Abstract
Routine strategies for the cell-free production of membrane proteins in the presence of detergent micelles and for their efficient co-translational solubilization have been developed. Alternatively, the expression in the presence of rationally designed lipid bilayers becomes interesting in particular for biochemical studies. The synthesized membrane proteins would be directed into a more native-like environment and cell-free expression of transporters, channels or other membrane proteins in the presence of supplied artificial membranes could allow their subsequent functional analysis without any exposure to detergents. In addition, lipid-dependent effects on activity and stability of membrane proteins could systematically be studied. However, in contrast to the generally efficient detergent solubilization, the successful stabilization of membrane proteins with artificial membranes appears to be more difficult. A number of strategies have therefore been explored in order to optimize the co-translational association of membrane proteins with different forms of supplied lipid bilayers including liposomes, bicelles, microsomes or nanodiscs. In this review, we have compiled the current state-of-the-art of this technology and we summarize parameters which have been indicated as important for the co-translational association of cell-free synthesized membrane proteins with supplied membranes.
Keywords::
Introduction
The rapidly increasing number of membrane proteins (MPs) including transporters, G-protein coupled receptors, channels or enzymes having been synthesized by cell-free (CF) expression systems demonstrates the strong potential of this technology in getting fast access to high quality samples of this critical class of proteins (Junge et al. Citation2011). By using the efficient continuous exchange cell-free (CECF) configuration, mg amounts of MPs can be synthesized in reaction volumes of few ml in less than 24 hours. CECF reactions are composed out of two compartments. A reaction mixture (RM) is separated from a feeding mixture (FM) providing a certain supply of low molecular weight precursors by a semi-permeable membrane ensuring efficient exchange in between the two compartments (Spirin et al. Citation1988, Kigawa and Yokoyama Citation1991). In the P-CF (precipitate forming) and D-CF (detergent based) expression modes, the synthesized MPs will be solubilized either post-translationally or co-translationally with detergents (Junge et al. Citation2011). However, several MPs do not fold properly in the artificial detergent environments, their function cannot be analyzed or they are not stable and might become even denatured.
The natural environment of an MP is the lipid bilayer and specific lipid interactions can be required for functional folding (van Dalen et al. Citation2002). Detergents, amphipols or other hydrophobic agents are only able to mimic the hydrophobicity of lipid bilayers, but not its shape, lateral pressure or topo-logy. In the L-CF (lipid based) mode, membranes of defined composition are therefore provided into the RM and the freshly synthesized MPs have the option to co-translationally insert into the lipid bilayers (). However, preparative scale CF systems are mostly devoid of any targeting or translocation machineries which are often required in cells for the efficient MP insertion into natural membranes (Drew et al. Citation2003). Nevertheless, the MP insertion in the artificial CF system could follow different mechanisms which could be modulated by variation of the expression conditions. Several approaches in order to improve the L-CF expression of MPs have been attempted so far. Modifications include the addition of different types of membranes such as microsomes, liposomes of defined compositions, bicelles or nanodiscs as well as the extra supply of chaperones which may improve translocation efficiencies (). Major advantages of L-CF expression could be: (i) The fast preparation of MP samples in membranes of defined composition which could instantly be used for specific applications, (ii) the unidirectional co-translational insertion of MPs into the provided membranes resulting into more homo-genous samples if compared with conventional non-directed in vitro reconstitution approaches resulting into random insertion, (iii) the systematic evaluation of lipid effects on MP structure and function, and (iv) the reconstitution of MPs into artificial membranes without any previous exposure to detergents.
Table I. Overview on L-CF expression approaches for MP production.
Figure 1. L-CF modes for membrane protein production. Lipids are provided in three basic forms, either as liposomes, lipid/detergent mixtures or nanodiscs. Illustrated is the recovery of the lipids together with the synthesized MPs after incubation in either supernatant or precipitated fraction of the reaction.
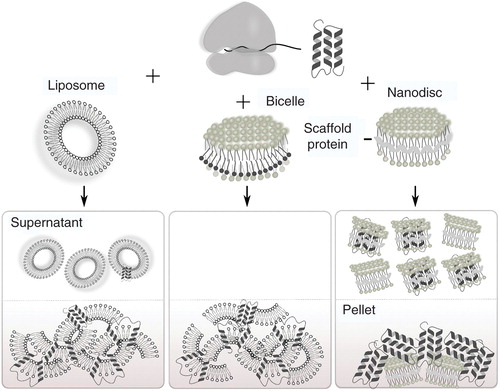
Evidence is accumulating that the co-translational integration mechanisms of the CF-expressed MPs into supplied membranes can be different from those observed in the complex environment of living cells. Concentration and composition of artificial membrane additives can easily be modulated in order to facilitate MP association. Empty membranes as well as in-homogeneities introduced by, e.g., mixing lipids with detergents or by solubilizing small membrane discs with the membrane scaffold protein could enforce MP integration by providing artificial entry sites. L-CF expression approaches can further provide excellent tools in order to study and to identify requirements of MPs for membrane insertion (Guilvout et al. Citation2008). In this article, we summarize the current knowledge on the L-CF production of MPs with special emphases on preparative scale expression using S30 extracts of Escherichia coli cells. We discuss tools and strategies for modulating the co-translational insertion of MPs into artificial membranes and we indicate parameters important for the optimization of L-CF expression approaches.
Getting started: Optimizing preparative scale MP expression
Frequent first bottlenecks in establishing L-CF expression protocols are too low expression yields of the MP target. As the complexity of protein production in CF systems is largely reduced to the basic transcription/translation process, the most common problems of low expression are: (i) Poor purity or quality of the template DNA, (ii) suboptimal Mg2+ ion concentrations in the CF reaction, or (iii) unfavorable design of the DNA template. While the first two critical parameters can be controlled by established routine screening approaches (Kai et al. Citation2012, Chumpolkulwong et al. Citation2006), optimization of the DNA template design requires particular consideration. Either plasmid DNA or linear PCR products can be used as templates in CECF reactions (Wu et al. Citation2007, Yabuki et al. Citation2007). For preparative scale CF expression using E. coli extracts, coupled transcription/translation controlled by T7 regulatory promoter and terminator regions is required (Schneider et al. Citation2010).
DNA templates may be improved by codon optimization implementing synthetic genes and/or by optimizing the initiation of translation upon modifying the 5-prime end of the MP coding region. As excess of tRNAs are present in CF reactions, codon-optimized genes of even eukaryotic targets are usually not mandatory for successful CF expression. However, problems with the initiation of translation are frequent and several strategies for optimization have been developed. The first few codons of mRNAs are crucial for the initiation process as their involvement in secondary structures could increasingly restrict accession to the nearby located ribosomal binding site. Therefore, fusion strategies for the general optimization of mRNA translation are highly successful (Esposito and Chatterjee Citation2006). N-terminal fusions with larger fusion proteins such as thioredoxin (Ishihara et al. Citation2005), peptidyl-prolyl cis-trans iso-merase B (Kralicek et al. Citation2011) or chloramphenicol acetyltransferase (Son et al. Citation2006) were effective in increasing CF protein expression yields.
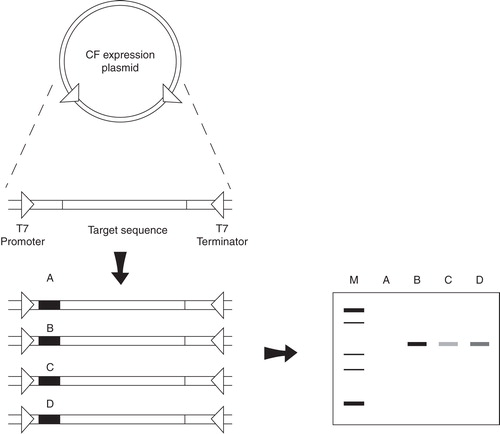
For MPs, smaller tags comprising only few codons such as the T7-tag (Schwarz et al. Citation2007, Junge et al. Citation2008) or OmpA signal sequences (Ahn et al. Citation2007) might be preferred. Small N-terminal tags comprising only few amino acids will have only minimal impacts on MP structure and such modifications can usually be tolerated in further applications, thus avoiding the often inefficient post-translational tag processing by protease cleavage. General rules for expression tags such as a triple A codon at position 1 and a general high AT content have been identified (Qing et al. Citation2003), but their particular effects still remain correlated with the individual coding sequence of the target MP. Therefore, an expression-enhancing tag of universal application can hardly be defined and rather empirical tag variation screens implementing a short list of optimized tags might be considered (Haberstock et al. Citation2012). Such a screen can be performed within few days by multistep PCR overlap protocols followed by CF expression screening of the generated set of linear DNA fragments (). Optimal combinations of expression tags and individual mRNAs can thus be identified with minimal workload as demonstrated for the preparative scale CF production of five G-protein coupled receptors (Haberstock et al. Citation2012).
Figure 2. Tag variation strategy for the optimization of CF expression efficiencies. A set of linear templates containing the MP coding sequence and short N-terminal expression tags (A–D) is generated by overlap PCR and subsequently screened for their CF expression efficiencies. The template having the most efficient expression tag (B) is then used for further preparative scale MP production (Haberstock et al. Citation2012).
Enabling throughput options: Fast monitoring of MP production
Sufficient yield in combination with high quality of the target MP are two key benchmarks which have to be approached by a multiple screening strategy (Junge et al. Citation2011). Fast and efficient monitoring systems would be helpful in order to accelerate protocol development. Derivatives of green fluorescent protein (GFP) have been proposed as expression and folding monitor for MPs synthesized in cellular systems (Drew et al. Citation2006). In MP-GFP fusions, the C-terminal GFP moiety appears to have even sometimes a correlated folding behavior with the attached MP. GFP fusions could thus be used: (i) For monitoring of MP expression rates, (ii) as preliminary folding indication of the attached MP, and (iii) for fast tracking and establishing MP purification protocols.
For the implementation of GFP as monitor for CF MP expression, potential effects of hydrophobic additives on the functional folding of GFP have to be considered. In L-CF mode approaches diverse types of lipids but also detergents could be present if, e.g., bicelles or lipid/detergent mixtures are supplied into the RM in order to optimize the membrane integration efficiency of the synthesized MPs (see paragraph below). The folding of GFP appears to be inhibited by the presence of a number of detergents (Schwarz et al. Citation2010). Almost complete inhibition was observed with many commonly used detergents such as DDM, Digitonin or TritonX-100 and only in the mild detergents Brij-58, Brij-78, Brij-98, in the amphipol Nvoy10 and in decyl-MNG some 30–60% of the fluorescence signal can be recovered while the rest of the GFP fraction still remains in a non-fluorescent conformation (). If artificial solubilization systems composed out of lipid/detergent mixtures should be combined with GFP monitoring, corresponding detergents tolerated by GFP must therefore be selected. Alternatively, the derivative superfolderGFP could be considered (Pedelacq et al. Citation2006). The overall detergent resistance of superfolderGFP is significantly increased and recovery of fluorescence signals exceeded 70% with most analyzed detergents ().
Figure 3. Functional folding of GFP derivatives in presence of detergents. TritonX-100; polyethylene-glycol, P-1,1,2,2-tetramethyl-butylphenyl-ether; Brij-35, polyoxyethylene-(23)-lauryl-ether (C12/23); Brij-58, polyoxyethylene-(20)-cetyl-ether (C16/20); Brij-78, polyoxyethylene-(20)-stearyl-ether (C18/20); Brij-98, polyoxyethylene-(20)-oleyl-ether (C18-1/20); DDM, dodecyl-β-D-maltoside; Lauryl-MNG, 2,2-didecylpropane-1,3-bis-β-D-maltopyranoside; Decyl-MNG, 2,2-dioctylpropane-1,3-bis-β-D-maltopyranoside; C6F-TAC, C6F13C2H4-S-poly[tris(hydroxymethyl)aminomethane; C8F-TAC, C8F17C2H4-S-poly[tris(hydroxymethyl); CHAPS, 3-[(3-Cholamidopropyl)dimethyl-ammonio]-1-propansulfat.
![Figure 3. Functional folding of GFP derivatives in presence of detergents. TritonX-100; polyethylene-glycol, P-1,1,2,2-tetramethyl-butylphenyl-ether; Brij-35, polyoxyethylene-(23)-lauryl-ether (C12/23); Brij-58, polyoxyethylene-(20)-cetyl-ether (C16/20); Brij-78, polyoxyethylene-(20)-stearyl-ether (C18/20); Brij-98, polyoxyethylene-(20)-oleyl-ether (C18-1/20); DDM, dodecyl-β-D-maltoside; Lauryl-MNG, 2,2-didecylpropane-1,3-bis-β-D-maltopyranoside; Decyl-MNG, 2,2-dioctylpropane-1,3-bis-β-D-maltopyranoside; C6F-TAC, C6F13C2H4-S-poly[tris(hydroxymethyl)aminomethane; C8F-TAC, C8F17C2H4-S-poly[tris(hydroxymethyl); CHAPS, 3-[(3-Cholamidopropyl)dimethyl-ammonio]-1-propansulfat.](/cms/asset/a8b8268f-e8c3-40bb-b6df-64bee2905935/imbc_a_693212_f0003_b.jpg)
In contrast to detergents, lipids generally do not affect the folding of GFP. However, some lipids might have negative effects on the protein expression efficiency depending on the supplied concentration. While transcription appears to mostly benefit from the presence of different types of liposomes, the translation was enhanced by anionic and neutral liposomes and inhibited by some cationic liposomes (Umakoshi et al. Citation2009). GFP fluorescence of MP-GFP fusions can certainly only be taken as first indication of MP folding which requires further subsequent verification by specific assays in each individual case. A relatively fast approach is the analysis of L-CF synthesized proteoliposome with confocal microscopes which could indicate the co-localization of the MP-GFP fusion with the liposomes (Moritani et al. Citation2010).
GFP fusions allow the real-time monitoring of soluble MP expression by direct measuring the RM without any purification. Several in vivo studies describe native activities of MPs having N- or C-terminal GFP-fusions, although potential impacts should be analyzed for each individual case (Marshall et al. Citation1995, Chen et al. Citation2000, Beckmann et al. Citation2002, Ban et al. Citation2007, Orban et al. Citation2008). Alternative monitoring strategies independent from protein fusions include radiolabelling with 35S-methionine or the in situ site-specific labeling of expressed MPs with fluorescently labeled amino acids using modified tRNAs as exemplified with bacteriorhodopsin (Ohtsuka et al. Citation2011). While this technique is more complex and might require defined CF systems such as the PURE system, it could also be used for the detection of lipid-associated MPs (Ohtsuka et al. Citation2011).
Approaching defined environments: Implementing artificial liposomes in L-CF reactions
Lipids as MP stabilizing agents have been applied in most known in vitro expression systems like E. coli-based S30 extract in the batch (Hovijitra et al. Citation2009, Chalmeau et al. Citation2011) or the CECF configuration (van Dalen et al. Citation2002), the PURE system (Kuruma et al. Citation2005, Moritani et al. Citation2010), wheat germ-based system (Goren and Fox Citation2008, Nomura et al. Citation2008) or in extracts of rabbit reticulocytes (Falk et al. Citation1997, Joseph et al. Citation1997, Lyford and Rosenberg Citation1999). The efficiencies of MP production are hard to compare as yields among the different CF expression systems can vary within several orders of magnitude. Systems based, e.g., on rabbit reticulocyte lysates (RRL) or the PURE system (Shimizu et al. Citation2001) composed out of purified enzymes of the translation machinery are designed for analytical scale expression purposes only, whereas systems based on robust E. coli S30 extracts are able to synthesize mg amounts of protein in one ml of RM.
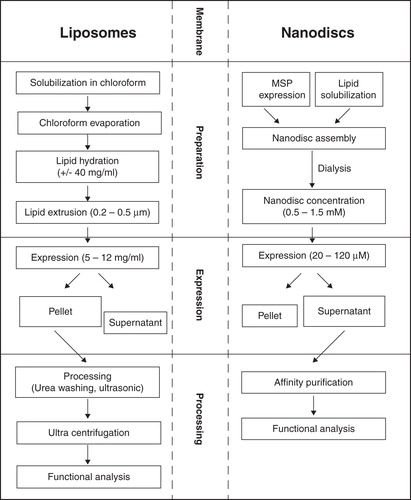
Artificial liposomes may be prepared either out of synthetic lipids or out of membrane fractions obtained from natural sources (). Frequently used are neutral phospholipids such as 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) or 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE) as well as the negatively charged lipids 1,2-dimyristoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (DMPG), 1,2-dioleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (DOPG), 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (POPG) or 1,2-dioleoyl-sn-glycero-3-phosphate (DOPA). Lipid extracts like total lipids, polar lipids or phosphatidylglycerols with different head groups extracted from different organism such as E. coli or soybean are used if more complex mixtures are required. Lipids are most frequently supplied as small unilamellar vesicles (SUV) as they provide optimal dispersion and membrane surface area. SUVs are produced by extrusion of lipid suspensions through membranes with defined pore sizes of 0.2–0.5 μm (). Increasing viscosity limits the initial lipid stock concentration to approximately 40 mg/ml.
Figure 4. Workflow of liposome and ND preparation. Preparation, application and analysis are compared. Liposome stocks hardly exceed 40 mg/ml due to high viscosity, while ND stocks of up to 1.5 mM (for e.g., MSP1 (DMPC) equal to 163 mg lipid/ml) are possible. Proteoliposomes usually precipitate after expression while MP-ND complexes remain mostly soluble.
Final lipid concentrations in CF reactions are therefore usually in between 1 and 10 mg/ml. However, significant rearrangements of the initially uniform liposomes occur during incubation and most of the liposomes are present in the precipitate after the CF reaction (). This observation is made by co-expression of different MPs (Rosenberg and East Citation1992, Berrier et al. Citation2011), but already synthetic liposomes alone without any MP expression tend to fuse and become insoluble during incubation in CF reaction mixtures under experimental conditions. This process could limit the lipid availability already in early states of MP expression and precipitation of artificial liposomes is almost generally observed throughout the different CF expression systems (). Tracking of the supplied lipids might be facilitated by including a fraction of approximately 1% lipids labeled with the fluorescent dye 7-nitro-2-1,3-benzoxadiazol (NBD).
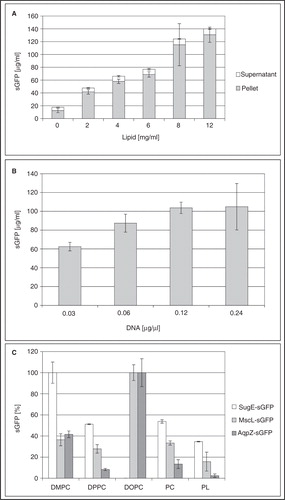
It should be considered that with preparative scale CF expression systems capable of synthesizing mg amounts of MPs in 1 ml or RM, only a minor fraction of the produced protein might associate with the liposomes in a functionally folded conformation while the majority of the protein will precipitate as more or less unfolded aggregate. For protocol development, the association/insertion of the synthesized MPs with the supplied liposomes can be monitored with MP-GFP fusions as exemplified for the L-CF expression of the multidrug transporter SugE (). Primary parameters important for modulation of the L-CF expression efficiencies are concentration and type of the supplied liposomes, DNA template concentration and incubation temperature. Increasing the liposome concentration in E. coli-based CF expression systems results in continuously increased MP-GFP fluorescence, indicating a higher efficiency of MP/liposome complex formation (). Similar results can be obtained by increasing the DNA template concentration in a certain range (). In particular, the type of lipid can have strong impacts on MP/liposome complex formation (). For SugE-sGFP, highest sGFP fluorescence can be obtained with liposomes composed out of the saturated lipid DMPC, while for fusions with the bacterial aquaporin AqpZ-sGFP and for the mechano-sensitive channel MscL-sGFP highest values were achieved with unsaturated DOPC lipids. Lowering expression temperature to, e.g., 25°C could further improve the MP insertion efficiencies, while total MP production rates might considerably be decreased.
Figure 5. Optimization of SugE-sGFP production in the L-CF mode. (A) Effect of DMPC concentration on the expression of SugE-sGFP. (B) Effect of DNA template concentration on SugE-sGFP production in presence of DMPC liposomes (4 mg/ml). (C) Modulation of fluorescent MP-sGFP production by the lipid type (4 mg/ml). Almost all sGFP is detected in the pellet.
Proteoliposomes are usually present as insoluble particles after the CF reaction and may contain in addition a large number of co-precipitated unfolded MP aggregates. For subsequent analysis, urea-washing in order to remove aggregates (Berrier et al. Citation2011) or density gradient ultra-centrifugation is often routinely performed (van Dalen et al. Citation2002, Liguori et al. Citation2008, Hovijitra et al. Citation2009). However, it generally remains hard to determine whether synthesized MPs are integrated into the provided lipid bilayers or whether they are only somehow attached to the surface. A strong evidence of appropriate membrane integration is the recording of MP functionality or of its structural integrity. Characteristic protease K cleavage patterns resulting from cleavage protection of TMS areas by the surrounding lipids are frequently taken as indication of MP integration (Kuruma et al. Citation2005, Wuu and Swartz Citation2008, Moritani et al. Citation2010). Common complementary approaches include freeze-fracture analysis in combination with electron microscopy (Berrier et al. Citation2011) or atomic force microscopy resulting in an image profile of, e.g., α-hemolysin pores embedded in the lipid bilayer (Chalmeau et al. Citation2011). Circular dichroism spectroscopy could indicate correct secondary structure formation of MPs due to lipid bilayer integration (Liguori et al. Citation2010).
Reconstitution with natural systems: L-CF expression with chaperones and microsomes
Artificial liposomes are completely devoid of any translocon or other helper systems. However, successful targeting and insertion of MPs into membranes often requires specific chaperones such as the bacterial SecA/B pathway or the signal recognition particle (SRP) receptor FtsY (de Keyzer et al. Citation2003, Koch et al. Citation2003). The SecA/B pathway is known to stabilize expressed MPs previous to post-translational membrane integration, while FtsY in combination with SRP directs the growing polypeptide chain already during translation towards the lipid bilayer. The translocation into the lipid bilayer is then mediated by the SecYEG-translocon (Muller et al. Citation2001, Luirink et al. Citation2005) under assistance of further proteins (e.g., YidC). Limitations in such chaperones could therefore represent a frequent problem in L-CF expression approaches (Underwood et al. Citation2005). Two strategies, either used alternatively or in combination, could address these potential bottlenecks. First, the addition of selected chaperones into CF reactions could already support translocation events and/or second, applying microsomes representing complex vesicles isolated from natural sources could bear the potential of providing complete translocation machineries that enable efficient MP integration.
The addition of purified FtsY increased the fraction of liposomes-associated MPs and providing Ffh, the protein component of SRP, resulted in formation of functional SRP by complex formation with the 4.5 S RNA from the supplemented tRNA (Kuruma et al. Citation2005, Hovijitra et al. Citation2009). However, the amount of functional folded fully integrated AqpZ into DOPC liposomes was not increased (Hovijitra et al. Citation2009). Accordingly, the insertion of the permease MtlA into synthetic liposomes was not supported by increased copies of FtsY and Ffh (Wuu and Swartz Citation2008). In contrast, the added chaperones increased integration of MtlA into complex E. coli microsomes (Kuruma et al. Citation2005, Nishiyama et al. Citation2006, Wuu and Swartz Citation2008). Lipid A derivatives were therefore proposed as additional insertion factors for MtlA integration (Nishiyama et al. Citation2006). As source of microsomes, E. coli inner membranes (De Vrije et al. Citation1987), canine and bovine pancreas (Awayda et al. Citation1995), thylakoids (Liguori et al. Citation2008) as well as soybean tissues (Goren and Fox Citation2008) have been considered. Interestingly, the above described commonly observed precipitation of artificial liposomes during CF reactions appears to be less pronounced with microsomes (Kuruma et al. Citation2005), although exceptions occur (Lyford and Rosenberg Citation1999, Liguori et al. Citation2008, Berrier et al. Citation2011). Furthermore, some microsome preparations could also inhibit CF reactions. The OmpA precursor protein (pOmpA) already integrated without additional chaperones into supplied microsomes (Kuruma et al. Citation2005). Solubility and MP integration into E. coli INV was further increased by addition of the SecA/B chaperons. Chaperone dependency of membrane integration clearly depends on the individual requirements of MP target. In the presence of SecA/B and/or FtsY, only 5% of the enzyme FtsQ is inserted into E. coli INV, whereas 55% of the expressed pOmpA integrated successfully (Kuruma et al. Citation2005). However, the production of functional MPs both with synthetic liposomes (Hovijitra et al. Citation2009, Moritani et al. Citation2010) and with complex microsomes (Lyford and Rosenberg Citation1999, Wuu and Swartz Citation2008) indicates that lipid bilayers alone can already be sufficient for a functional integration and for supporting the folding of MPs into their functional state (Bogdanov and Dowhan Citation1998, Shanmugavadivu et al. Citation2007). In any case, the chaperone-assisted L-CF expression requires intensive optimization for each single MP.
The L-CF expression of a diverse variety of MPs with α-helical or β-strand topology including channels (Rosenberg and East Citation1992, van Dalen et al. Citation2002, Hovijitra et al. Citation2009, Berrier et al. Citation2011), pores (De Vrije et al. Citation1987, Kuruma et al. Citation2005, Guilvout et al. Citation2008), receptors (Joseph et al. Citation1997) or transporters (Kalmbach et al. Citation2007, Wuu and Swartz Citation2008) has been reported and examples with subsequent quality evaluation are indicated in . A standard technique for verifying MP integration into the provided membranes is freeze fracture analysis by EM. If eukaryotic microsomes have been supplied, the monitoring of glycosylation patterns of integrated MPs could be an additional tool (Rosenberg and East Citation1992, Joseph et al. Citation1997, Lyford and Rosenberg Citation1999). The functionality of L-CF produced proteoliposomes was analyzed with a variety of complementary techniques. Spectral characterization of Rhodobacter capsulatus light-harvesting complex (Troschel and Muller Citation1990) and photo cycle measurements of bacteriorhodopsin (Kalmbach et al. Citation2007) indicated functional folding with properly incorporated co-factors. The black lipid membrane assay was used to demonstrate activity of ion channels such as the ShakerH4 potassium channel (Rosenberg and East Citation1992) or the ligand gated ion-channel nAChR (Lyford and Rosenberg Citation1999). Patch clamp approaches indicated the function of VDAC (Liguori et al. Citation2008) and of the mechanosensitive channel MscL (Berrier et al. Citation2011). Here it was shown that higher speed centrifugation during E. coli cell extract preparation is necessary in order to remove residual endogenous porin contaminations that result in high background signals. The quality of L-CF expressed PulD (Guilvout et al. Citation2008) or Cx43 (Moritani et al. Citation2010) was monitored by permease activity, cross-link experiments as well as by EM or AFM analysis. Transport activity was further shown for the secondary active transporter TetA by taking advantage of the proton gradient formed by the endogenous electron transport chain in the supplied microsomes (Wuu and Swartz Citation2008). Ligand binding was performed for the G-protein coupled receptor β2AR (Kobilka Citation1990) and enzymatic assays demonstrated the quality of the lipid desaturase SCD1 expressed in wheat germ extracts (Goren and Fox Citation2008).
Only few examples have been discussed and additional approaches of quality control are indicated in . In summary, the origin of the implemented cell extract, i.e., the used CF expression system, appears to have only minor overall effects on the quality of L-CF expressed MPs. In contrast, the expression efficiencies can vary in several orders of magnitude within the different systems and prepa-rative scale expression is currently only possible in extracts of E. coli and to some extend in wheat germ extracts. However, high impacts on the quality of the synthesized MPs can be expected from the selected expression conditions and significant time investments in protocol development, e.g., in selecting the appropriate lipid type or the nature and origin of supplied vesicles, must be considered in each individual case.
Artificial systems: Improving MP translocation with lipid/detergent mixtures
Complete or partial solubilization of lipid bilayers by detergents could improve their availability and interaction with synthesized MPs. Corresponding lipid/detergent mixtures are prepared in vitro in fixed ratios () and they can be implemented for the soluble CF expression of MPs in two different approaches. One strategy is to add a certain amount of detergent only into the RM in order to initially solubilize the supplied lipids. During incubation, the detergent will than constantly be diluted out from the RM by diffusion into the FM. The kinetics of dilution will depend on the specific CMC and micelle size of the detergent. Mixtures of lipids and detergents form water-soluble assemblies most likely containing solubilized individual lipids as well as variable patterns and stretches of solubilized bilayers. The first 6 h of CECF reactions are most productive and some 80% of the final MP will be synthesized during that time (Kai et al. Citation2012). The detergent will support the solubilization of the synthesized MPs and could promote their association with the solubilized lipids. During progress of the reaction, the detergent concentration will constantly be reduced and the forming liposomes may include the associated MPs. With this approach, a better folding of bacteriorhodopsin was obtained if initially steroid detergents such as cholate, CHAPS or digitonin have been provided (Shimono et al. Citation2009).
Table II. Preparation of lipid/detergent mixtures for L(D)-CF expression approaches.
Alternatively, the detergent concentration can be kept constant by its addition in RM and FM. Relatively well analyzed are bicelles formed by mixtures of long-chain (e.g., DMPC, C-14) and short-chain (e.g., DHPC, C-6/7) phospholipids and consisting of central lipid discs surrounded by the detergent (Jiang et al. Citation2010). For preparing bicelles, the lipid ratio q (the molar ratio of long- to short-chain lipids or detergent) is crucial for the size of bicelles and need to be optimized for specific MP targets (De Angelis and Opella Citation2007). One of the most important parameters for structural studies is the stability of the samples. MPs in bicelles tend to precipitate with halftimes of some 3–4 days for q = 0.33 bicelles. One reason for this instability could be ester hydrolysis of phospholipids. This can be solved by using the more stable ether-linked analogues of DMPC and DHPC (Ottiger and Bax Citation1999). Another alternative solution is introducing a fraction of negative charged phospholipids, which will increase the repulsion between bicelles and minimize protein aggregation (Poget and Girvin Citation2007). Several MPs like, e.g., ATP synthase were functionally L-CF produced in the presence of bicelles (Uhlemann et al. Citation2012). In addition, recent studies showed that NMR analysis of MPs in bicelles is feasible (Poget and Girvin Citation2007, Dittmer et al. Citation2009, Lyukmanova et al. Citation2012).
Combining technologies: Targeting of MPs into nanodiscs
The emerging technique of using nanodiscs (NDs) for MP stabilization provides a perfect synergy with CF expression. NDs consist of two copies of the membrane scaffold protein (MSP) surrounding a small lipid bilayer disc. The assembly of NDs of different sizes in between 10 and 15 nm and loaded with different lipids has been described (Denisov et al. Citation2004, Grinkova et al. Citation2010). NDs combine all potential benefits of L-CF expression with high solubility of the resulting MP/ND particles. NDs could be supplied as preformed assemblies in which the synthesized MPs can co-translationally integrate. Alternatively, the NDs could be formed directly in the CF reaction by simultaneous expression of the MSP in the presence of lipids (Cappuccio et al. Citation2008). In contrast to the L-CF expression in the presence of liposomes, the NDs are more stable and do not precipitate during the reaction if supplied in sufficient concentrations (Lyukmanova et al. Citation2012) ().
Only recently few but very promising attempts have been started to analyze the CF expression of MPs in the presence of NDs. Functionally folded bacteriorhodopsin could be monitored after co-production of DMPC filled NDs during MP synthesis in a CF expression reaction (Cappuccio et al. Citation2008). Less complex is the addition of preformed empty NDs and the solubilization of a small MP library including bacteriorhodopsin and the small multidrug transporter EmrE could be shown (Katzen et al. Citation2008). Ligand-binding activity of derivatives of the β2AR receptor expressed in the presence of preformed NDs was detectable (Yang et al. Citation2011). CF produced MP/ND complexes could also become suitable for structural studies by using Nuclear Magnetic Resonance (NMR) spectroscopy (Lyukmanova et al. Citation2012). However, more detailed studies on ND-specific integration mechanisms and on the effects of different lipids on the functional folding of MPs are still missing.
Acknowledgements
This work was supported by the Collaborative Research Center (SFB) 807 of the German Research Foundation (DFG). We further thank the European Drug Initiative on Channels and Transporters (EDICT), contract number HEALTH-F4-2007-201924, the European initiative on Structural Biology of Membrane Proteins (SBMP), contract number PITN-GA-2008-211800 and the NIH (grant number U54 GM094608) for funding.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ahn JH, Hwang MY, Lee KH, Choi CY, Kim DM. 2007. Use of signal sequences as an in situ removable sequence element to stimulate protein synthesis in cell-free extracts. Nucleic Acids Res 35:e21.
- Awayda MS, Ismailov I, Berdiev BK, Benos DJ. 1995. A cloned renal epithelial Na+ channel protein displays stretch activation in planar lipid bilayers. Am J Physiol 268:1450–1459.
- Ban H, Inoue M, Griesenbach U, Munkonge F, Chan M, Iida A, 2007. Expression and maturation of Sendai virus vector-derived CFTR protein: Functional and biochemical evidence using a GFP-CFTR fusion protein. Gene Ther 14:1688–1694.
- Bayle D, Weeks D, Sachs G. 1995. The membrane topology of the rat sarcoplasmic and endoplasmic reticulum calcium ATPases by in vitro translation scanning. J Biol Chem 270:25678–25684.
- Beckmann R, Toye AM, Smythe JS, Anstee DJ, Tanner MJ. 2002. An N-terminal GFP tag does not alter the functional expression to the plasma membrane of red cell and kidney anion exchanger (AE1) in mammalian cells. Mol Membr Biol 19:187–200.
- Berrier C, Guilvout I, Bayan N, Park KH, Mesneau A, Chami M, 2011. Coupled cell-free synthesis and lipid vesicle insertion of a functional oligomeric channel MscL MscL does not need the insertase YidC for insertion in vitro. Biochim Biophys Acta 1808:41–46.
- Bogdanov M, Dowhan W. 1998. Phospholipid-assisted protein folding: Phosphatidylethanolamine is required at a late step of the conformational maturation of the polytopic membrane protein lactose permease. EMBO J 17:5255–5264.
- Cappuccio JA, Blanchette CD, Sulchek TA, Arroyo ES, Kralj JM, Hinz AK, 2008. Cell-free co-expression of functional membrane proteins and apolipoprotein, forming soluble nanolipoprotein particles. Mol Cell Proteomics 7:2246–2253.
- Chalmeau J, Monina N, Shin J, Vieu C, Noireaux V. 2011. alpha-Hemolysin pore formation into a supported phospholipid bilayer using cell-free expression. Biochim Biophys Acta 1808:271–278.
- Chen R, Mukhin YV, Garnovskaya MN, Thielen TE, Iijima Y, Huang C, 2000. A functional angiotensin II receptor-GFP fusion protein: Evidence for agonist-dependent nuclear translocation. Am J Physiol Renal Physiol 279:440–448.
- Chumpolkulwong N, Sakamoto K, Hayashi A, Iraha F, Shinya N, Matsuda N, 2006. Translation of ‘rare' codons in a cell-free protein synthesis system from Escherichia coli. J Struct Funct Genomics 7:31–36.
- De Angelis AA, Opella SJ. 2007. Bicelle samples for solid-state NMR of membrane proteins. Nat Protoc 2:2332–2338.
- De Keyzer J, Van Der Does C, Driessen AJ. 2003. The bacterial translocase: A dynamic protein channel complex. Cell Mol Life Sci 60:2034–2052.
- De Vrije T, Tommassen J, De Kruijff B. 1987. Optimal posttranslational translocation of the precursor of PhoE protein across Escherichia coli membrane vesicles requires both ATP and the protonmotive force. Biochim Biophys Acta 900:63–72.
- Denisov IG, Grinkova YV, Lazarides AA, Sligar SG. 2004. Directed self-assembly of monodisperse phospholipid bilayer Nanodiscs with controlled size. J Am Chem Soc 126:3477–3487.
- Dittmer J, Thogersen L, Underhaug J, Bertelsen K, Vosegaard T, Pedersen JM, 2009. Incorporation of antimicrobial peptides into membranes: A combined liquid-state NMR and molecular dynamics study of alamethicin in DMPC/DHPC bicelles. J Phys Chem B 113:6928–6937.
- Drew D, Froderberg L, Baars L, De Gier JW. 2003. Assembly and overexpression of membrane proteins in Escherichia coli. Biochim Biophys Acta 1610:3–10.
- Drew D, Lerch M, Kunji E, Slotboom DJ, De Gier JW. 2006. Optimization of membrane protein overexpression and purification using GFP fusions. Nat Methods 3:303–313.
- Esposito D, Chatterjee DK. 2006. Enhancement of soluble protein expression through the use of fusion tags. Curr Opin Biotechnol 17:353–358.
- Falk MM, Buehler LK, Kumar NM, Gilula NB. 1997. Cell-free synthesis and assembly of connexins into functional gap junction membrane channels. EMBO J 16:2703–2716.
- Goren MA, Fox BG. 2008. Wheat germ cell-free translation, purification, and assembly of a functional human stearoyl-CoA desaturase complex. Protein Expr Purif 62:171–178.
- Grinkova YV, Denisov IG, Sligar SG. 2010. Engineering extended membrane scaffold proteins for self-assembly of soluble nanoscale lipid bilayers. Protein Eng Des Sel 23:843–848.
- Guilvout I, Chami M, Berrier C, Ghazi A, Engel A, Pugsley AP, 2008. In vitro multimerization and membrane insertion of bacterial outer membrane secretin PulD. J Mol Biol 382:13–23.
- Haberstock S, Roos C, Hoevels Y, Dotsch V, Schnapp G, Pautsch A, 2012. A systematic approach to increase the efficiency of membrane protein production in cell-free expression systems. Protein Expr Purif 82:308–316.
- Hovijitra NT, Wuu JJ, Peaker B, Swartz JR. 2009. Cell-free synthesis of functional aquaporin Z in synthetic liposomes. Biotechnol Bioeng 104:40–49.
- Ishihara G, Goto M, Saeki M, Ito K, Hori T, Kigawa T, 2005. Expression of G protein coupled receptors in a cell-free translational system using detergents and thioredoxin-fusion vectors. Protein Expr Purif 41:27–37.
- Jiang Y, Wang H, Kindt JT. 2010. Atomistic simulations of bicelle mixtures. Biophys J 98:2895–2903.
- Joseph SK, Boehning D, Pierson S, Nicchitta CV. 1997. Membrane insertion, glycosylation, and oligomerization of inositol trisphosphate receptors in a cell-free translation system. J Biol Chem 272:1579–1588.
- Junge F, Haberstock S, Roos C, Stefer S, Proverbio D, Dotsch V, 2011. Advances in cell-free protein synthesis for the functional and structural analysis of membrane proteins. N Biotechnol 28:262–271.
- Junge F, Schneider B, Reckel S, Schwarz D, Dotsch V, Bernhard F. 2008. Large-scale production of functional membrane proteins. Cell Mol Life Sci 65:1729–1755.
- Kai L, Roos C, Haberstock S, Proverbio D, Ma Y, Junge F, 2012. Systems for the cell-free synthesis of proteins. Methods Mol Biol 800:201–225.
- Kalmbach R, Chizhov I, Schumacher MC, Friedrich T, Bamberg E, Engelhard M. 2007. Functional cell-free synthesis of a seven helix membrane protein: In situ insertion of bacteriorhodopsin into liposomes. J Mol Biol 371:639–648.
- Katz FN, Rothman JE, Lingappa VR, Blobel G, Lodish HF. 1977. Membrane assembly in vitro: Synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci USA 74:3278–3282.
- Katzen F, Fletcher JE, Yang JP, Kang D, Peterson TC, Cappuccio JA, 2008. Insertion of membrane proteins into discoidal membranes using a cell-free protein expression approach. J Proteome Res 7:3535–3542.
- Kawashima Y, Miyazaki E, Muller M, Tokuda H, Nishiyama K. 2008. Diacylglycerol specifically blocks spontaneous integration of membrane proteins and allows detection of a factor-assisted integration. J Biol Chem 283:24489–24496.
- Kigawa T, Yokoyama S. 1991. A continuous cell-free protein synthesis system for coupled transcription-translation. J Biochem 110:166–168.
- Kobilka BK. 1990. The role of cytosolic and membrane factors in processing of the human beta-2 adrenergic receptor following translocation and glycosylation in a cell-free system. J Biol Chem 265:7610–7618.
- Koch HG, Moser M, Muller M. 2003. Signal recognition particle-dependent protein targeting, universal to all kingdoms of life. Rev Physiol Biochem Pharmacol 146:55–94.
- Kralicek AV, Radjainia M, Mohamad Ali NA, Carraher C, Newcomb RD, Mitra AK. 2011. A PCR-directed cell-free approach to optimize protein expression using diverse fusion tags. Protein Expr Purif 80:117–124.
- Kuruma Y, Nishiyama K, Shimizu Y, Muller M, Ueda T. 2005. Development of a minimal cell-free translation system for the synthesis of presecretory and integral membrane proteins. Biotechnol Prog 21:1243–1251.
- Kuruma Y, Suzuki T, Ueda T. 2010. Production of multi-subunit complexes on liposome through an E. coli cell-free expression system. Methods Mol Biol 607:161–171.
- Liguori L, Blesneac I, Madern D, Vivaudou M, Lenormand JL. 2010. Single-step production of functional OEP24 proteoliposomes. Protein Expr Purif 69:106–111.
- Liguori L, Marques B, Villegas-Mendez A, Rothe R, Lenormand JL. 2008. Liposomes-mediated delivery of pro-apoptotic therapeutic membrane proteins. J Control Release 126:217–227.
- Locker JK, Rose JK, Horzinek MC, Rottier PJ. 1992. Membrane assembly of the triple-spanning coronavirus M protein. Individual transmembrane domains show preferred orientation. J Biol Chem 267:21911–21918.
- Luirink J, Von Heijne G, Houben E, De Gier JW. 2005. Biogenesis of inner membrane proteins in Escherichia coli.. Annu Rev Microbiol 59:329–355.
- Lyford LK, Rosenberg RL. 1999. Cell-free expression and functional reconstitution of homo-oligomeric alpha7 nicotinic acetylcholine receptors into planar lipid bilayers. J Biol Chem 274:25675–25681.
- Lyukmanova EN, Shenkarev ZO, Khabibullina NF, Kopeina GS, Shulepko MA, Paramonov AS, 2012. Lipid-protein nanodiscs for cell-free production of integral membrane proteins in a soluble and folded state: Comparison with detergent micelles, bicelles and liposomes. Biochim Biophys Acta 1818:349–358.
- Ma Y, Munch D, Schneider T, Sahl HG, Bouhss A, Ghoshdastider U, 2011. Preparative scale cell-free production and quality optimization of MraY homologues in different expression modes. J Biol Chem 286:38844–38853.
- Marques B, Liguori L, Paclet MH, Villegas-Mendez A, Rothe R, Morel F, 2007. Liposome-mediated cellular delivery of active gp91(phox). PLoS One 2:e856.
- Marshall J, Molloy R, Moss GW, Howe JR, Hughes TE. 1995. The jellyfish green fluorescent protein: A new tool for studying ion channel expression and function. Neuron 14:211–215.
- Moritani Y, Nomura SM, Morita I, Akiyoshi K. 2010. Direct integration of cell-free-synthesized connexin-43 into liposomes and hemichannel formation. FEBS J 277:3343–3352.
- Muller M, Koch HG, Beck K, Schafer U. 2001. Protein traffic in bacteria: Multiple routes from the ribosome to and across the membrane. Prog Nucleic Acid Res Mol Biol 66:107–157.
- Nagamori S, Vazquez-Ibar JL, Weinglass AB, Kaback HR. 2003. In vitro synthesis of lactose permease to probe the mechanism of membrane insertion and folding. J Biol Chem 278:14820–14826.
- Nishiyama K, Ikegami A, Moser M, Schiltz E, Tokuda H, Muller M. 2006. A derivative of lipid A is involved in signal recognition particle/SecYEG-dependent and -independent membrane integrations. J Biol Chem 281:35667–35676.
- Nomura SM, Kondoh S, Asayama W, Asada A, Nishikawa S, Akiyoshi K. 2008. Direct preparation of giant proteo-liposomes by in vitro membrane protein synthesis. J Biotechnol 133:190–195.
- Nozawa A, Nanamiya H, Miyata T, Linka N, Endo Y, Weber AP, 2007. A cell-free translation and proteoliposome reconstitution system for functional analysis of plant solute transporters. Plant Cell Physiol 48:1815–1820.
- Ohtsuka T, Neki S, Kanai T, Akiyoshi K, Nomura SM, Ohtsuki T. 2011. Synthesis and in situ insertion of a site-specific fluorescently labeled membrane protein into cell-sized liposomes. Anal Biochem 418:97–101.
- Orban TI, Seres L, Ozvegy-Laczka C, Elkind NB, Sarkadi B, Homolya L. 2008. Combined localization and real-time functional studies using a GFP-tagged ABCG2 multidrug transporter. Biochem Biophys Res Commun 367:667–673.
- Ottiger M, Bax A. 1999. Bicelle-based liquid crystals for NMR-measurement of dipolar couplings at acidic and basic pH values. J Biomol NMR 13:187–191.
- Pedelacq JD, Cabantous S, Tran T, Terwilliger TC, Waldo GS. 2006. Engineering and characterization of a superfolder green fluorescent protein. Nat Biotechnol 24:79–88.
- Poget SF, Girvin ME. 2007. Solution NMR of membrane proteins in bilayer mimics: Small is beautiful, but sometimes bigger is better. Biochim Biophys Acta 1768:3098–3106.
- Qing G, Xia B, Inouye M. 2003. Enhancement of translation initiation by A/T-rich sequences downstream of the initiation codon in Escherichia coli.. J Mol Microbiol Biotechnol 6:133–144.
- Ridder AN, Van De Hoef W, Stam J, Kuhn A, De Kruijff B, Killian JA. 2002. Importance of hydrophobic matching for spontaneous insertion of a single-spanning membrane protein. Biochemistry 41:4946–4952.
- Rosenberg RL, East JE. 1992. Cell-free expression of functional Shaker potassium channels. Nature 360:166–169.
- Schneider B, Junge F, Shirokov VA, Durst F, Schwarz D, Dotsch V, 2010. Membrane protein expression in cell-free systems. Methods Mol Biol 601:165–186.
- Schwarz D, Daley D, Beckhaus T, Dotsch V, Bernhard F. 2010. Cell-free expression profiling of E. coli inner membrane proteins. Proteomics 10:1762–1779.
- Schwarz D, Junge F, Durst F, Frolich N, Schneider B, Reckel S, 2007. Preparative scale expression of membrane proteins in Escherichia coli-based continuous exchange cell-free systems. Nat Protoc 2:2945–2957.
- Sen K, Nikaido H. 1991. Trimerization of an in vitro synthesized OmpF porin of Escherichia coli outer membrane. J Biol Chem 266:11295–11300.
- Sevova ES, Goren MA, Schwartz KJ, Hsu FF, Turk J, Fox BG, 2010. Cell-free synthesis and functional characterization of sphingolipid synthases from parasitic trypanosomatid protozoa. J Biol Chem 285:20580–20587.
- Shanmugavadivu B, Apell HJ, Meins T, Zeth K, Kleinschmidt JH. 2007. Correct folding of the beta-barrel of the human membrane protein VDAC requires a lipid bilayer. J Mol Biol 368:66–78.
- Shimizu Y, Inoue A, Tomari Y, Suzuki T, Yokogawa T, Nishikawa K, 2001. Cell-free translation reconstituted with purified components. Nat Biotechnol 19:751–755.
- Shimono K, Goto M, Kikukawa T, Miyauchi S, Shirouzu M, Kamo N, 2009. Production of functional bacteriorhodopsin by an Escherichia coli cell-free protein synthesis system supplemented with steroid detergent and lipid. Protein Sci 18:2160–2171.
- Son JM, Ahn JH, Hwang MY, Park CG, Choi CY, Kim DM. 2006. Enhancing the efficiency of cell-free protein synthesis through the polymerase-chain-reaction-based addition of a translation enhancer sequence and the in situ removal of the extra amino acid residues. Anal Biochem 351:187–192.
- Spirin AS, Baranov VI, Ryabova LA, Ovodov SY, Alakhov YB. 1988. A continuous cell-free translation system capable of producing polypeptides in high yield. Science 242:1162–1164.
- Troschel D, Muller M. 1990. Development of a cell-free system to study the membrane assembly of photosynthetic proteins of Rhodobacter capsulatus. J Cell Biol 111:87–94.
- Uhlemann EM, Pierson HE, Fillingame RH, Dmitriev OY. 2012. Cell-free synthesis of membrane subunits of ATP synthase in phospholipid bicelles: NMR shows subunit a fold similar to the protein in the cell membrane. Protein Sci 21:279–288.
- Umakoshi H, Suga K, Bui HT, Nishida M, Shimanouchi T, Kuboi R. 2009. Charged liposome affects the translation and folding steps of in vitro expression of green fluorescent protein. J Biosci Bioeng 108:450–454.
- Underwood KA, Swartz JR, Puglisi JD. 2005. Quantitative polysome analysis identifies limitations in bacterial cell-free protein synthesis. Biotechnol Bioeng 91:425–435.
- Van Dalen A, Hegger S, Killian JA, De Kruijff B. 2002. Influence of lipids on membrane assembly and stability of the potassium channel KcsA. FEBS Lett 525:33–38.
- Wu PS, Ozawa K, Lim SP, Vasudevan SG, Dixon NE, Otting G. 2007. Cell-free transcription/translation from PCR-amplified DNA for high-throughput NMR studies. Angew Chem Int Ed Engl 46:3356–3358.
- Wuu JJ, Swartz JR. 2008. High yield cell-free production of integral membrane proteins without refolding or detergents. Biochim Biophys Acta 1778:1237–1250.
- Yabuki T, Motoda Y, Hanada K, Nunokawa E, Saito M, Seki E, 2007. A robust two-step PCR method of template DNA production for high-throughput cell-free protein synthesis. J Struct Funct Genomics 8:173–191.
- Yang JP, Cirico T, Katzen F, Peterson TC, Kudlicki W. 2011. Cell-free synthesis of a functional G protein-coupled receptor complexed with nanometer scale bilayer discs. BMC Biotechnol 11:57.
- Zhou X, Baker NK, Arakaki RF. 1993. In vitro translation of the human insulin proreceptor results in N-linked glycosylation without dimer formation. Biochem Biophys Res Commun 192:1453–1459.