored in these studies were not characterized based on the presence of specific marker proteins for DRMs and DSMs. Since composition and properties of membrane domains are often influenced by the method used (Banerjee et al. Citation1995, Schuck et al. Citation2003), it is important to characterize them based on the presence of specific marker proteins and lipids. In the present study, we have explored the localization of the human serotonin1A receptor in DRMs, based on the presence of specific marker proteins such as caveolin-1 and flotillin-1 and lipids (GM1) and its modulation by membrane cholesterol content. For this, cell membranes isolated from CHO cells stably expressing 5-HT1AR-EYFP were treated with 1% cold Triton X-100 and fractionated on a discontinuous sucrose density gradient. It is important to mention here that we have previously characterized CHO-5-HT1AREYFP and shown that the EYFP-tagged receptor is essentially similar to the native receptor (Pucadyil et al. Citation2004). Specifically, we previously showed that EYFP fusion to the serotonin1A receptor does not affect receptor function, i.e., ligand binding, G-protein coupling and signaling (Pucadyil et al. Citation2004). A typical pattern of membrane fractions on a sucrose density gradient is shown in . A total of 12 fractions were collected from top of the centrifuge tube and analyzed for the presence of flotillin-1 and caveolin-1 by immunoblotting. Fractions containing DRMs or DSMs were characterized by the presence or absence of specific marker proteins such as flotillin-1 and caveolin-1. Since no detectable amounts of protein were found in fractions 1–3, these fractions were discarded. shows that fractions 4–7 are characterized by distinctively higher flotillin-1 and caveolin-1 contents and were therefore termed as DRMs. The remaining fractions (8–12) were assigned as DSMs. We therefore pooled fractions 4–7 and 8–12 for DRMs and DSMs, respectively, for further analysis. It should be noted that DRMs are typically low-density membrane fractions and DSMs are mostly the high-density membrane fractions. Interestingly, we observed a distribution of the human serotonin1A receptor in different fractions, although the receptor was found to be predominantly present in DSM fractions.
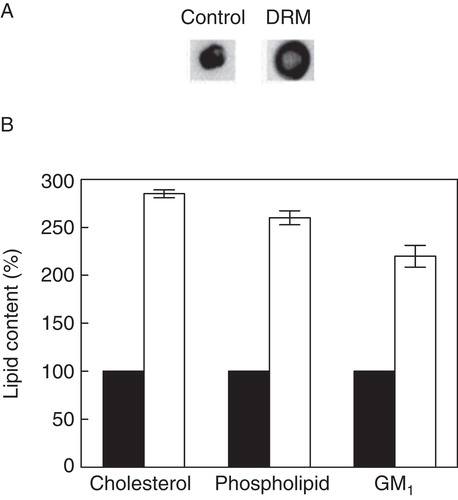
In order to estimate the serotonin1A receptor content in DRMs and DSMs, we performed densitometric analysis of immunoblots of the serotonin1A receptor and marker proteins of DRMs and DSMs (). We observed that ∼ 28% of serotonin1A receptors are localized in DRMs. This is in excellent agreement with previous reports on the localization of the serotonin1A receptors in DRMs (Kalipatnapu and Chattopadhyay Citation2004, Renner et al. Citation2007). In addition, ∼ 95% flotillin-1 and ∼ 83% caveolin-1 were found to be present in DRMs. It is known from earlier literature that the transferrin receptor (a single transmembrane domain) exhibits solubility in Triton X-100 and therefore is often used as a control for DSM (Mayor and Maxfield Citation1995). shows that ∼ 96% transferrin receptor was found to be present in DSMs. The presence of higher contents of flotillin-1 and caveolin-1 in pooled fractions for DRM and transferrin receptor in pooled fractions for DSM reinforces our analysis. Importantly, the glycosphingolipid GM1 and cholesterol are commonly enriched in DRMs and frequently used as markers to characterize DRMs (Brown and London Citation2000). Cholera toxin B (CTXB) is known to bind specifically to GM1 with high affinity (Fishman Citation1982). In order to quantitate GM1 level, we performed dot blot analysis of DRM fractions on nitrocellulose membrane and probed GM1 with CTXB (). We observed ∼ 2-fold enrichment of GM1 in DRM fractions relative to control membranes (see ). In addition, both cholesterol and phospholipids exhibited ∼ 3-fold increase in DRMs relative to control membranes (). Cholesterol, GM1 and phospholipids were not estimated in DSM fractions due to their poor recovery during centrifugation.
Figure 2. Quantitative analysis of serotonin1A receptors in DRM and DSM fractions of cell membranes. (A) Representative immunoblots showing distribution of serotonin1A receptors, flotillin-1, caveolin-1 and transferrin receptors in control membranes, and DRM and DSM fractions. (B) Contents of serotonin1A receptors, flotillin-1, caveolin-1 and transferrin receptors in DRM (white bars) and DSM (gray bars). Values are expressed as percentages of total protein content in DRM and DSM fractions. Protein contents were estimated by densitometric analysis of their respective bands on immunoblots using Bio-2D+ software (Bio-Rad). Data represent means ± SE of at least three independent experiments. See Methods for other details.
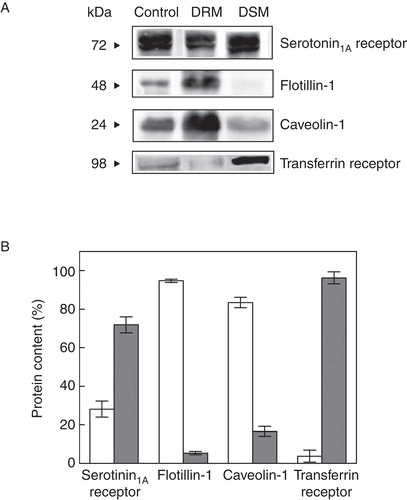
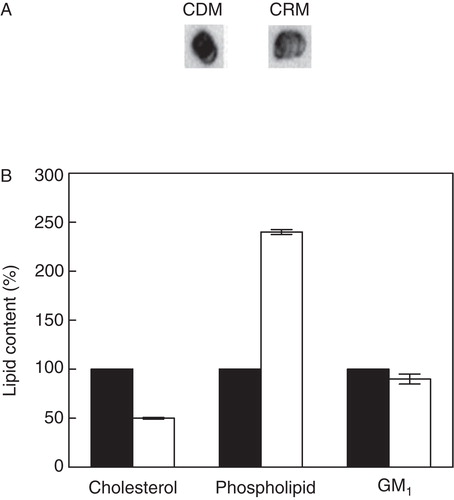
Figure 3. Quantitative analysis of lipid contents in DRM fractions of cell membranes. (A) Representative dot blots of GM1 in control membranes and DRMs. (B) Lipid contents in control membranes (black bars) and DRM fractions (white bars). Values are expressed as percentages of lipid contents in control membranes, normalized to total protein content of respective membranes. GM1 was estimated by densitometric analysis of dot blots using Bio-2D+ software (Bio-Rad). Data represent means ± SE of at least three independent experiments. See Methods for other details.
We (Pucadyil and Chattopadhyay Citation2007b, Shrivastava et al. Citation2010) and others (Sjögren et al. Citation2008) previously reported that membrane cholesterol is crucial for the function of serotonin1A receptor. It has been reported earlier that DRMs are enriched with cholesterol (Brown and London Citation1998). We therefore explored the distribution of serotonin1A receptors in DRMs and DSMs of CDMs and the results are shown in . In order to deplete cholesterol, we treated cell membranes with MβCD. MβCD is a water-soluble cyclic oligosaccharide, and has earlier been shown to extract cholesterol from membranes in a selective and efficient manner by including it in a central nonpolar cavity (Zidovetzki and Levitan Citation2007). We observed ∼ 90% of cholesterol was depleted from cell membranes upon MβCD treatment (data not shown). On the other hand, there was negligible loss of phospholipids and GM1 contents from these membranes under these conditions (not shown). The effect of cholesterol depletion on DRM localization of serotonin1A receptors was monitored by immunoblotting the receptor and marker proteins for DRMs and DSMs, followed by densitometric analysis. Representative immunoblots of the serotonin1A receptor and marker proteins in CDMs, and in DRMs and DSMs isolated from these membranes are shown in . As shown in , cholesterol depletion resulted in a decrease in the pool of serotonin1A receptors in DRMs isolated from CDMs in comparison to DRMs isolated from control membranes (cf. ). shows that serotonin1A receptor content was reduced to ∼ 7% in DRMs accompanied by a concominant increase in DSM fractions. Similarly, levels of flotillin-1 and caveolin-1 also displayed reduction to ∼ 45% in DRM fractions and their corresponding levels were increased in DSM fractions. Such reduction in the localization of specific marker proteins from DRMs upon cholesterol depletion has earlier been reported (Troost et al. Citation2004, Hajduch et al. Citation2008). Since glycosphingolipids are known to preferentially interact with cholesterol (Ramstedt and Slotte Citation2006), we monitored the effect of cholesterol depletion on lipids contents, particularly GM1 in DRMs isolated from CDMs. shows representative dot blots of GM1 in CDMs and DRMs isolated from them. Cholesterol level in DRMs was reduced to ∼ 50% of its level in CDMs (see ). Interestingly, we observed a decrease in GM1 content in DRMs isolated from CDMs relative to DRMs isolated from control membranes. In addition, the level of phospholipids in DRMs remained more or less invariant with cholesterol depletion ().
Figure 4. Estimation of serotonin1A receptors in DRM and DSM fractions of cholesterol-depleted membranes. (A) Representative immunoblots showing the distribution of serotonin1A receptors, flotillin-1, caveolin-1 and transferrin receptors in cholesterol-depleted membranes and the corresponding DRM and DSM fractions. (B) Contents of serotonin1A receptors, flotillin-1, caveolin-1 and transferrin receptors in DRM (white bars) and DSM (gray bars). Values are expressed as percentages of total protein contents in DRM and DSM fractions. Estimation of proteins was performed by densitometric analysis of respective bands on immunoblots using Bio-2D+software (Bio-Rad). Data represent means ± SE of at least three independent experiments. See Methods for other details.
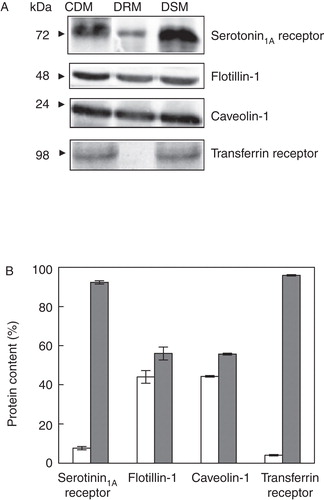
Figure 5. Estimation of lipid contents in DRM and DSM fractions of cholesterol-depleted membranes. (A) Representative immunoblots showing distribution of GM1 in cholesterol-depleted membranes and corresponding DRM fractions. (B) Lipid contents in cholesterol-depleted membranes (black bars) and DRM fractions (white bars). Values are expressed as percentages of lipid contents in cholesterol-depleted membranes, normalized to total protein content of respective membranes. GM1 was estimated by densitometric analysis of dot blots using Bio-2D+ software (Bio-Rad). Data represent means ± SE of at least three independent experiments. See Methods for other details.
Discussion
Insolubility of membrane components in non-ionic detergents such as Triton X-100 at low temperature represents an extensively used biochemical criterion to identify, isolate and characterize certain types of membrane domains (Brown and Rose Citation1992, Hooper Citation1999). Evidence from model membrane studies shows that enrichment with lipids such as sphingolipids (with high melting temperature) and cholesterol serves as an important determinant for the phenomenon of detergent resistance (Schroeder et al. Citation1998). The tight acyl chain packing in cholesterol-sphingolipid-rich membrane regions is thought to confer detergent resistance to membrane regions enriched in these lipids and to the proteins which reside in them. Several GPI-anchored proteins, few transmembrane proteins and certain G-proteins have been found to reside in DRMs (Brown and Rose Citation1992, Chamberlain Citation2004). In spite of reported concerns on the possibility of membrane perturbation due to the use of detergents (Heerklotz Citation2002), resistance to detergent extraction continues to be a principal tool to study membrane domains since the need for relatively simple and straightforward biochemical methods for detecting membrane domains persists. Information obtained from this extensively used biochemical approach can often form the basis for a more detailed analysis of membrane domains utilizing other specialized approaches.
In this work, we monitored the detergent in solubility of the serotonin1A receptor in CHO cell membranes and its modulation by membrane cholesterol. Our results show that a significant fraction (∼ 28%) of the serotonin1A receptor resides in DRMs and this distribution is altered upon membrane cholesterol depletion. In addition, we show that contents of DRM markers such as flotillin-1, caveolin-1 and GM1 are altered in DRMs upon cholesterol depletion. These results gain relevance in the context of our previous results that the function of the serotonin1A receptor is affected by membrane cholesterol and sphingolipids (Pucadyil and Chattopadhyay Citation2004, Paila et al. Citation2008, Citation2010, Shrivastava et al. Citation2010). Our results assume broader significance since membrane organization of the serotonin1A receptor is crucial for a comprehensive understanding of its function (Björk et al. Citation2010; Saxena and Chattopadhyay Citation2011).
We report here the change in the membrane organization of the serotonin1A receptor upon cholesterol depletion. The organization of the serotonin1A receptor under low cholesterol condition is relevant since reduced membrane cholesterol results in manifestation of several physiological effects. For example, it has been previously shown that cholesterol depletion affects sorting (Hansen et al. Citation2000), distribution (Pike and Casey Citation2002), endocytosis (Subtil et al. Citation1999) and trafficking (Pediconi et al. Citation2004) of membrane proteins. Importantly, we recently reported that chronic cholesterol depletion impairs the function of the serotonin1A receptor, which could have important implications in mood disorders (Shrivastava et al. Citation2010).
We have previously reported, utilizing a green fluorescent protein-based microscopic approach, that the detergent insoluble fraction of the serotonin1A receptor in CHO cells exhibits a small increase upon cholesterol depletion using MβCD (Kalipatnapu and Chattopadhyay Citation2005). In the present work, our results show that the detergent insoluble fraction of the serotonin1A receptor is reduced upon membrane cholesterol depletion. Although these results appear contradictory, it can be rationalized as follows. In the case of detergent insolubility using green fluorescent protein-based microscopic approach, the results referred to live cells and cholesterol depletion was carried out in intact cells (Kalipatnapu and Chattopadhyay Citation2005). In the present case, we have biochemically isolated cell membranes and depleted cholesterol from the isolated membranes. A possible reason for this difference could be due to reorganization of membrane cholesterol upon MβCD treatment in live cells which is clearly absent when isolated cell membranes were used for MβCD treatment. We conclude that caution should be exercised in interpreting results of Triton X-100 insolubility experiments, specifically in cases where cholesterol depletion is involved.
Acknowledgements
This work was supported by the Council of Scientific and Industrial Research, Govt. of India. S.K.S. thanks the Department of Biotechnology, Govt. of India for the award of a Postdoctoral Fellowship. R.S. thanks the Council of Scientific and Industrial Research for the award of a Senior Research Fellowship. A.C. gratefully acknowledges J.C. Bose Fellowship (Department of Science and Technology, Govt. of India). A.C. is an Adjunct Professor at the Special Centre for Molecular Medicine of Jawaharlal Nehru University (New Delhi, India) and Indian Institute of Science Education and Research (Mohali, India), and Honorary Professor at the Jawaharlal Nehru Centre for Advanced Scientific Research (Bangalore, India). We thank Md. Jafurulla and Pushpendra Singh for helpful discussions and members of our laboratory for critically reading the manuscript.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Amundson DM, Zhou M. 1999. Fluorometric method for the enzymatic determination of cholesterol. J Biochem Biophys Meth 38:43–52.
- Banerjee P, Joo JB, Buse JT, Dawson G. 1995. Differential solubilization of lipids along with membrane proteins by different classes of detergents. Chem Phys Lipids 77:65–78.
- Björk K, Sjögren B, Svenningsson P. 2010. Regulation of serotonin receptor function in the nervous system by lipid rafts and adaptor proteins. Exp Cell Res 316:1351–1356.
- Brown DA, London E. 1998. Structure and origin of ordered lipid domains in biological membranes. J Membr Biol 164:103–114.
- Brown DA, London E. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem 275:17221–17224.
- Brown DA, Rose JK. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533–544.
- Chamberlain LH. 2004. Detergents as tools for the purification and classification of lipid rafts. FEBS Lett 559:1–5.
- Fishman PH. 1982. Role of membrane ganglioides in the binding and action of bacterial toxins. J Membr Biol 69:85–97.
- Ganguly S, Chattopadhyay A. 2010. Cholesterol depletion mimics the effect of cytoskeletal destabilization on membrane dynamics of the serotonin1A receptor: A zFCS study. Biophys J 99:1397–1407.
- Hajduch E, Turban S, Le Liepvre X, Le Lay S, Lipina C, Dimopoulos N, 2008. Targeting of PKCζ and PKB to caveolin-enriched microdomains represents a crucial step underpinning the disruption in PKB-directed signalling by ceramide. Biochem J 410:369–379.
- Hansen GH, Niels-Christiansen L, Thorsen E, Immerdal L, Danielsen EM. 2000. Cholesterol depletion of enterocytes: Effect on the golgi complex and apical membrane trafficking. J Biol Chem 275:5136–5142.
- Heerklotz H. 2002. Triton promotes domain formation in lipid raft mixtures. Biophys J 83:2693–2701.
- Heilker R, Wolff M, Tautermann CS, Bieler M. 2009. G-protein-coupled receptor-focused drug discovery using a target class platform approach. Drug Discov Today 14:231–240.
- Hooper NM. 1999. Detergent-insoluble glycosphingolipid/cholesterol-rich membrane domains, lipid rafts and caveolae. Mol Membr Biol 16:145–156.
- Jacobson K, Mouritsen OG, Anderson RGW. 2007. Lipid rafts: At a crossroad between cell biology and physics. Nat Cell Biol 9:7–14.
- Kalipatnapu S, Chattopadhyay A. 2004. A GFP fluorescence-based approach to determine detergent insolubility of the human serotonin1A receptor. FEBS Lett 576:455–460.
- Kalipatnapu S, Chattopadhyay A. 2005. Membrane organization of the human serotonin1A receptor monitored by detergent insolubility using GFP fluorescence. Mol Membr Biol 22:539–547.
- Kalipatnapu S, Chattopadhyay A. 2007a. Membrane organization of the serotonin1A receptor monitored by a detergent-free approach. Cell Mol Neurobiol 27:463–474.
- Kalipatnapu S, Chattopadhyay A. 2007b. Membrane organization and function of the serotonin1A receptor. Cell Mol Neurobiol 27:1097–1116.
- Kalipatnapu S, Pucadyil TJ, Harikumar KG, Chattopadhyay A. 2004. Ligand binding characteristics of the human serotonin1A receptor heterologously expressed in CHO cells. Biosci Rep 24:101–115.
- Lingwood D, Simons K. 2010. Lipid rafts as a membrane-organizing principle. Science 327:46–50.
- Mayor S, Maxfield FR. 1995. Insolubility and redistribution of GPI-anchored proteins at the cell surface after detergent treatment. Mol Biol Cell 6:929–944.
- McClare CWF. 1971. An accurate and convenient organic phosphorus assay. Anal Biochem 39:527–530.
- Mukherjee S, Maxfield FR. 2004. Membrane domains. Annu Rev Cell Dev Biol 20:839–866.
- Müller CP, Carey RJ, Huston JP, De Souza Silva MA. 2007. Serotonin and psychostimulant addiction: Focus on 5-HT1A-receptors. Prog Neurobiol 81:133–178.
- Ostrom RS, Insel PA. 2004. The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: Implications for molecular pharmacology. Br J Pharmacol 143:235–245.
- Paila YD, Chattopadhyay A. 2010. Membrane cholesterol in the function and organization of G-protein coupled receptors. Subcell Biochem 51:439–466.
- Paila YD, Ganguly S, Chattopadhyay A. 2010. Metabolic depletion of sphingolipids impairs ligand binding and signaling of human serotonin1A receptors. Biochemistry 49:2389–2397.
- Paila YD, Murty MRVS, Vairamani M, Chattopadhyay A. 2008. Signaling by the human serotonin1A receptor is impaired in cellular model of Smith-Lemli-Opitz syndrome. Biochim Biophys Acta 1778:1508–1516.
- Pediconi MF, Gallegos CE, De Los Santos EB, Barrantes FJ. 2004. Metabolic cholesterol depletion hinders cell-surface trafficking of the nicotinic acetylcholine receptor. Neuroscience 128:239–249.
- Pierce KL, Premont RT, Lefkowitz RJ. 2002. Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3:639–650.
- Pike LJ, Casey L. 2002. Cholesterol levels modulate EGF receptor-mediated signaling by altering receptor function and trafficking. Biochemistry 41:10315–10322.
- Pucadyil TJ, Chattopadhyay A. 2004. Cholesterol modulates the ligand binding and G-protein coupling to serotonin1A receptors from bovine hippocampus. Biochim Biophys Acta 1663:188–200.
- Pucadyil TJ, Chattopadhyay A. 2006. Role of cholesterol in the function and organization of G-protein coupled receptors. Prog Lipid Res 45:295–333.
- Pucadyil TJ, Chattopadhyay A. 2007a. Cholesterol: A potential therapeutic target in Leishmania infection? Trends Parasitol 23:49–53.
- Pucadyil TJ, Chattopadhyay A. 2007b. Cholesterol depletion induces dynamic confinement of the G-protein coupled serotonin1A receptor in the plasma membrane of living cells. Biochim Biophys Acta 1768:655–668.
- Pucadyil TJ, Kalipatnapu S, Chattopadhyay A. 2005. The serotonin1A receptor: A representative member of the serotonin receptor family. Cell Mol Neurobiol 25:553–580.
- Pucadyil TJ, Kalipatnapu S, Harikumar KG, Rangaraj N, Karnik SS, Chattopadhyay A. 2004. G-protein-dependent cell surface dynamics of the human serotonin1A receptor tagged to yellow fluorescent protein. Biochemistry 43:15852–15862.
- Ramstedt B, Slotte JP. 2006. Sphingolipids and the formation of sterol-enriched ordered membrane domains. Biochim Biophys Acta 1758:1945–1956.
- Renner U, Glebov K, Lang T, Papusheva E, Balakrishnan S, Keller B, 2007. Localization of the mouse 5-hydroxytryptamine1Areceptor in lipid microdomains depends on its palmitoylation and is involved in receptor-mediated signaling. Mol Pharmacol 72:502–513.
- Rosenbaum DM, Rasmussen SGF, Kobilka BK. 2009. The structure and function of G protein-coupled receptors. Nature 459:356–363.
- Saxena R, Chattopadhyay A. 2011. Membrane organization and dynamics of the serotonin1A receptor in live cells. J Neurochem 116:726–733.
- Schlyer S, Horuk R. 2006. I want a new drug: G-protein-coupled receptors in drug development. Drug Discov Today 11:481–493.
- Schroeder RJ, Ahmed SN, Zhu Y, London E, Brown DA. 1998. Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J Biol Chem 273:1150–1157.
- Schuck S, Honsho M, Ekroos K, Shevchenko A, Simons K. 2003. Resistance of cell membranes to different detergents. Proc Natl Acad Sci USA 100:5795–5800.
- Shrivastava S, Pucadyil TJ, Paila YD, Ganguly S, Chattopadhyay A. 2010. Chronic cholesterol depletion using statin impairs the function and dynamics of human serotonin1A receptors. Biochemistry 49:5426–5435.
- Sjögren B, Csöregh L, Svenningsson P. 2008. Cholesterol reduction attenuates 5-HT1A receptor-mediated signaling in human primary neuronal cultures. Naunyn-chmiedeberg's Arch Pharmacol 378:441–446.
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, 1985. Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85.
- Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE. 1999. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci USA 96:6775–6780.
- Troost J, Lindenmaier H, Haefeli WE, Weiss J. 2004. Modulation of cellular cholesterol alters P-glycoprotein activity in multidrug-resistant cells. Mol Pharmacol 66:1332–1339.
- Zhang Y, DeVries ME, Skolnick J. 2006. Structure modeling of all identified G protein coupled receptors in the human genome. PLoS Comput Biol 2:88–99.
- Zidovetzki R, Levitan I. 2007. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochim BiophysActa 1768:1311–1324.