Abstract
Protein kinase C (PKC) activation induced by diacylglycerols (DAGs) is one of the sequels of the dysregulation of intramuscular lipid metabolism and is thought to play an important role in the development of insulin resistance (IR). We tested the hypothesis that DAGs with different acyl chains have different biological effects and that DAG species enriched in monounsaturated fatty acids (MUFA) act as better activators of PKC. The experiments were performed in vitro on C2C12 myotubes treated with palmitate (16:0), stearate (18:0) or oleate (18:1) and in vivo on the skeletal muscles of rats fed high-fat (HF), high-tristearin (TS) or high-triolein (TO) diets. To define the importance of endogenously synthesized MUFA on DAG-induced PKCθ activation, we performed experiments on stearoyl-CoA desaturase 1 knockout mice (SCD1-/-) as well. The results show that the content of total DAGs and the levels of saturated DAG species are significantly increased in both insulin-resistant (16:0, HF and TO) and highly insulin-sensitive (18:0 and TS) groups. An increase in MUFA-containing DAGs levels was most constantly related to increase in PKCθ membrane translocation and IR. In the muscles of MUFA-deficient SCD1-/- mice, the DAG content and the induction of PKCθ translocation by the HF diet were significantly reduced. Collectively, our data from both the cell and animal experiments show that DAGs composed of 16:1 and/or 18:1, rather than the levels of total or saturated DAGs, are related to PKCθ membrane translocation. Moreover, our results show that the availability of dietary MUFA and/or the activity of endogenous desaturases play an important role in muscle DAG accumulation.
Introduction
Skeletal muscle insulin resistance (IR) as linked to type 2 diabetes and obesity is among the most common metabolic defects, affecting over 5% of the population in western countries. While the exact pathogenic basis of IR remains poorly defined, it is clear that intramuscular lipid over-accumulation is a critical factor in its etiology. Free fatty acids (FFA), fatty acyl-CoAs, 1,2 diacylglycerols (DAGs) and ceramides are lipid intermediates that are widely believed to be the lipotoxic culprits underlying the association between muscle lipid accumulation and IR. All of these lipid molecules have the potential to reduce insulin-mediated glucose uptake, partially via selective interference with insulin signaling (reviewed in Bosma et al. Citation2012). However, while increased levels of intramyocellular ceramides and FFA are consistently associated with obesity-induced IR, the role of skeletal muscle total DAG accumulation in the development of type 2 diabetes is still unclear.
Intramuscular DAGs are generated during the synthesis and breakdown of triglycerides (TAG) and during the degradation of phospholipids (PL). DAGs and their metabolite phosphatidic acid are important intracellular second messengers involved in metabolic responses, and changes in DAG levels can lead to impaired intracellular signaling (van Blitterswijk and Houssa Citation2000, Chibalin et al. Citation2008). By activating protein kinase C (PKC), DAGs seem to reduce the activity of insulin receptor substrate 1 and thus suppress the activity of its downstream targets phosphatidyl-inositol-3 kinase and Akt, leading to impaired translocation of insulin-induced glucose transporter 4 (GLUT4) and glucose uptake (review in Turban and Hajduch Citation2011). Elevated intramuscular DAG levels have been reported in several animal models of obesity-induced IR (Avignon et al. Citation1996, Schmitz-Peiffer et al. Citation1997) and in diabetic humans (Itani et al. Citation2002). Moreover, lipid infusions in humans have been shown to induce insulin resistance in parallel with increased intramyocellular DAG levels and PKC activation (Itani et al. Citation2002). On the other hand, a number of studies have shown that total DAGs content is not elevated in insulin-resistant muscles of obese subjects (Coen et al. Citation2010, Jocken et al. Citation2010) and in prediabetic men (Perreault et al. Citation2010). Increased levels of DAGs have been reported in lean insulin-sensitive subjects compared with obese volunteers (Jocken et al. Citation2010) and in highly insulin-sensitive skeletal muscles of endurance-trained subjects (Dobrzyn et al. Citation2010, Amati et al. Citation2011). Furthermore, strategies that resulted in improved insulin sensitivity in obese humans, e.g., weight loss (Bajaj et al. Citation2010) and treatment with pioglitazone (Anastasiou et al. Citation2010), did not consistently reduce total DAG levels. Collectively, these results indicate that increased intramuscular total DAG levels per se do not promote IR.
A number of recent studies suggest a role for specific molecular species of DAGs and possibly their subcellular location in the development of IR (Montell et al. Citation2001, Bruce et al. Citation2006, van Hees et al. Citation2011). To date, DAGs enriched in saturated fatty acids have been universally considered to have a negative effect on muscle insulin action (Bergman et al. Citation2009, Citation2010, Citation2012). However, the recent study of Amati et al. (Citation2011) showed that saturated DAG content was significantly higher in highly insulin-sensitive muscles compared with insulin-resistant skeletal muscles. Similar results were observed for DAG species in which one of the fatty acids was unsaturated. In contrast, insulin-resistant obese subjects had elevated DAG species containing monounsaturated fatty acids (MUFA), mainly oleate (18:1) and palmitoleate (16:1), in both positions compared with both subjects of normal weight and endurance-trained subjects (Amati et al. Citation2011). The above data suggest that MUFA-enriched DAGs are most closely related to IR, in accord with our previous studies showing that a deficiency of stearoyl-CoA desaturase 1 (SCD1), the rate-limiting enzyme in 18:1 and 16:1 synthesis, is associated with increased insulin sensitivity in skeletal muscle (Rahman et al. Citation2003, Dobrzyn et al. Citation2005a). Thus, DAGs with different acyl chains might have different biological effects, and DAG species enriched in MUFA might act as better activators of PKC than DAG species enriched in saturated and/or polyunsaturated fatty acids. In the present study, we tested the hypothesis that the availability of MUFA is required for PKCθ membrane translocation induced by lipids in skeletal muscles. We also examined whether dietary and endogenously synthesized 18:1 can stimulate DAGs synthesis and whether this stimulation is linked to IR.
Materials and methods
Animals and diets
Male Wistar rats at 6 weeks of age were placed on a high-fat (HF) (containing 60% calories from fat) (n = 8), high-tristearin (TS) (n = 8) or high-triolein (TO) (n = 8) diet for 8 weeks. TS and TO diets were custom manufactured by supplementing a fat-free basal mix (Harland-Teklad, Madison, WI, USA) with 20% by weight of tristearin or triolein (36% fat calories) (Ssniff, Germany). Dietary fat absorption was assessed as described previously (Sampath et al. Citation2007). The control group was fed the standard chow diet (Ssniff, Germany). Animals were given ad libitum access to food and water. The generation of SCD1-/- mice has been described previously (Miyazaki et al. Citation2001). At 3 weeks of age, pre-bred homozygous (SCD1-/-) and wild-type (SCD1+/+) male mice on a C57Bl/6 background were fed ad libitum a standard laboratory chow diet or a HF diet (60% fat calories) (Harlan-Teklad, Madison, WI) for 12 weeks. At the end of each of the feeding periods, the animals were euthanized, and the red and white sections of the gastrocnemius muscles were extracted, rapidly frozen in liquid nitrogen and stored at −80°C. The experiments were approved by the Ethical Committee for Animal Experiments at the Nencki Institute of Experimental Biology, Polish Academy of Sciences, Warsaw, Poland.
Materials
SCD1, β-actin, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), GLUT4 and PKC-θ antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Akt and pAkt (S473) antibodies were obtained from Cell Signaling (Hertfordshire, UK). Cell culture media and other chemicals were purchased from Sigma (St Louis, MO, USA).
Cell culture and fatty acid treatment
C2C12 skeletal muscle cells were maintained in high-glucose DMEM containing 10% FBS, 1% Pen/Strep mix and 1% L-Glutamine at 37°C in a 5% CO2 humidified atmosphere. To differentiate C2C12 cells into contractile myotubes, the medium was replaced with high-glucose DMEM supplemented with 2% horse serum at 90–100% confluence. All experiments were carried out 7 days after inducing differentiation. Myotubes were treated for 24 h in serum-free medium with 0.5 mM fatty acids (FA), palmitate, stearate or oleate. Control cells were incubated for the same period of time with high-glucose serum-free DMEM supplemented with a corresponding concentration of BSA. The myotubes were stimulated with 100 nM insulin for 10 min to assess their insulin sensitivity.
Subcellular fractionation
Plasma membranes were isolated from both the muscles of experimental animals and FA-treated C2C12 cells by differential centrifugation, as described previously (Schmitz-Peiffer et al. Citation1997). Briefly, muscle samples were extracted in ice-cold homogenizing buffer (20 mM Tris-Cl, pH 7.4; 2 mM EGTA; 2 mM EDTA; 2 mM Na3VO4; 1 mM phenylmethylsulfonyl fluoride (PMSF); 10 mM β-mercaptoethanol and protease and phosphatase inhibitor mix) at 4°C. C2C12 myotubes were scraped into a detergent-free buffer (20 mM Tris HCl, pH 7.4; 0.5 mM EDTA; 0.5 mM EGTA; 10 mM β-mercaptoethanol; 1 mM PMSF; 2 mM Na3VO4 and protease and phosphatase inhibitors) and lysed by passing through a 25 G needle on ice. Cell lysates and muscle homogenates were centrifuged at 100,000 g for 1 h at 4°C, and the supernatant, representing the cytosolic fraction, was saved and stored at −80°C. The pellet, representing the crude membrane fraction, was rinsed twice with ice-cold PBS and resuspended in a buffer containing Triton X-100 at a final concentration of 0.5%. The pellet was disrupted by passing it through a 25 G needle on ice. The membranes were then centrifuged at 18,000 g for 15 min at 4°C in order to remove the insoluble material. The supernatant was preserved in the same manner as the solubilized membrane fraction.
Measurement of lipids
Lipids were extracted from the C2C12 myotubes and muscle samples and quantitated as previously described (Dobrzyn et al. Citation2005b). The lipids extracted from myotubes and muscle tissues were separated by thin layer chromatography on silica gel 60 plates (Merck) in heptane/isopropyl ether/glacial acetic acid (60/40/4, v/v/v) with authentic standards. The bands corresponding to the TAG, DAG, FFA and PL standards were scraped off the plate and transferred to screw-cap glass tubes. FAs were transmethylated in the presence of 14% boron trifluoride in methanol. The resulting methyl esters were extracted with hexane and analyzed by gas-liquid chromatography. Methylpentadecanoic acid was used as an internal standard.
Western blot analysis
Protein levels of SCD1, Akt and the extent of phosphorylation of Akt at Ser473 were determined in clarified C2C12 myotube lysates or crude muscle homogenates using specific antibodies. In brief, C2C12 myotubes were rinsed with ice-cold PBS and solubilized in ice-cold lysis buffer containing 150 mM NaCl, 50 mM Tris (pH 7.4), 1% Triton X-100, 5 mM EDTA, 1 mM PMSF, 10 mM NaF and 2 mM Na3VO4. To enhance solubilization, cells were passed through a 25 G needle on ice, rapidly frozen and thawed. The whole-cell lysates were centrifuged at 12,000 rpm and 4°C for 15 min in order to remove the insoluble material. Samples of the animal tissues were homogenized and centrifuged at 3000 g for 10 min in ice-cold homogenization buffer (20 mM Tris-Cl, pH 7.4; 2 mM EGTA; 2 mM EDTA; 2 mM Na3VO4; 1 mM PMSF; 10 mM β-mercaptoethanol and protease and phosphatase inhibitor mix). Plasma membranes were separated as described above, and GLUT4 and PKCθ levels were determined as described previously (Rahman et al. Citation2003). Prepared samples were loaded onto 10% SDS-PAGE. The separated proteins were transferred to PVDF membranes (Millipore, Billerica, MA, USA), which were then blotted using appropriate antibodies. The proteins were visualized using ECL (Pierce, Rockford, IL, USA) as described by the manufacturer and quantified by densitometry.
Protein content
Protein concentrations were determined using Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA) using BSA as the standard.
Statistical analysis
Results were analyzed using a one-way ANOVA with the Student-Newman post-hoc test. A value of p < 0.05 was considered significant. Values are presented as means ± SD.
Results
16:0 and 18:1 treatment increases total TAG and DAG levels and affects the saturation of neutral lipids in C2C12 myotubes
Total levels of FFA were significantly increased (by ∼ 50%) in 16:0-treated cells and significantly reduced (by ∼ 30%) in both the 18:0- and 18:1-treated groups when compared with the controls (). Treatment with 16:0 resulted in a more than 2-fold increase in total DAG content and an approximately 40-fold increase in TAG in C2C12 myotubes (). Similarly, total levels of DAGs and TAG were significantly increased (by ∼ 70% and more then 25-fold, respectively) in 18:1-treated cells compared with the controls (). In 18:0-treated myotubes, total DAG and TAG levels were also significantly elevated although the changes were not as pronounced as in the 16:0- and 18:1-treated groups (). PL levels were similar in all groups studied ().
Figure 1. Total content (A) and the level of monounsaturated (MUFA) (B) and saturated (SFA) (C) species of free fatty acids (FFA), diacylglycerols (DAGs), triacylglycerols (TAG) and phospholipids (PL) in C2C12 myotubes treated with albumin (BSA – control cells), palmitate (16:0), stearate (18:0) or oleate (18:1). *p < 0.05 vs. BSA-treated cells; # p < 0.05 vs. 16:0-treated cells; & p < 0.05 vs. 18:0-treated cells. The results presented are the average ± SD from three independent experiments.
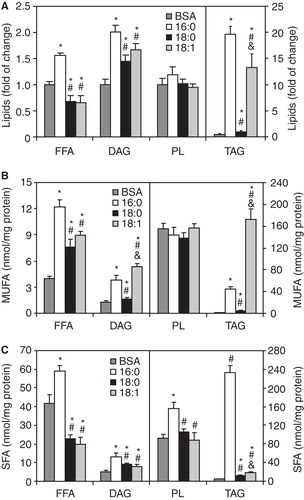
To determine whether overall lipid saturation differed by group, we calculated the sum of species with saturated (SFA) acyl chains (myristate, palmitate, stearate, arachidate, behenate) and monounsaturated (MUFA) acyl chains (palmitoleate, oleate, vaccinate) in FFA, DAGs, TAG and PL. As shown in , the highest concentration of MUFA was found in DAGs and TAG in the 16:0- and 18:1-treated C2C12 myotubes. MUFA contents in FFA were significantly increased in all experimental groups compared with the control (). The levels of SFA acyl species were significantly increased in all analyzed lipid fractions in the 16:0-treated C2C12 cells relative to the control group (). SFA contents in the 18:0- and 18:1-treated groups were decreased in FFA but increased in TAG and DAGs compared with the control ().
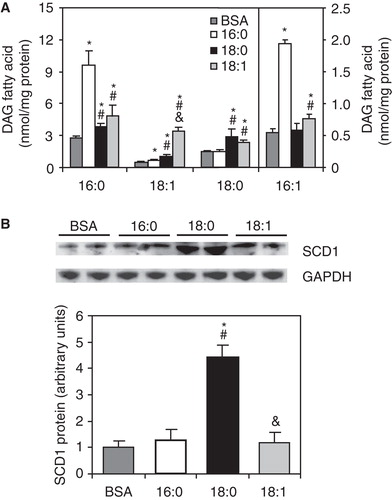
Levels of 16:1- and 18:1-enriched DAG are significantly increased in myotubes treated with high doses of 16:0 and 18:1, respectively
The levels of DAG species that contained 18:1 and 16:1 were increased by more then 6-fold and about 25%, respectively, in 18:1-treated C2C12 myotubes compared with the control cells (). The levels of 16:0- and 18:0-containing DAGs were also significantly higher in myotubes treated with 18:1 (). The levels of DAG species with 16:0 and 16:1 were significantly increased in 16:0-treated C2C12 myotubes compared with the control group (). Treatment with 18:0 resulted in slightly increased levels of DAGs containing 16:0, 18:0 and 18:1 in C2C12 myotubes (). The higher level of 18:1-containing DAG in 18:0-treated C2C12 myotubes was coupled with increased protein levels of SCD1 (). SCD1 protein levels in C2C12 myotubes were not affected by treatment with 16:0 and 18:1 ().
Figure 2. Individual diacylglycerol (DAG) molecular species (A) and protein level of stearoyl-CoA desaturase 1 (SCD1) (B) in C2C12 myotubes treated with palmitate (16:0), stearate (18:0) or oleate (18:1). *p < 0.05 vs. BSA-treated cells; # p < 0.05 vs. 16:0-treated cells; & p < 0.05 vs. 18:0-treated cells. The results presented are the average ± SD from three independent experiments.
PKCθ membrane translocation is significantly increased in C2C12 cells treated with 16:0 and 18:1
To test whether the increased level of MUFA-enriched DAGs was coupled with increased PKC-theta membrane translocation, we analyzed the PKCθ protein levels in the membrane and cytosolic fractions of FA-treated C2C12 myotubes. The level of PKCθ was significantly higher in membranes and significantly reduced in the cytosol of 16:0- and 18:1-treated C2C12 myotubes compared with the control cells (). The ratio of membrane to cytosolic PKC content (mem./cyt. ratio) is a common marker of activation, because PKC is thought to be active in the membrane fraction (Schmitz-Peiffer and Biden Citation2008). The PKCθ mem./cyt. ratio was significantly increased in 16:0- and 18:1-treated cells (by more than 3-fold and 4-fold, respectively) compared with the control group (). Both PKCθ subcellular protein levels and the PKCθ mem./cyt. ratio in C2C12 myotubes treated with 18:0 did not differ from those of the control cells ().
Figure 3. Membrane (mem.) and cytosolic (cyt.) content and cell distribution of PKC-theta (A) and fold of insulin-stimulated Akt phosphorylation change from baseline state (B) in C2C12 myotubes treated with palmitate (16:0), stearate (18:0) or oleate (18:1). *p < 0.05 vs. BSA-treated cells; # p < 0.05 vs. 16:0-treated cells; & p < 0.05 vs. 18:0-treated cells. The results presented are the average ± SD from three independent experiments.
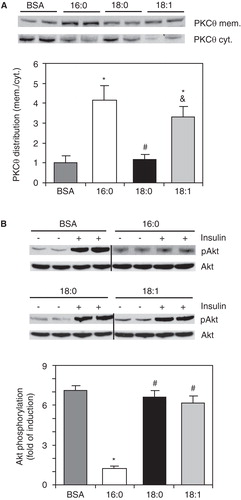
To determine the impact of PKCθ membrane translocation on insulin sensitivity, we analyzed insulin-stimulated phosphorylation of Akt, a central protein of the insulin pathway. As shown in , the fold of insulin-stimulated Akt phosphorylation change from baseline state was significantly reduced (by 83%) in 16:0-treated cells compared with the control group. The increase in phosphorylation of Akt upon insulin stimulation in C2C12 myotubes was not changed in 18:0-treated cells (). Also, the insulin-stimulated pAkt-to-unstimulated pAkt ratio was not significantly different between the control and 18:1-treated groups ().
HF and TO feeding increases MUFA-containing DAG levels in skeletal muscles
To examine whether dietary fats can affect the synthesis and fatty acid composition of DAGs, we fed Wistar rats a high-fat (HF), high-tristearin (TS) or high-triolein (TO) diet. In red gastrocnemius, the total DAG levels were ∼ 50% higher in HF- and TO-fed rats and ∼ 25% higher in TS-fed rats compared with the chow-fed controls (). Each of the experimental diets also triggered increased total DAG accumulation in white sections of gastrocnemius muscle ().
Figure 4. Total content of muscle diacylglycerol (DAG) (A), individual DAG molecular species in red (B) and white (C) sections of gastrocnemius muscle, and muscle stearoyl-CoA desaturase (SCD1) protein levels (D) in rats fed chow, high-fat (HF), high-tristearate (TS) or high-trioleate (TO) diets. *p < 0.05 vs. chow-fed rats; # p < 0.05 vs. HF-fed rats; & p < 0.05 vs. TS-fed rats; n = 8.
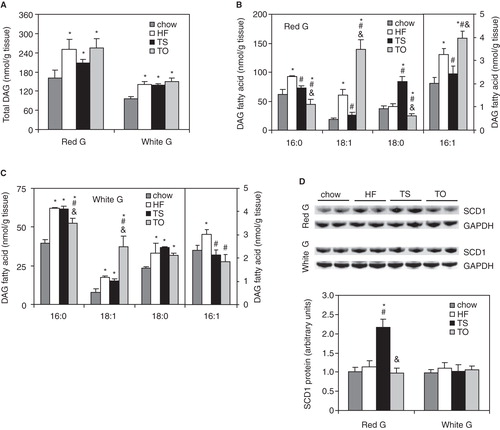
Across the individual DAG species, increased 18:1 contents were found in DAGs of red and white gastrocnemius in HF- and TO-fed groups compared with chow-fed rats (). Additionally, 18:1-enriched DAG levels were increased in white gastrocnemius of TS-fed rats. Levels of DAG containing 16:1 were elevated in HF- and TO-fed rats in red gastrocnemius and in HF-fed rats in white gastrocnemius muscle compared with the chow-fed controls (). Levels of DAG containing 16:0 and 18:0 were increased in white gastrocnemius after HF, TS and TO feeding. In red sections of the muscle, 16:0-DAG levels were elevated only after HF feeding while 18:0-DAG levels were only increased after TS feeding compared with the chow-fed controls ().
In red gastrocnemius, higher levels of 18:1-containing DAG in TS-fed rats were coupled with an increase in SCD1 protein content (). SCD1 protein levels in HF-, TS- and TO-fed rats were not changed in white gastrocnemius when compared with the chow-fed rats ().
PKCθ membrane translocation is increased in the gastrocnemius of HF- and TO-fed rats
PKCθ membrane translocation (mem./cyt. ratio) was significantly higher in the red and white gastrocnemius of HF- and TO-fed rats compared with the chow-fed rats (). TS feeding did not change that ratio in either of the analyzed sections of the gastrocnemius muscle ().
Figure 5. PKC-theta membrane (mem.) and cytosolic (cyt.) content and distribution (A), phosphorylation of Akt (B) and GLUT4 membrane localization (C) in red and white sections of gastrocnemius muscle of rats fed chow, high-fat (HF), high-tristearin (TS) or high-triolein (TO) diets. *p < 0.05 vs. chow-fed rats; # p < 0.05 vs. HF-fed rats; & p < 0.05 vs. TS-fed rats; n = 8.
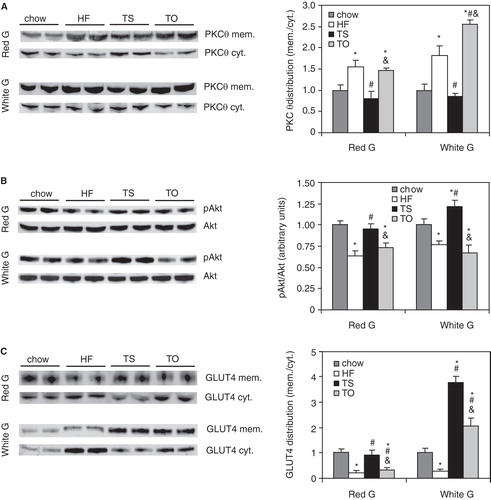
Increased PKCθ membrane translocation in HF- and TO-fed rats was coupled with a reduced basal level of phosphorylation of Akt in both red and white gastrocnemius muscle (). Plasma membrane GLUT4 levels were decreased in the red gastrocnemius of HF- and TO-fed rats compared with the chow-fed controls (). In the white gastrocnemius, TO feeding resulted in increased levels of membrane GLUT4 (). Furthermore, both pAkt and plasma membrane GLUT4 levels were significantly increased in the white gastrocnemius of TS-fed rats compared with the chow-fed controls (). The levels of basal pAkt and membrane GLUT4 were not affected by TS feeding in the red gastrocnemius ().
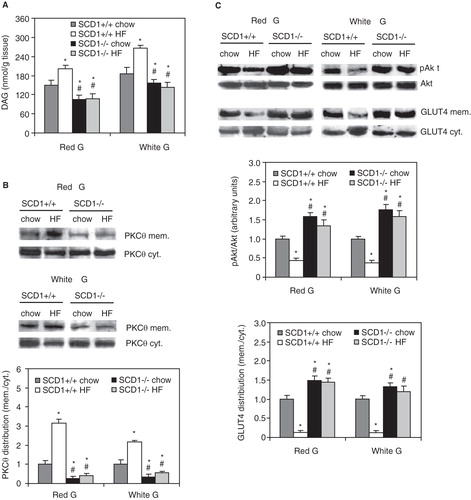
Membrane translocation of PKCθ induced by HF feeding is diminished in the muscles of SCD1-/- mice
The muscles of SCD1-/- mice are deficient in MUFA in all neutral lipid fractions including DAGs (Dobrzyn et al. Citation2005a, Citation2005b). Therefore, we used this animal model to test the hypothesis that MUFA deficiency would reduce PKCθ membrane translocation induced by HF diet. While HF feeding significantly increased both the total DAG level () and the content of MUFA-enriched DAGs (data not shown) in skeletal muscles of wild-type mice, the level of total DAGs was significantly reduced in the red and white gastrocnemius of SCD1-/- mice fed both chow and HF diet compared with the respective SCD1+/+ groups (). In the skeletal muscles of SCD1-/- mice, HF feeding did not result in PKCθ membrane translocation (). Furthermore, the activity of muscle PKCθ, the estimation of which was based on the PKCθ mem./cyt. ratio (Schmitz-Peiffer and Biden Citation2008), was reduced by more than 70% in chow-fed and more than 80% in HF-fed SCD1-/- mice compared with the respective SCD1+/+ controls (). Basal Akt phosphorylation levels and the protein level of GLUT4 in membrane fractions were significantly reduced in the white and red gastrocnemius of SCD1+/+ mice consuming the HF diet (). In SCD1-/- mice, the basal level of pAkt and the level of plasma membrane GLUT4 were significantly increased in both the chow- and HF-fed mice compared with the SCD1+/+ controls, indicating higher insulin sensitivity ().
Figure 6. Total diacylglycerol (DAG) level (A), PKC-theta membrane (mem.) and cytosolic (cyt.) content and distribution (B) and phosphorylation of Akt and GLUT4 membrane localization (C) in red and white sections of gastrocnemius muscle of wild-type (SCD1+/+) and SCD1-deficient (SCD1-/-) mice fed chow or a high-fat (HF) diet. *p < 0.05 vs. SCD1+/+ chow-fed mice; # p < 0.05 vs. SCD1+/+ HF-fed mice; n = 6.
Discussion
While over the last two decades DAGs have commonly been considered to have a negative effect on muscle insulin action, recent studies suggest that it is not the overall concentration of DAGs that is deleterious but that particular DAG moieties may be lipotoxic even in relatively smaller amounts (reviewed in Bosma et al. Citation2012). Consistent with this finding, the total intramuscular DAG concentration was not universally correlated with IR development in this study. We found a positive correlation between total DAG levels and IR in 16:0-treated C2C12 myotubes and skeletal muscles of HF-fed animals. This finding is in agreement with a number of studies reporting increased DAG concentrations in IR related to obesity, aging and type 2 diabetes (Erion and Shulman Citation2010). In contrast, we found increased levels of total DAGs in insulin-sensitive C2C12 myotubes treated with 18:0 and 18:1 and in the red and white gastrocnemius muscles of TS-fed animals. Together with recent findings showing that total DAGs content is significantly increased in insulin-sensitive muscles in athletic humans (Amati et al. Citation2011) and animals (Dobrzyn et al. Citation2010), our data support the notion that total DAG levels cannot explain IR development.
To dissect the effect of individual DAG species on muscle insulin sensitivity, we analyzed the fatty acid profile of DAGs isolated from experimental C2C12 myotubes and skeletal muscles. Consistent with previous investigations (Montell et al. Citation2001, Bruce et al. Citation2006, van Hees et al. Citation2011), IR induced by treatment with 16:0 or the HF diet was associated with increased levels of intramuscular DAGs that contained SFA (mainly 16:0 and 18:0) in our study. However, we also found increased levels of DAG containing 16:0 and 18:0 in highly insulin-sensitive C2C12 cells treated with 18:0 and in the skeletal muscles of TS-fed rats. Thus, our data show that increased levels of DAGs containing SFA are not universally related to IR. This finding is supported by Amati et al. (Citation2011), who found increased levels of saturated DAGs in highly insulin-sensitive muscles of athletes and reduced amounts of saturated DAGs in insulin-resistant muscles of obese people. Current data on the role of DAG saturation in IR development are inconsistent. Some studies have identified DAGs containing SFA to be an important predictor of IR (Bergman et al. Citation2009, Citation2010, Citation2012), other studies indicate that DAGs enriched in unsaturated fatty acids are most directly associated with obesity-induced IR (Amati et al. Citation2011), and still others do not show any correlation between various DAG species and IR (Coen et al. Citation2010, Dubé et al. Citation2011). An important role for 16:1 in insulin sensitivity regulation was proposed by Cao et al. (Citation2008) who showed that adipose tissue-delivered 16:1 strongly stimulates muscle insulin action. Interestingly, in all experimental models with reduced insulin sensitivity, we found significantly increased amounts of MUFA in DAGs. Our data from both cell and animal experiments show that DAG moieties that seem to be most consistently related to lipid-induced reduction in insulin sensitivity are DAG containing 16:1 and/or 18:1. Notably, increased 16:1- and 18:1-enriched DAG species have also been found in insulin-resistant muscles of obese humans (Amati et al. Citation2011).
Because DAG induces insulin resistance by activating PKC in membranes, we hypothesized that an increased level of MUFA-enriched DAGs is followed by increased membrane translocation of PKCθ, the predominant PKC isoform in skeletal muscles (Osada et al. Citation1992). Corroborative data were reported in a recent study demonstrating that increased PKCε activation in the muscles of type 2 diabetic patients is correlated to the amount of MUFA-containing DAGs in plasma membranes (Bergman et al. Citation2012) and that PKCθ activity is increased in the muscles of obese insulin-resistant animals (Marková et al. Citation2010) and 16:0-treated L6 myocytes (Kewalramani et al. Citation2011). In our study, we found a positive correlation between the amount of MUFA-enriched DAGs and protein levels of PKCθ in plasma membranes in both cell and animal experimental models. The PKCθ mem./cyt. ratio was significantly increased in 16:0-treated C2C12 myotubes and in the white and red gastrocnemius of HF- and TO-fed animals. In each of these groups, increased PKCθ membrane distribution was concomitant with either IR or reduced basal phosphorylation of Akt. In MUFA-deficient SCD1-/- mice, the level of membrane PKCθ in skeletal muscles was significantly reduced in both chow- and HF-fed animals. Concomitantly, the muscles of SCD1-/- animals exhibited increased insulin sensitivity and were protected against HF diet-induced IR. These results are in accord with previous studies in which we (Rahman et al. Citation2003, Dobrzyn et al. Citation2005a) and others (Warensjö et al. Citation2007, Bjermo and Risérus Citation2010) showed inverse relationships between SCD activity and insulin sensitivity. Surprisingly, increased PKCθ translocation induced by 18:1 treatment did not significantly affect insulin sensitivity in C2C12 myotubes. The discrepancy between PKCθ activation and IR development in 18:1-treated cells may be due to 18:1-induced changes in membrane properties that affect the interaction between PKC and the insulin receptor (Borkman et al. Citation1993). Other possibilities of this discrepancy are that, unlike 18:1 treatment, HF diets or exposure to 16:0 activate other PKC isoforms such as PKCβ, PKCε and PKCδ (Itani et al. Citation2002), which we did not measure, and/or implicate non-PKC mechanisms involved in IR. These results could also suggest that DAG-induced PKCθ activation per se plays a less important role in lipid-induced inhibition of insulin signaling and that the development of IR may require the involvement of other lipotoxic mediators, such as ceramides or FFA. Future mechanistic studies are needed to further elucidate these findings.
We have shown for the first time that diet-delivered 18:1 partitions into DAGs in skeletal muscles. Also the exposure of C2C12 myotubes (the present study) and human SMC cells (Oram and Bornfeldt Citation2004) to 18:1 was shown to increase 18:1-containing DAG levels. 18:1 may enhance DAG accumulation both by activating phospholipase D and by inhibiting DAG kinase (Askari et al. Citation2002). Skeletal muscle oxidative capacity plays an important role in regulation of lipid metabolism and maintaining insulin sensitivity (Phielix and Mensink Citation2008). We found higher dietary fat-induced DAG accumulation and PKCθ membrane translocation in red gastrocnemius (consisting mainly of oxidative type I fibers) than in white gastrocnemius (consisting mainly of glycolytic type IIB muscle fibers). This finding suggests that lipid-induced DAG accumulation may be fiber-type dependent. Further investigation is needed to confirm this. In addition, complementary observations that both MUFA-enriched DAG accumulation and lipid-induced IR are more pronounced in red gastrocnemius provide insight into muscle heterogeneity in DAG metabolism in insulin resistance. It has been suggested that type I myofibers may have a greater capacity to buffer increases in FFA, mainly 18:1, by partitioning into lipid droplets whereas type II fibers have a reduced capacity to incorporate excess fatty acids into intramuscular TAG (Coen et al. Citation2010). Thus, red gastrocnemius might be more inclined to incorporate MUFA-enriched DAG species. Consistent with this possibility, the effect of HF diets on PKCθ translocation was more pronounced in red than in white gastrocnemius. Our study showed that the 18:1-deficient TS diet increases intramuscular SCD protein content and activity and simultaneously increases both total DAG content as well as 18:1- and 16:1-enriched DAGs levels in skeletal muscles. Although the effect of endogenously synthesized 18:1 on DAG synthesis was not as pronounced as in the case of exogenous 18:1, global SCD1 deficiency resulted in a significant reduction in intramuscular DAGs. Moreover, the lack of SCD1 function protected against HF diet-induced DAG accumulation. Thus, the basal levels of DAGs in muscles might be influenced by both the MUFA content in the diet and the activity of endogenous desaturases. Further studies are needed to address this attractive hypothesis.
Conclusion
The content of total DAGs and the levels of saturated fatty acids-containing DAG species were significantly increased in 16:0-treated myotubes and HF-fed animals in conjunction with IR development. However, increased levels of both total and saturated DAGs were also found in highly insulin-sensitive 18:0-treated cells and in muscles of TS-fed rats. These findings indicate that neither the total DAGs nor SFA-containing DAGs are predictors of IR. In our study, activation of PKCθ was most constantly related to increase in MUFA-containing DAG levels. In the muscles of MUFA-deficient SCD1-/- mice, the DAG content and the induction of PKCθ membrane translocation by the HF diet were significantly diminished. Consistently, SCD1-/- mice exhibited increased insulin sensitivity and were protected from HF diet-induced IR. Collectively, our data show that DAG composed of 16:1 and/or 18:1, rather than the total or saturated DAG levels, are related to PKCθ membrane translocation and IR induced by lipid overload. Furthermore, our findings suggest that dietary MUFA and/or endogenous desaturases play important roles in the regulation of intramuscular DAG levels and activation of PKCθ.
Acknowledgements
This work was supported by Polish Ministry of Science and Higher Education grant no. N N301 0402 36 (to P.D.), EMBO Installation Grant no. 1643 (to A.D.) and Polish Science Foundation grant TEAM/2010-5/2 (to A.D.).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Amati F, Dubé JJ, Alvarez-Carnero E, Edreira MM, Chomentowski P, Coen PM, 2011. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: Another paradox in endurance-trained athletes?. Diabetes 60:2588–2597.
- Anastasiou CA, Kavouras SA, Lentzas Y, Gova A, Sidossis LS, Melidonis A. 2010. Moderate weight loss depletes intramyocellular triglycerides but has no effect on diglycerides in type II diabetes. Eur J Clin Nutr 64:328–330.
- Askari B, Carroll MA, Capparelli M, Kramer F, Gerrity RG, Bornfeldt KE. 2002. Oleate and linoleate enhance the growth-promoting effects of insulin-like growth factor-I through a phospholipase D-dependent pathway in arterial smooth muscle cells. J Biol Chem 277:36338–36344.
- Avignon A, Yamada K, Zhou X, Spencer B, Cardona O, Saba-Siddique S, 1996. Chronic activation of protein kinase C in soleus muscles and other tissues of insulin-resistant type II diabetic Goto-Kakizaki (GK), obese/aged, and obese/Zucker rats. A mechanism for inhibiting glycogen synthesis. Diabetes 45:1396–1404.
- Bajaj M, Baig R, Suraamornkul S, Hardies LJ, Coletta DK, Cline GW, 2010. Effects of pioglitazone on intramyocellular fat metabolism in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 95:1916–1923.
- Bergman BC, Hunerdosse DM, Kerege A, Playdon MC, Perreault L. 2012. Localization and composition of skeletal muscle diacylglycerol predicts insulin resistance in humans. Diabetologia 55:1140–50.
- Bergman BC, Perreault L, Hunerdosse DM, Koehler MC, Samek AM, Eckel RH. 2010. Increased intramuscular lipid synthesis and low saturation relate to insulin sensitivity in endurance-trained athletes. J Appl Physiol 108:1134–1141.
- Bergman BC, Perreault L, Hunerdosse DM, Koehler MC, Samek AM, Eckel RH. 2009. Intramuscular lipid metabolism in the insulin resistance of smoking. Diabetes 58:2220–2227.
- Bjermo H, Risérus U. 2010. Role of hepatic desaturases in obesity-related metabolic disorders. Curr Opin Clin Nutr Metab Care 13:703–708.
- Borkman M, Storlien LH, Pan DA, Jenkins AB, Chisholm DJ, Campbell LV. 1993. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med 328:238–244.
- Bosma M, Kersten S, Hesselink MK, Schrauwen P. 2012. Re-evaluating lipotoxic triggers in skeletal muscle: Relating intramyocellular lipid metabolism to insulin sensitivity. Prog Lipid Res 51:36–49.
- Bruce CR, Thrush AB, Mertz VA, Bezaire V, Chabowski A, Heigenhauser GJ, 2006. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am J Physiol Endocrinol Metab 291:E99–E107.
- Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. 2008. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134:933–944.
- Chibalin AV, Leng Y, Vieira E, Krook A, Björnholm M, Long YC, 2008. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell 132:375–386.
- Coen PM, Dubé JJ, Amati F, Stefanovic-Racic M, Ferrell RE, Toledo FG, 2010. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes 59:80–88.
- Dobrzyn A, Dobrzyn P, Lee SH, Miyazaki M, Cohen P, Asilmaz E, 2005a. Stearoyl-CoA desaturase-1 deficiency reduces ceramide synthesis by downregulating serine palmitoyltransferase and increasing beta-oxidation in skeletal muscle. Am J Physiol Endocrinol Metab 288:E599–E607.
- Dobrzyn A, Dobrzyn P, Miyazaki M, Sampath H, Chu K, Ntambi JM. 2005b. Stearoyl-CoA desaturase 1 deficiency increases CTP:choline cytidylyltransferase translocation into the membrane and enhances phosphatidylcholine synthesis in liver. J Biol Chem 280:23356–23362.
- Dobrzyn P, Pyrkowska A, Jazurek M, Szymanski K, Langfort J, Dobrzyn A. 2010. Endurance training-induced accumulation of muscle triglycerides is coupled to upregulation of stearoyl-CoA desaturase 1. J Appl Physiol 109:1653–1661.
- Dubé JJ, Amati F, Toledo FG, Stefanovic-Racic M, Rossi A, Coen P, 2011. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia 54:1147–1156.
- Erion DM, Shulman GI. 2010. Diacylglycerol-mediated insulin resistance. Nat Med 16:400–402.
- Itani SI, Ruderman NB, Schmieder F, Boden G. 2002. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 51:2005–2011.
- Jocken JW, Moro C, Goossens GH, Hansen D, Mairal A, Hesselink MK, 2010. Skeletal muscle lipase content and activity in obesity and type 2 diabetes. J Clin Endocrinol Metab 95:5449–5453.
- Kewalramani G, Fink LN, Asadi F, Klip A. 2011. Palmitate-activated macrophages confer insulin resistance to muscle cells by a mechanism involving protein kinase C θ and ε. PLoS One 6:e26947.
- Marková I, Zídek V, Musilová A, Simáková M, Mlejnek P, Kazdová L, 2010. Long-term pioglitazone treatment augments insulin sensitivity and PKC-epsilon and PKC-theta activation in skeletal muscles in sucrose fed rats. Physiol Res 59:509–516.
- Miyazaki M, Kim HJ, Man WC, Ntambi JM. 2001. Oleoyl-CoA is the major de novo product of stearoyl-CoA desaturase 1 gene isoform and substrate for the biosynthesis of the Harderian gland 1-alkyl-2,3-diacylglycerol. J Biol Chem 276:39455–39461.
- Montell E, Turini M, Marotta M, Roberts M, Noé V, Ciudad CJ, 2001. DAG accumulation from saturated fatty acids desensitizes insulin stimulation of glucose uptake in muscle cells. Am J Physiol Endocrinol Metab 280:E229–E237.
- Oram JF, Bornfeldt KE. 2004. Direct effects of long-chain non-esterified fatty acids on vascular cells and their relevance to macrovascular complications of diabetes. Front Biosci 9:1240–1253.
- Osada S, Mizuno K, Saido TC, Suzuki K, Kuroki T, Ohno S. 1992. A new member of the protein kinase C family, nPKC theta, predominantly expressed in skeletal muscle. Mol Cell Biol 12:3930–3938.
- Perreault L, Bergman BC, Hunerdosse DM, Eckel RH. 2010. Altered intramuscular lipid metabolism relates to diminished insulin action in men, but not women, in progression to diabetes. Obesity (Silver Spring) 18:2093–2100.
- Phielix E, Mensink M. 2008. Type 2 diabetes mellitus and skeletal muscle metabolic function. Physiol Behav 94:252–258.
- Rahman SM, Dobrzyn A, Dobrzyn P, Lee SH, Miyazaki M, Ntambi JM. 2003. Stearoyl-CoA desaturase 1 deficiency elevates insulin-signaling components and down-regulates protein-tyrosine phosphatase 1B in muscle. Proc Natl Acad Sci USA 100:11110–11115.
- Sampath H, Miyazaki M, Dobrzyn A, Ntambi JM. 2007. Stearoyl-CoA desaturase-1 mediates the pro-lipogenic effects of dietary saturated fat. J Biol Chem 282:2483–2493.
- Schmitz-Peiffer C, Biden TJ. 2008. Protein kinase C function in muscle, liver, and beta-cells and its therapeutic implications for type 2 diabetes. Diabetes 57:1774–1783.
- Schmitz-Peiffer C, Browne CL, Oakes ND, Watkinson A, Chisholm DJ, Kraegen EW, 1997. Alterations in the expression and cellular localization of protein kinase C isozymes epsilon and theta are associated with insulin resistance in skeletal muscle of the high-fat-fed rat. Diabetes 46:169–178.
- Turban S, Hajduch E. 2011. Protein kinase C isoforms: Mediators of reactive lipid metabolites in the development of insulin resistance. FEBS Lett 585:269–274.
- van Blitterswijk WJ, Houssa B. 2000. Properties and functions of diacylglycerol kinases. Cell Signal 12:595–605.
- van Hees AM, Jans A, Hul GB, Roche HM, Saris WH, Blaak EE. 2011. Skeletal muscle fatty acid handling in insulin resistant men. Obesity (Silver Spring) 19:1350–1359.
- Warensjö E, Ingelsson E, Lundmark P, Lannfelt L, Syvänen AC, Vessby B, 2007. Polymorphisms in the SCD1 gene: Associations with body fat distribution and insulin sensitivity. Obesity 15:1732–1740.