Abstract
Water passes through cell membranes relatively slowly by diffusion. In order to maintain water homeostasis, the rapid and specific regulation of cellular water flow is mediated by the aquaporin (AQP) family of membrane protein water channels. The wide range of tissues that are known to express AQPs is reflected by their involvement in many physiological processes and diseases; thirteen human AQPs have been identified to date and the majority are highly specific for water while others show selectivity for water, glycerol and other small solutes. Receptor mediated translocation, via hormone activation, is an established method of AQP regulation, especially for AQP2. There is now an emerging consensus that the rapid and reversible translocation of other AQPs from intracellular vesicles to the plasma membrane, triggered by a range of stimuli, confers altered membrane permeability thereby acting as a regulatory mechanism. This review examines the molecular components that may enable such AQP regulation; these include cytoskeletal proteins, kinases, calcium and retention or localization signals. Current knowledge on the dynamic regulation of sub-cellular AQP translocation in response to a specific trigger is explored in the context of the regulation of cellular water flow.
Introduction
Over 50% of total body water content is found inside cells. The water content of different tissues is diverse and ranges from about 10% in adipocytes to 75% in muscle tissue (Martini and Naith Citation2009). Consequently, homeostasis of the cytosol, interstitial fluid, plasma and other tissues is physiologically essential (Martini and Naith Citation2009). In order to maintain this physiological homeostasis, the control of transcellular water flow must be tightly regulated. In mammals, water can pass into and out of cells by diffusion through the plasma membrane and may also be co-transported passively with other ions or solutes (Loo et al. Citation1996). Neither of these routes permits the rapid, regulated and selective water permeability that is evident in tissues such as kidney and secretory glands. Rather, cellular water flow is mediated by aquaporin water channels (AQPs), which were first discovered in 1993 (Agre et al. Citation1993). Since then, 13 members of this membrane protein family have been identified in humans (AQP0-12), which are distributed throughout a wide range of tissues. For example, AQP1 has been shown to be highly expressed in red blood cells, kidney proximal tubule cells and many other tissues (Magni et al. Citation2006), while AQP11 has been found at low levels in smooth muscle or cells of the immune system (Ishibashi Citation2009). A number of studies have also shown that AQPs play a role in various disorders from renal disease to cerebral oedema (Schrier and Cadnapaphornchai Citation2003, Benarroch Citation2007) and even cancer (Chae et al. Citation2008, Yin et al. Citation2008). Consequently, AQPs have been highlighted as key drug targets (Wang et al. Citation2006, Huber et al. Citation2012), although drugs that specifically target AQPs for clinical use remain to be discovered. This diverse tissue distribution and involvement in many physiological and pathological processes, is summarized in .
Table I. Tissue distribution and functional roles of the 13 human AQPs. The Table summarizes the distribution and function of AQPs in specific cells and tissues of the human body. Immunohistochemistry has revealed the diversity of AQP distribution within cells and tissues. For example, AQPs 1, 2, 3, 4, 6, 7, 8, 9, 10 and 11 have all been shown to be expressed in tissues of the kidney whereas AQP0 is almost exclusively expressed in lens epithelial cells. AQP11 expression in leucocytes may explain the immunohistochemical identification of AQP11 in other tissues.
Structure and function of AQPs
The high resolution structures of several AQPs have been resolved (see Tornroth-Horsefield et al. Citation2010 for a recent review) including the structures of human AQP1, 4 and 5 (Murata et al. Citation2000, Horsefield et al. Citation2008, Ho et al. Citation2009); these studies have revealed that the family shares a conserved architecture (). AQPs exist as homotetramers with four AQP subunits per functional AQP tetramer; selective transport of water molecules, glycerol and/or ions occurs through each of the four pores. The carboxyl and amino termini of an AQP monomer is orientated towards the cytoplasm with six transmembrane α-helices linked by alternating extracellular and intracellular loops (designated A–E). The six α-helices are arranged in a right-handed bundle forming the highly water selective central pore, which resembles an hour-glass (Scheuring et al. Citation2000).
Figure 1. Schematic diagram of the shared structural architecture of aquaporins. Six transmembrane α-helices are connected by alternating extracellular and intracellular loops with the carboxyl and amino termini orientated towards the cytoplasm. Two of these connecting loops (B and E) fold into the transmembrane pore so that the protein resembles an hour-glass. Loops B and E each form a helical region containing highly conserved Asn-Pro-Ala (NPA) motifs that facilitate selectivity of the pore. The approximate positions of serine or threonine protein kinase phosphorylation sites involved in AQP translocation are indicated as follows; ▴ = Ser235 in AQP0; ⧫ = Thr157 and ○ = Thr239 in AQP1; □ = Ser256 in AQP2; = Ser111 and
= Ser180 in AQP4;
= Ser152 in AQP5;
= Ser11 and ▵ = Ser222 in AQP9.
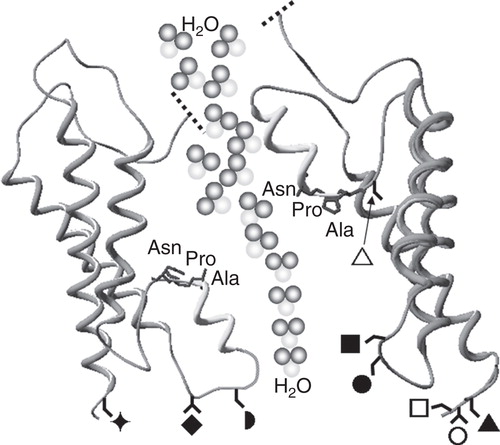
The pore of mammalian AQPs is widely acknowledged to be constitutively open and highly specific (Walz et al. Citation2009). Pore specificity involves two selection stages (de Groot and Grubmuller Citation2001); the first is due to the fact that intracellular loop B and extracellular loop E fold into the transmembrane pore, each forming a helical region containing a highly conserved Asn-Pro-Ala (NPA) motif. Consequently, passage of the majority of ions, such as Na+ is prohibited (Wu et al. Citation2009). The second stage is due to the aromatic/arginine (ar/R) constriction site region and facilitates water selectivity through re-positioning of water molecules and the exclusion of protons (de Groot et al. Citation2001).
AQPs 0, 1, 2, 4, 5 and 8 are thought to be permeable only to water, whilst AQPs 3, 7, 9 and 10 are known as aquaglyceroporins because they are permeable to both water and small non-ionic molecules such as glycerol, urea and ammonia (de Groot and Grubmuller Citation2001, de Groot et al. Citation2003b, Litman et al. Citation2009). AQP6 is highly specific for water but may also be permeable to ionic molecules (Beitz et al. Citation2006). AQPs 11 and 12 are thought to function as water channels but have yet to be fully characterized (Ishibashi Citation2009); notably, the sequence of the NPA motifs within AQP11 and 12 deviates from the conserved NPA of the other AQPs (Itoh et al. Citation2005, Ikeda et al. Citation2010).
It is clear that the structural features of the AQP family and the mechanism of selective water passage through the AQP pore are now established (de Groot and Grubmuller Citation2001, de Groot et al. Citation2003a). However, less information is available describing how AQPs regulate cellular water flow in order to meet the constant and rapid changes in the local cellular environment that challenge them.
What regulates AQP function?
Eukaryotes have evolved to fine-tune water transport through AQPs by three main regulatory mechanisms: (i) At the transcriptional/translational level (Matsumura et al. Citation1997); (ii) by conformational change or ‘gating' (Walz et al. Citation2009); and (iii) by translocation to the membrane in response to a trigger (Nedvetsky et al. Citation2009). Regulation by AQP gene expression can be achieved over a timescale from minutes to days (Gunnarson et al. Citation2004, Zelenina Citation2010). However, a gene expression model for regulation of membrane permeability is contingent upon constitutive membrane expression, which does not account for the established receptor-mediated regulation of AQP2 sub-cellular localization (Nedvetsky et al. Citation2009) or the dynamic control of AQPs that may be necessary to rapidly alter membrane water permeability.
Water permeability through the AQP pore can be inhibited by non-specific, usually mercury-based compounds (Savage and Stroud Citation2007). Alterations in pH and calcium concentration, through interaction with specific histidine residues within the AQP pore, have also been shown to cause different effects on water permeability in bovine and fish AQPs, despite sharing approximately 70% of their sequence (Nemeth-Cahalan et al. Citation2004). Regulation via gating mechanisms, which allow conformationally distinct open and closed states, has specifically been reported for plant and microbial AQPs (Tornroth-Horsefield et al. Citation2006). Structures of gated AQPs have revealed the molecular details of gating by phosphorylation, pH and Ca2+ for the spinach aquaporin, SoPIP2;1 (Johansson et al. Citation1996), and mechanosensivity for the yeast aquaporin, AQY1 (Fischer et al. Citation2009). Direct phosphorylation has also been suggested to cause an increase in water permeability of AQP1 (Han and Patil Citation2000), whereas the water permeability of phosphorylated AQP5 has been suggested to be indistinguishable from that of non-phosphorylated AQP5 (Sidhaye et al. Citation2005, Woo et al. Citation2008). The role of gating in the regulation of mammalian AQP function therefore remains to be conclusively established, but it is not a widely-accepted regulatory mechanism for water homeostasis in mammalian AQPs (Wang and Tajkhorshid Citation2007).
In contrast to regulation by AQP gene expression, and in the absence of an established gating mechanism, a more dynamic, rapid and selective means of regulation could be achieved by translocation of AQPs: The regulation of membrane permeability is possible by altering AQP abundance in response to a specific trigger. and summarize current knowledge of triggers and mechanisms of post-translational AQP translocation. It is possible that the regulation of basal membrane expression following translation and Golgi processing of nascent AQP proteins shares mechanisms with such post-translational, induced subcellular localization.
Table II. Triggers of aquaporin translocation to the plasma membrane (PM). The Table summarizes known stimuli of the dynamic translocation of AQPs, where possible avoiding observations solely linked to water permeability (WP) and/or transcription/translation. The experimental system used to determine the AQP translocation is included for each trigger.
Table III. Intracellular components involved in the translocation of specific aquaporins to the plasma membrane (PM). The Table summarizes the components of dynamic translocation of AQPs avoiding, where possible, observations solely linked to water permeability (WP) and/or transcription/translation. Arrows indicate the direction of the effect. The experimental system used to determine the AQP translocation is included for each trigger and the residue that the component acts upon is also included where known. †Residues in PKC. *AQP2 is not added in more detail than PKA-linked as this has been very well reviewed elsewhere (Valenti et al. Citation2005).
What triggers AQP translocation?
The most well established trigger of post-translational AQP translocation is the G protein-coupled receptor (GPCR) agonist, vasopressin, which induces translocation of AQP2 to promote water reabsorption in the kidney (). AQP2 is retained in sub-cellular compartments and translocation occurs as a result of vasopressin binding and activating the vasopressin V2 GPCR. Subsequently, the G protein, Gs, initiates adenylate cyclase activation leading to increases in cyclic AMP which mediate activation of protein kinase A (PKA) and result in phosphorylation of serine 256 on the C-terminus of AQP2. This facilitates AQP2 translocation to the plasma membrane and water reabsorption in order to maintain osmotic homeostasis (see Valenti et al. Citation2005 for review). More recently, AQP2 has been found to play a role in water homeostasis in the uterus (Aralla et al. Citation2009) and in the inner ear (Nishioka et al. Citation2010).
A number of other GPCR agonists are also thought to regulate AQP translocation events (). For example, early studies demonstrated that secretin, a hormone involved in osmoregulation, promotes osmotic water movement in cholangiocytes by inducing exocytic insertion of AQP1 into the cell membrane (Marinelli et al. Citation1997). Quantitative immunoblotting assays revealed an inverse correlation between the presence of AQP1 at the plasma membrane and within the microsomal intracellular compartment in response to secretin. In the case of AQP3, the beta-adrenergic receptor agonist, isoprenaline, and the adrenergic receptor agonist, adrenaline, have been shown to trigger its translocation in human adipocytes and Caco-2 cells, respectively (Yasui et al. Citation2008, Rodriguez et al. Citation2011b). A decrease in cell water permeability was discovered to be linked to the internalization of AQP4 from the cell surface following histamine stimulation (Carmosino et al. Citation2007). Recently, it was determined that short-term regulation of AQP4 is also mediated by vasopressin (Moeller et al. Citation2009); in the presence of vasopressin, a reduction in water permeability was detected. The water permeability of astrocytes has also been shown to be affected by glutamate activation of metabotropic glutamate receptors that may mediate AQP4 translocation (Gunnarson et al. Citation2008). Understanding how induced translocation affects water permeability of a cell is also likely to be crucial in physiological processes such as maintenance of the blood brain barrier (Saadoun and Papadopoulos Citation2009). Furthermore, the membrane-localization of AQP4 in astrocytes is a physiological requirement for the formation of cytotoxic brain oedema following stroke, blunt-force trauma or meningitis (Papadopoulos and Verkman Citation2007). Finally, AQP5, which has a major role in the generation of saliva, tears and pulmonary secretions, has been shown to translocate to the plasma membrane of rat parotid cells upon stimulation with the M3 muscarinic GPCR agonist, acetylcholine, or the adrenergic receptor agonist, adrenaline (Ishikawa et al. Citation1999). Internalization of AQP5 was observed following prolonged exposure to acetylcholine (Ishikawa et al. Citation1998a, Citation2002). A 4-h stimulation with the agonist, isoprenaline, was also shown to mediate the translocation of AQP7 from the cytoplasm compartment to the plasma membrane (Rodriguez et al. Citation2011b). Vasoactive intestinal polypeptide and lipopolysacharide has been shown to trigger the translocation of AQP5 to the plasma membrane of duodenal Brunner's gland cells (Parvin et al. Citation2005) and mouse lung epithelial cells (Ohinata et al. Citation2005), respectively.
As AQPs are water channels, it is not surprising that water itself might act as a trigger for AQP translocation: A recent study described how immunofluorescent staining revealed cytoplasmic localization of AQP3 and its translocation to the cell membrane of cultured, human keratinocytes following 2-h osmotic stress (Garcia et al. Citation2011). Hypertonicity has also been shown to trigger the translocation of AQPs 1, 2, 3, 4, 5 and 9 (Hoffert et al. Citation2000, Matsuzaki et al. Citation2001, Arima et al. Citation2003, Hasler et al. Citation2005, Loitto et al. Citation2007) whilst hypotonicity has been shown to be involved in translocation of AQPs 1, 3 and 8 (Qi et al. Citation2009, Conner et al. Citation2010, Citation2012, Garcia et al. Citation2011) ().
What are the molecular components of triggered translocation mechanisms?
Many different triggers of AQP translocation have been identified; however, the pathways involved are very diverse. Nonetheless, the mechanisms of AQP translocation share similar characteristics, for example, kinase-mediated phosphorylation (see ). Notably AQP translocation to or from the membrane appears to be the most universal mechanism employed, involving microtubule-dependent vesicle trafficking. It may be that the molecular mechanisms involved in AQP translocation, highlighted below, have evolved to convey diverse triggers or stimuli to the specific translocation of particular AQPs.
Cytoskeletal proteins
Following transcription and translation, several mechanisms are thought to mediate AQP trafficking. AQPs may traffic within vesicles (Garcia et al. Citation2001), often via a microtubule network and sometimes with the involvement of the actin cytoskeleton, whereby re-arrangement of the actin network assists in AQP membrane integration (Riethmuller et al. Citation2008). Once fully integrated into the membrane, newly synthesized AQPs exhibit certain chemical properties. The most noticeable is the clustering of positively charged lysine and arginine residues at the cytoplasmic side of the channel, over the external region. This ‘positive inside rule' assists in facilitating insertion into the membrane (Lerch-Bader et al. Citation2008).
Elements of the mechanisms of post-translational translocation of AQPs may be different from constitutive AQP membrane expression mechanisms, although both actin and microtubules have been implicated in the regulation of the latter. Colchicine-induced disruption of microtubules suggested secretin-induced AQP1 translocation was mediated by the microtubule network (Marinelli et al. Citation1997). The microtubule network has been further implicated in the induced translocation of AQP1 from intracellular compartments to the cell membrane following diverse stimuli (Tietz et al. Citation2006, Conner et al. Citation2010). Studies have determined which proteins and mechanisms regulate AQP1 trafficking in cholangiocytes. Following addition of secretin or activation of messenger cAMP, AQP1 was shown to co-localize with cytoskeletal motor proteins; dynein and kinesin in cholangiocytes. Partial reorganization of actin upon vesicular contact at the apical membrane was thought to facilitate fusion with the cell membrane (Tietz et al. Citation2006).
Similarly to studies on AQPs 1 and 2, inhibitors of the microtubule network and actin microfilaments implicated cytoskeletal proteins in the mechanism of AQP5 trafficking to the plasma membrane (Tada et al. Citation1999). However, trafficking of AQP5 via microtubules (but not actin filaments) has been reported in Madin-Darby canine kidney cells (Karabasil et al. Citation2009). Depolymerization of the F-actin cytoskeleton using cytochalasin-D has also been shown to impair AQP4 plasma membrane localization (Nicchia et al. Citation2008). Application of microtubule inhibitor colchicine was discovered to be sufficient to prevent cAMP stimulated translocation, emphasising reliance of AQP8 translocation on a fully functional microtubule network (Koyama et al. Citation1998).
Calcium
Calcium is ubiquitous in the mechanisms regulating cell signalling and function, so it is not surprising that calcium has been shown to be essential in the mechanism of translocation of AQPs. The role of calcium in trafficking of vesicles containing AQP5 has been demonstrated in human salivary gland cells in which thapsigargin and calcium ionophores induced AQP5 trafficking (Ishikawa et al. Citation1998a). A putative calmodulin binding site at the N-terminus of AQP6 has also been identified (Rabaud et al. Citation2009). Calmodulin binding may have a role in the translocation of AQP6 to the cell surface; it is likely that trafficking of the majority of AQPs is stimulated by calcium elevations (Rabaud et al. Citation2009).
Extracellular calcium influx and calmodulin have been shown to be involved in the hypotonicity-induced translocation of AQP1 to the plama membrane of human embryo kidey cells (HEK) and interestingly, there is a high degree of sequence homology between the AQP1 C-terminus and EF-hands from Ca2+-binding proteins belonging to the calmodulin superfamily (Fotiadis et al. Citation2002).
Protein kinase A
Protein kinases are key regulators of vesicle and protein trafficking mechanisms (Pearce et al. Citation2010). The involvement of PKA phosphorylation in the vasopressin-triggered translocation of AQP2 is well documented (Procino et al. Citation2003, Noda and Sasaki Citation2006) and the AGC group of protein kinases is also involved in the regulation of other AQPs (). For example, putative phosphorylation sites in the C-terminus of AQP0 have been implicated in membrane translocation. Studies involving oocyte swelling assays revealed that truncation of the AQP0 C-terminus at residue 243 resulted in a 15% decrease in water permeability compared to full length AQP0, while surface protein expression analysis showed that impaired water permeability was a result of less efficient membrane trafficking. Truncation of the C-terminus at residue 234 or 238 resulted in completely impaired trafficking but interestingly, mutation of serine 235 to alanine had little effect on water permeability (Ball et al. Citation2003). Serine 235 in the C-terminus of AQP0 has been shown to be located in a PKA consensus sequence. Nuclear magnetic resonance data demonstrated that serine 235 phosphorylation resulted in inhibition of calmodulin binding to AQP0, and this calcium-dependent calmodulin binding to AQP0 resulted in decreased membrane water permeability (Reichow and Gonen Citation2008).
AQP4 is particularly highly expressed in the astrocytic glial cells of the brain with a role in maintaining osmotic potential across the blood brain barrier (Pasantes-Morales and Cruz-Rangel Citation2010). Crucially, AQP4 knock-out models are protected from cytotoxic brain oedema (Manley et al. Citation2000) and AQP4 antibodies are thought to be key to the onset of neuromyelitis optica (Chan et al. Citation2012). Fluorescence localization studies on histamine-mediated AQP4 internalization have shown AQP4 co-localization with the mannose-6-phosphate receptor, a protein that assists in intracellular trafficking of lysosomal digestive enzymes from the Golgi sorting apparatus to the lysosome cell surface. During the internalization period, AQP4 underwent enhanced, specific phosphorylation via PKA; this was thought to be essential for preventing redirection of AQP4 to the lysosomes. Removal of the stimulus enabled free AQP4 translocation from late endosomes back to the basolateral cell surface after a brief time delay (Carmosino et al. Citation2007). To confirm whether a vasopressin-triggered decrease in water permeability (Moeller et al. Citation2009) was due to alterations in a putative gating mechanism or endocytosis of the channel from the plasma membrane, measurements of capacitive currents were performed using a voltage-clamp technique on Xenopus oocytes. The gating possibility was rejected and verification of AQP4 endocytosis was obtained via immunoblotting in combination with immunofluorescence analyses. Contrary to the situation for AQP2, vasopressin was found not to mediate translocation of AQP4 from internal stores to the plasma membrane, but to induce internalization of AQP4 from the cell surface. It was also discovered that vasopressin regulation could be mediated by protein kinase C (PKC), which phosphorylates serine 180 of loop D. This is believed to be the critical residue required for internalization of AQP4. Interestingly, a second serine residue at position 111 was discovered which antagonized the actions of serine 180 as it serves to increase water permeability when activated by phosphorylation (Gunnarson et al. Citation2008).
Using calcium ionophores to stimulate AQP5 plasma membrane localization, it was deduced that activation of M3 muscarinic receptors by acetylcholine, leads to a signalling cascade occurring via intermediates, such as inositol 1,4,5-trisphosphate, triggering release of calcium from intracellular stores (Ishikawa et al. Citation1998b). Dephosphorylation of a PKA consensus sequence starting at serine residue 152 increased translocation of AQP5 to the membrane of Madin-Darby canine kidney cells (Karabasil et al. Citation2009). Although serine 156 of AQP5 has been shown to be phosphorylated in human bronchial epithelial cells, AQP5 targeting to the membrane may require additional mechanisms besides cAMP-dependent PKA phosphorylation (Woo et al. Citation2008). Constitutive membrane localization of an AQP5-C-terminal GFP chimera in Madin-Darby canine kidney cells has also been observed, whereas an AQP5-N-terminal GFP chimera was localized in intracellular vesicles. Translocation of the AQP5-N-terminal GFP chimera in response to PKA activation was independent of phosphorylation of the putative PKA phosphorylation residue, threonine 259 (Kosugi-Tanaka et al. Citation2006). This suggests that the PKA phosphorylation of the C-terminus may not be involved in AQP5 translocation; however, AQP5 has been shown to be translocated to the plasma membrane in a PKA-dependent fashion in duodenal Brunner's gland tissue (Parvin et al. Citation2005). The PKA pathway has also been shown to be involved in AQP5 regulation through cAMP-induced AQP5 translocation to the plasma membrane of murine lung epithelial cells (Yang et al. Citation2003).
AQP6 is thought to be retained within intracellular vesicles and recent studies have focused on identifying the mechanism and function of AQP6 retention in intracellular stores (Beitz et al. Citation2006). Localization studies on AQP6-GFP chimeras have revealed that residues at the N-terminal sequence of the protein are necessary and sufficient for cytosolic retention. Substitution of the N-terminal sequence on AQP1 for that of AQP6 resulted in intracellular retention of the AQP1/AQP6-N-terminus chimera. A putative AQP6 C-terminal PKA phosphorylation site was also identified but activation studies failed to demonstrate a significant role of this site in AQP6 localization. Interestingly, continuous expression of AQP6 at the cell surface led to apoptosis of the cell (Beitz et al. Citation2006).
AQP8 is expressed in a number of tissues but noted for its role in the colon (Liu et al. Citation2011). The expression and distribution of AQP8 in amnion epithelial cells were regulated by osmotic loads, suggesting a role for AQP8 in intramembranous water transport and the balance of amniotic fluid (Qi et al. Citation2009). AQP8 expression may also be stimulated to increase at the cell surface by cAMP in hepatocytes; cells that normally exhibit low water permeability in their resting state due to intracellular sequestration of AQP8 (Garcia et al. Citation2001). Evidence for this was provided through labelling experiments, which showed an inverse correlation between AQP8 numbers located within microsomes and those on the plasma membrane, with an increase in the latter following stimulation by cAMP (Koyama et al. Citation1998).
Although AQP8 shares conserved NPA sequences and 6 transmembrane architecture with other members of the aquaporin family (Koyama et al. Citation1998), it is interesting to note that despite cAMP-induced activation, AQP8 lacked the conserved regions required for phosphorylation via a PKA- or PKC-mediated mechanism. The significance of this points towards the possibility of an intermediate protein that is independent of the PKA pathway being involved in phosphorylation of AQP8.
Protein kinase C
AQP1 was the first AQP identified (Agre et al. Citation1993) and has a diverse tissue distribution; including expression in red blood cells, kidney proximal tubule cells and the bile duct. Early studies suggested that AQP1 was constitutively expressed in cell membranes in the kidney (Knepper et al. Citation1996); however, recent studies have shown that AQP1 is expressed in both the cytoplasm and in the membrane and can be induced to undergo rapid and reversible translocation to the plasma membrane upon hypotonic stimulation mediated by PKC and microtubules (Conner et al. Citation2010). This dynamic mechanism of AQP1 translocation in human embryo kidney cells involves extracellular calcium influx through stretch activated transient receptor potential channels and calmodulin-mediated PKC phosphorylation of two specific threonine residues at positions 157 and 239 (Conner et al. Citation2012). These residues of AQP1 have previously been shown to be phosphorylated by PKC. Furthermore, stimulation of PKC resulted in an increase of AQP1-dependent water permeability and this was reduced in AQP1 mutants lacking either threonine 157 or threonine 239 and abolished in an AQP1 mutant lacking both threonine residues (Zhang et al. Citation2007).
AQP0 is the major protein in the membrane of the lens and has a role in cataract formation. Inhibition of PKC or mutation of serine 235 to alanine resulted in retention of AQP0, but not AQP1, in the cytoplasm suggesting that PKC phosphorylation of serine 235, following translation and ER/Golgi sorting, mediates specific AQP0 targeting to the cell surface (Golestaneh et al. Citation2008).
The biochemical analysis of epinephrine-induced AQP3-translocation after 60 min in Caco-2 colonic epithelia cells has been described. The use of inhibitors and activators for phospholipase C and PKC has suggested involvement of the PKC pathway (Yasui et al. Citation2008). Threonine at position 514 in PKC was found to be phosphorylated with time upon the treatment of the cells with epinephrine while the phosphorylation of serine 660 in PKC was shown significantly following 60 min of the treatment. This suggests epinephrine mediated post-translational modification of PKC threonine 514 may act as a signal for translocation of AQP3 to the basolateral domain, and that perhaps phosphorylation of PKC serine 660 activates the process of AQP3 recycling.
AQP9 has greater sequence similarity to AQP3 and AQP7 than other AQPs and it has been suggested that in leukocytes AQP9 may be selective for water and urea but not glycerol (Ishibashi et al. Citation1998). It is thought to be involved in glycerol metabolism in the liver and adipocytes (Maeda et al. Citation2009). Potential AQP9 PKC binding or phosphorylation sites have been identified as serine residues 11 and 222 (Loitto et al. Citation2007). AQP9 has also been shown to be phosphorylated in human neutrophils. Expression of AQP9 or a phosphomimetic mutation of the putative PKC phosphorylation site, serine 11, to aspartate dynamically localized to the plasma membrane and changed cell volume regulation as a response to hyperosmotic changes. However when serine 11 was mutated to alanine to create a phosphorylation deficient mutant, AQP9 failed to localize to the plasma membrane. AQP9 translocation to the membrane was also shown to be regulated by the G protein Rac1 (Karlsson et al. Citation2011).
Protein kinase G
The use of specific inhibitors has revealed that the mechanism of acetylcholine-induced AQP5 translocation is mediated by cGMP, calmodulin and protein kinase G (Ishikawa et al. Citation2002).
Phosphatidylinositol 3-kinase
The aquaglyceroporin, AQP7, is responsible (with AQP3) for the majority of glycerol transport across the membranes of adipose tissue (Rodriguez et al. Citation2011a) and signalling pathways, including phosphatidylinositol 3-kinase pathway, have been associated with the regulation of aquaglyceroporin transcription (Kishida et al. Citation2001). Evidence for negative feedback regulation in lipolytic states to restrict glycerol release from fat cells by restricting AQP7 expression comes from treatment of cells with leptin, isoprenaline and carboxymethyl chitin (Rodriguez et al. Citation2011a). It is also thought that insulin regulates AQP7 levels, but the evidence is contradictory and there may be divergent effects of insulin on the regulation of all aquaglyceroporins in humans compared to rodents (Kishida et al. Citation2001, Rodriguez et al. Citation2011b). Whilst it has been proposed that PKA may be involved in the translocation of AQP7 (Kishida et al. Citation2000), experimental evidence for such a process is currently lacking.
Localization and retention signals
The AQP0 mouse model CatFr synthesises dysfunctional AQP0; AQP0-LTR in which LTR is translated into a peptide and causes retention of AQP0-LTR in sub-cellular compartments of lens epithelial cells producing cataracts (Kalman et al. Citation2006).
AQP3, an aquaglyceroporin, is a water and glycerol transporter, generally thought to be expressed in the basolateral membrane of cells. This has been demonstrated for AQP3-rich, polarized epithelial (Zhang et al. Citation2011) and kidney collecting duct cells (Langaa et al. Citation2012). Analysis of the AQP3 protein sequence revealed the presence of a conserved localization Tyr-Arg-Leu-Leu (YRLL) motif at the N-terminus. Mutations within this YRLL motif of either tyrosine or the di-leucine residues alone led to partial disruption of the protein translocation mechanism in vitro in polarized Madin-Darby canine kidney type II cells, whereas a complete mutation of the YRLL motif caused the complete absence of protein trafficking. The combined presence of the tyrosine, together with the di-leucine residues is essential for proper localization at the basolateral domain (Rai et al. Citation2006). Chimeric proteins involving substitution of the AQP2 N-terminus for that of the AQP3 were able to re-direct localization of AQP2 from its original site at the apical membrane to its new site at the basolateral membrane. Interestingly, despite this clear amino acid-sorting motif, membrane expression of AQP3 is not polarized in red blood cells (Roudier et al. Citation1998), epidermal keratinocytes or epidermis basal cells; AQP3 may be predominantly intracellular (Sougrat et al. Citation2002).
Systematic deletion analysis of C-terminal residues of AQP4 identified a region necessary for correct localization of AQP4 in epithelial cells (Madrid et al. Citation2001). Two separate localization signals have been identified within the C-terminal tail; a specific sequence containing tyrosine 277 and its adjacent glycine residue was associated with correct localization of AQP4 to the basolateral membrane. The second region consisted of a combined acidic cluster Glu-Thr-Glu-Asp (ETED) and a Leu-Ile-Leu (LIL) sequence motif, causing reduced localization at the apical domain. The tyrosine sorting signal Try-Met-Glu-Val (WMEV) doubles its function as a mediator of endocytosis via clathrin-coated vesicles.
AQP11 and 12 appear to lack the conserved NPA motifs, being NPC and NPT in AQPs 11 (Ikeda et al. Citation2010) and 12 (Itoh et al. Citation2005), respectively. Interestingly mutation of the alanine of the AQP4 NPA motif to threonine to mimic AQP12 resulted in retention within the endoplasmic reticulum of mammalian cell lines, whereas mutation of this alanine to cysteine, mimicking AQP11, expresses normally in the plasma membrane (Guan et al. Citation2010).
Conclusions: Implications for disease therapies
The ability of cells to adapt and respond to diverse stimuli, triggers or changes in their environment is a requirement for maintaining water homeostasis; one of the key mechanisms enabling this is the translocation of AQPs. This review has highlighted that the mechanisms leading to AQP translocation are varied but share certain characteristics (); some or all of the components shown may be involved in the translocation of different AQPs. For example, a specific trigger, which may be hormonal or a change in tonicity, leads to a cascade culminating in calmodulin-mediated phosphorylation of specific AQP residues. This results in microtubule-dependent AQP translocation.
Figure 2. Trigger-induced aquaporin translocation: a regulatory mechanism for cellular water flow. Some or all of the components shown may be involved in the translocation of different AQPs. For example, AQP1 translocation is known to be triggered by hypotonicity, which causes calcium influx through transient receptor potential (TRP) channels and subsequent calmodulin-mediated PKC phosphorylation of specific AQP1 threonine residues, resulting in microtubule-dependent AQP1 translocation. Other protein kinases are thought to be involved in AQP translocation such as PKA-mediated translocation of AQP2 and AQP5 following activation of vasopressin V2 and M3 muscarinic GPCRs, respectively.
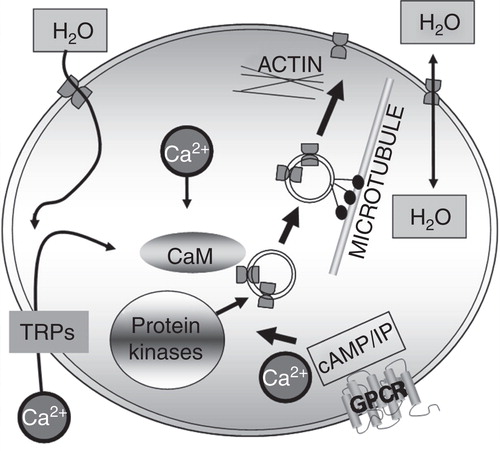
It is clear that the rapid, regulation of sub-cellular localization of AQPs can be targeted to specific areas of the cell in order to increase water, ion or small molecule permeability. Manipulation of AQP abundance may therefore provide novel methods for treating specific diseases. For example, AQP4 knockout mice show an increased protection and reduced accumulation of water in the brain in models of ischemic stroke, cerebral injury and meningitis (Manley et al. Citation2000, Papadopoulos and Verkman Citation2005). The opposite is the case for hydrocephalus and vasogenic oedema, where AQP4 null mice show greater water accumulation in the brain and a worse clinical outcome (Papadopoulos et al. Citation2004). AQP5 is expressed in the ciliated epithelia that line the upper airways and is involved in near-isomolar fluid secretion by the epithelium of airway submucosal glands (Verkman Citation2003). Thus, the manipulation of AQP4 and AQP5 translocation and subsequent subcellular localization appears to be a potential target for the development of novel drugs for cerebral oedema and cystic fibrosis (Song and Verkman Citation2001, Zador et al. Citation2009).
AQPs are an extremely important group of proteins and their role in diverse disease states is becoming more evident. Identification of the stimuli and mechanisms of dynamic-induced translocation may offer a new avenue for drug targets and eventual disease therapy.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Agre P, Preston GM, Smith BL, Jung JS, Raina S, Moon C, 1993. Aquaporin CHIP: The archetypal molecular water channel. Am J Physiol 265:F463–F476.
- Aralla M, Borromeo V, Groppetti D, Secchi C, Cremonesi F, Arrighi S. 2009. A collaboration of aquaporins handles water transport in relation to the estrous cycle in the bitch uterus. Theriogenology 72:310–321.
- Arima H, Yamamoto N, Sobue K, Umenishi F, Tada T, Katsuya H, 2003. Hyperosmolar mannitol simulates expression of aquaporins 4 and 9 through a p38 mitogen-activated protein kinase-dependent pathway in rat astrocytes. J Biol Chem 278:44525–44534.
- Ball LE, Little M, Nowak MW, Garland DL, Crouch RK, Schey KL. 2003. Water permeability of C-terminally truncated aquaporin 0 (AQP0 1-243) observed in the aging human lens. Invest Ophthalmol Vis Sci 44:4820–4828.
- Beitz E, Liu K, Ikeda M, Guggino WB, Agre P, Yasui M. 2006. Determinants of AQP6 trafficking to intracellular sites versus the plasma membrane in transfected mammalian cells. Biol Cell 98:101–109.
- Benarroch EE. 2007. Aquaporin-4, homeostasis, and neurologic disease. Neurology 69:2266–2268.
- Carmosino M, Procino G, Tamma G, Mannucci R, Svelto M, Valenti G. 2007. Trafficking and phosphorylation dynamics of AQP4 in histamine-treated human gastric cells. Biol Cell 99:25–36.
- Chae YK, Woo J, Kim MJ, Kang SK, Kim MS, Lee J, 2008. Expression of aquaporin 5 (AQP5) promotes tumor invasion in human non small cell lung cancer. PLoS One 3:e2162.
- Chan KH, Kwan JS, Ho PW, Ho JW, Chu AC, Ramsden DB. 2012. Aquaporin-4 autoantibodies in neuromyelitis optica spectrum disorders: Comparison between tissue-based and cell-based indirect immunofluorescence assays. J Neuroinflammation 7:50.
- Conner MT, Conner AC, Bland CE, Taylor LH, Brown JE, Parri HR, 2012. Rapid aquaporin translocation regulates cellular water flow: The mechanism of hypotonicity-induced sub-cellular localization of the aquaporin 1 water channel. J Biol Chem 30 287(14):11516–25.
- Conner MT, Conner AC, Brown JE, Bill RM. 2010. Membrane trafficking of aquaporin 1 is mediated by protein kinase C via microtubules and regulated by tonicity. Biochemistry 49:821–823.
- De Groot BL, Engel A, Grubmuller H. 2001. A refined structure of human aquaporin-1. FEBS Lett 504:206–211.
- De Groot BL, Engel A, Grubmuller H. 2003a. The structure of the aquaporin-1 water channel: A comparison between cryo-electron microscopy and X-ray crystallography. J Mol Biol 325:485–493.
- De Groot BL, Frigato T, Helms V, Grubmuller H. 2003b. The mechanism of proton exclusion in the aquaporin-1 water channel. J Mol Biol 333:279–293.
- De Groot BL, Grubmuller H. 2001. Water permeation across biological membranes: Mechanism and dynamics of aquaporin-1 and GlpF. Science 294:2353–2357.
- Fischer G, Kosinska-Eriksson U, Aponte-Santamaria C, Palmgren M, Geijer C, Hedfalk K, 2009. Crystal structure of a yeast aquaporin at 1.15 angstrom reveals a novel gating mechanism. PLoS Biol 7:e1000130.
- Fotiadis D, Suda K, Tittmann P, Jeno P, Philippsen A, Muller DJ, 2002. Identification and structure of a putative Ca2+-binding domain at the C terminus of AQP1. J Mol Biol 318:1381–1394.
- Garcia F, Kierbel A, Larocca MC, Gradilone SA, Splinter P, Larusso NF, 2001. The water channel aquaporin-8 is mainly intracellular in rat hepatocytes, and its plasma membrane insertion is stimulated by cyclic AMP. J Biol Chem 276:12147–12152.
- Garcia N, Gondran C, Menon G, Mur L, Oberto G, Guerif Y, 2011. Impact of AQP3 inducer treatment on cultured human keratinocytes, ex vivo human skin and volunteers. Int J Cosmet Sci 33:432–442.
- Golestaneh N, Fan JG, Zelenka P, Chepelinsky AB. 2008. PKC putative phosphorylation site Ser(235) is required for MIP/AQP0 translocation to the plasma membrane. Mol Vis 14:1006–1014.
- Guan XG, Su WH, Yi F, Zhang D, Hao F, Zhang HG, 2010. NPA motifs play a key role in plasma membrane targeting of aquaporin-4. IUBMB Life 62:222–226.
- Gunnarson E, Zelenina M, Aperia A. 2004. Regulation of brain aquaporins. Neuroscience 129:947–955.
- Gunnarson E, Zelenina M, Axehult G, Song Y, Bondar A, Krieger P, 2008. Identification of a molecular target for glutamate regulation of astrocyte water permeability. Glia 56:587–596.
- Han Z, Patil RV. 2000. Protein kinase A-dependent phosphorylation of aquaporin-1. Biochem Biophys Res Commun 273:328–332.
- Hasler U, Vinciguerra M, Vandewalle A, Martin PY, Feraille E. 2005. Dual effects of hypertonicity on aquaporin-2 expression in cultured renal collecting duct principal cells. J Am Soc Nephrol 16:1571–1582.
- Ho JD, Yeh R, Sandstrom A, Chorny I, Harries WE, Robbins RA, 2009. Crystal structure of human aquaporin 4 at 1.8 A and its mechanism of conductance. Proc Natl Acad Sci USA 106:7437–7442.
- Hoffert JD, Leitch V, Agre P, King LS. 2000. Hypertonic induction of aquaporin-5 expression through an ERK-dependent pathway. J Biol Chem 275:9070–9077.
- Horsefield R, Norden K, Fellert M, Backmark A, Tornroth-Horsefield S, Terwisscha Van Scheltinga AC, 2008. High-resolution X-ray structure of human aquaporin 5. Proc Natl Acad Sci USA 105:13327–13332.
- Huber VJ, Tsujita M, Nakada T. 2012. Aquaporins in drug discovery and pharmacotherapy. Mol Aspects Med 33(5–6):691–703.
- Ikeda M, Andoo A, Shimono M, Takamatsu N, Taki A, Muta K, 2010. The NPC motif of aquaporin-11, unlike the NPA motif of known aquaporins, is essential for full expression of molecular function. J Biol Chem 286:3342–3350.
- Ishibashi K. 2009. New members of mammalian aquaporins: AQP10–AQP12. Handb Exp Pharmacol 251–262.
- Ishibashi K, Kuwahara M, Gu Y, Tanaka Y, Marumo F, Sasaki S. 1998. Cloning and functional expression of a new aquaporin (AQP9) abundantly expressed in the peripheral leukocytes permeable to water and urea, but not to glycerol. Biochem Biophys Res Commun 244:268–274.
- Ishikawa Y, Eguchi T, Skowronski MT, Ishida H. 1998a. Acetylcholine acts on M3 muscarinic receptors and induces the translocation of aquaporin5 water channel via cytosolic Ca2+ elevation in rat parotid glands. Biochem Biophys Res Commun 245:835–840.
- Ishikawa Y, Eguchi T, Skowronski MT, Ishida H. 1998b. Acetylcholine acts on M-3 muscarinic receptors and induces the translocation of aquaporin5 water channel via cytosolic Ca2+ elevation in rat parotid glands. Biochem Biophys Res Communic 245:835–840.
- Ishikawa Y, Iida H, Ishida H. 2002. The muscarinic acetylcholine receptor-stimulated increase in aquaporin-5 levels in the apical plasma membrane in rat parotid acinar cells is coupled with activation of nitric oxide/cGMP signal transduction. Mol Pharmacol 61:1423–1434.
- Ishikawa Y, Skowronski MT, Inoue N, Ishida H. 1999. alpha(1)-adrenoceptor-induced trafficking of aquaporin-5 to the apical plasma membrane of rat parotid cells. Biochem Biophys Res Commun 265:94–100.
- Itoh T, Rai T, Kuwahara M, Ko SB, Uchida S, Sasaki S, 2005. Identification of a novel aquaporin, AQP12, expressed in pancreatic acinar cells. Biochem Biophys Res Commun 330:832–838.
- Johansson I, Larsson C, Ek B, Kjellbom P. 1996. The major integral proteins of spinach leaf plasma membranes are putative aquaporins and are phosphorylated in response to Ca2+ and apoplastic water potential. Plant Cell 8:1181–1191.
- Kalman K, Nemeth-Cahalan KL, Froger A, Hall JE. 2006. AQP0-LTR of the Cat Fr mouse alters water permeability and calcium regulation of wild type AQP0. Biochim Biophys Acta 1758:1094–1099.
- Karabasil MR, Hasegawa T, Azlina A, Purwanti N, Purevjav J, Yao C, 2009. Trafficking of GFP-AQP5 chimeric proteins conferred with unphosphorylated amino acids at their PKA-target motif ((152)SRRTS) in MDCK-II cells. J Med Invest 56:55–63.
- Karlsson T, Glogauer M, Ellen RP, Loitto VM, Magnusson KE, Magalhaes MA. 2011. Aquaporin 9 phosphorylation mediates membrane localization and neutrophil polarization. J Leukoc Biol 90:963–973.
- Kishida K, Kuriyama H, Funahashi T, Shimomura I, Kihara S, Ouchi N, 2000. Aquaporin adipose, a putative glycerol channel in adipocytes. J Biol Chem 275:20896–20902.
- Kishida K, Shimomura I, Kondo H, Kuriyama H, Makino Y, Nishizawa H, 2001. Genomic structure and insulin-mediated repression of the aquaporin adipose (AQPap), adipose-specific glycerol channel. J Biol Chem 276:36251–36260.
- Knepper MA, Wade JB, Terris J, Ecelbarger CA, Marples D, Mandon B, 1996. Renal aquaporins. Kidney Int 49:1712–1717.
- Kosugi-Tanaka C, Li X, Yao C, Akamatsu T, Kanamori N, Hosoi K. 2006. Protein kinase A-regulated membrane trafficking of a green fluorescent protein-aquaporin 5 chimera in MDCK cells. Biochim Biophys Acta 1763:337–344.
- Koyama N, Ishibashi K, Kuwahara M, Inase N, Ichioka M, Sasaki S, 1998. Cloning and functional expression of human aquaporin8 cDNA and analysis of its gene. Genomics 54:169–172.
- Langaa S, Bloksgaard M, Bek S, Neess D, Norregaard R, Hansen PB, 2012. Mice with targeted disruption of the acyl-CoA binding protein display attenuated urine concentrating ability and diminished renal aquaporin-3 abundance. Am J Physiol Renal Physiol 2 302(8):F1034–44.
- Lerch-Bader M, Lundin C, Kim H, Nilsson I, Von Heijne G. 2008. Contribution of positively charged flanking residues to the insertion of transmembrane helices into the endoplasmic reticulum. Proc Natl Acad Sci USA 105:4127–4132.
- Litman T, Sogaard R, Zeuthen T. 2009. Ammonia and urea permeability of mammalian aquaporins. Handb Exp Pharmacol 327–358.
- Liu J, Tian DA, Wang JP, Zhang SZ, Feng J, Zhao ZZ, 2011. Expression of aquaporin 8 and its relationship with Melanosis coli. Chin Med J (Engl) 124:3061–3065.
- Loitto VM, Huang C, Sigal YJ, Jacobson K. 2007. Filopodia are induced by aquaporin-9 expression. Exp Cell Res 313:1295–1306.
- Loo DD, Zeuthen T, Chandy G, Wright EM. 1996. Cotransport of water by the Na+/glucose cotransporter. Proc Natl Acad Sci USA 93:13367–13370.
- Madrid R, Le Maout S, Barrault MB, Janvier K, Benichou S, Merot J. 2001. Polarized trafficking and surface expression of the AQP4 water channel are coordinated by serial and regulated interactions with different clathrin-adaptor complexes. Embo J 20:7008–7021.
- Maeda N, Hibuse T, Funahashi T. 2009. Role of aquaporin-7 and aquaporin-9 in glycerol metabolism; involvement in obesity. Handb Exp Pharmacol 233–249.
- Magni F, Sarto C, Ticozzi D, Soldi M, Bosso N, Mocarelli P, 2006. Proteomic knowledge of human aquaporins. Proteomics 6:5637–5649.
- Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, 2000. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med 6:159–163.
- Marinelli RA, Pham L, Agre P, Larusso NF. 1997. Secretin promotes osmotic water transport in rat cholangiocytes by increasing aquaporin-1 water channels in plasma membrane – evidence for a secretin-induced vesicular translocation of aquaporin-1. J Biologic Chem 272:12984–12988.
- Martini F, Naith JL. 2009. Fundamentals of anatomy and physiology. San Francisco, CA: Bejamin-Cummings Publishing.
- Matsumura Y, Uchida S, Rai T, Sasaki S, Marumo F. 1997. Transcriptional regulation of aquaporin-2 water channel gene by cAMP. J Am Soc Nephrol 8:861–867.
- Matsuzaki T, Suzuki T, Takata K. 2001. Hypertonicity-induced expression of aquaporin 3 in MDCK cells. Am J Physiol Cell Physiol 281:C55–C63.
- Moeller HB, Fenton RA, Zeuthen T, Macaulay N. 2009. Vasopressin-dependent short-term regulation of aquaporin 4 expressed in Xenopus oocytes. Neuroscience 164:1674–1684.
- Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, 2000. Structural determinants of water permeation through aquaporin-1. Nature 407:599–605.
- Nedvetsky PI, Tamma G, Beulshausen S, Valenti G, Rosenthal W, Klussmann E. 2009. Regulation of aquaporin-2 trafficking. Handb Exp Pharmacol 133–157.
- Nemeth-Cahalan KL, Kalman K, Hall JE. 2004. Molecular basis of pH and Ca2+ regulation of aquaporin water permeability. J Gen Physiol 123:573–580.
- Nicchia GP, Rossi A, Mola MG, Procino G, Frigeri A, Svelto M. 2008. Actin cytoskeleton remodeling governs aquaporin-4 localization in astrocytes. Glia56(16):1755–66.
- Nishioka R, Takeda T, Kakigi A, Okada T, Takebayashi S, Taguchi D, 2010. Expression of aquaporins and vasopressin type 2 receptor in the stria vascularis of the cochlea. Hear Res 260:11–19.
- Noda Y, Sasaki S. 2006. Regulation of aquaporin-2 trafficking and its binding protein complex. Biochim Biophys Acta 1758:1117–1125.
- Ohinata A, Nagai K, Nomura J, Hashimoto K, Hisatsune A, Miyata T, 2005. Lipopolysaccharide changes the subcellular distribution of aquaporin 5 and increases plasma membrane water permeability in mouse lung epithelial cells. Biochem Biophys Res Commun 326:521–526.
- Papadopoulos MC, Manley GT, Krishna S, Verkman AS. 2004. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. Faseb J 18:1291–1293.
- Papadopoulos MC, Verkman AS. 2005. Aquaporin-4 gene disruption in mice reduces brain swelling and mortality in pneumococcal meningitis. J Biol Chem 280:13906–13912.
- Papadopoulos MC, Verkman AS. 2007. Aquaporin-4 and brain edema. Pediatr Nephrol 22:778–784.
- Parvin MN, Kurabuchi S, Murdiastuti K, Yao C, Kosugi-Tanaka C, Akamatsu T, 2005. Subcellular redistribution of AQP5 by vasoactive intestinal polypeptide in the Brunner's gland of the rat duodenum. Am J Physiol Gastrointest Liver Physiol 288:G1283–G1291.
- Pasantes-Morales H, Cruz-Rangel S. 2010. Brain volume regulation: Osmolytes and aquaporin perspectives. Neuroscience 168:871–884.
- Pearce LR, Komander D, Alessi DR. 2010. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 11:9–22.
- Procino G, Carmosino M, Marin O, Brunati AM, Contri A, Pinna LA, 2003. Ser-256 phosphorylation dynamics of Aquaporin 2 during maturation from the ER to the vesicular compartment in renal cells. Faseb J 17:1886–1888.
- Qi H, Li L, Zong W, Hyer BJ, Huang J. 2009. Expression of aquaporin 8 is diversely regulated by osmotic stress in amnion epithelial cells. J Obstet Gynaecol Res 35:1019–1025.
- Rabaud NE, Song L, Wang Y, Agre P, Yasui M, Carbrey JM. 2009. Aquaporin 6 binds calmodulin in a calcium-dependent manner. Biochem Biophys Res Commun 383:54–57.
- Rai T, Sasaki S, Uchida S. 2006. Polarized trafficking of the aquaporin-3 water channel is mediated by an NH2-terminal sorting signal. Am J Physiol Cell Physiol 290:C298–C304.
- Reichow SL, Gonen T. 2008. Noncanonical binding of calmodulin to aquaporin-0: Implications for channel regulation. Structure 16:1389–1398.
- Riethmuller C, Oberleithner H, Wilhelmi M, Franz J, Schlatter E, Klokkers J, 2008. Translocation of aquaporin-containing vesicles to the plasma membrane is facilitated by actomyosin relaxation. Biophys J 94:671–678.
- Rodriguez A, Catalan V, Gomez-Ambrosi J, Fruhbeck G. 2011a. Aquaglyceroporins serve as metabolic gateways in adiposity and insulin resistance control. Cell Cycle 10:1548–1556.
- Rodriguez A, Catalan V, Gomez-Ambrosi J, Garcia-Navarro S, Rotellar F, Valenti V, 2011b. Insulin- and leptin-mediated control of aquaglyceroporins in human adipocytes and hepatocytes is mediated via the PI3K/Akt/mTOR signaling cascade. J Clin Endocrinol Metab 96:E586–E597.
- Roudier N, Verbavatz JM, Maurel C, Ripoche P, Tacnet F. 1998. Evidence for the presence of aquaporin-3 in human red blood cells. J Biol Chem 273:8407–8412.
- Saadoun S, Papadopoulos MC. 2009. Aquaporin-4 in brain and spinal cord oedema. Neuroscience168(4):1036–46 .
- Savage DF, Stroud RM. 2007. Structural basis of aquaporin inhibition by mercury. J Mol Biol 368:607–617.
- Scheuring S, Tittmann P, Stahlberg H, Ringler P, Borgnia M, Agre P, 2000. The aquaporin sidedness revisited. J Mol Biol 299:1271–1278.
- Schrier RW, Cadnapaphornchai MA. 2003. Renal aquaporin water channels: From molecules to human disease. Prog Biophys Mol Biol 81:117–131.
- Sidhaye V, Hoffert JD, King LS. 2005. cAMP has distinct acute and chronic effects on aquaporin-5 in lung epithelial cells. J Biol Chem 280:3590–3596.
- Song Y, Verkman AS. 2001. Aquaporin-5 dependent fluid secretion in airway submucosal glands. J Biol Chem 276:41288–41292.
- Sougrat R, Morand M, Gondran C, Barre P, Gobin R, Bonte F, 2002. Functional expression of AQP3 in human skin epidermis and reconstructed epidermis. J Invest Dermatol 118:678–685.
- Tada J, Sawa T, Yamanaka N, Shono M, Akamatsu T, Tsumura K, 1999. Involvement of vesicle-cytoskeleton interaction in AQP5 trafficking in AQP5-gene-transfected HSG cells. Biochem Biophys Res Commun 266:443–447.
- Tietz PS, Mcniven MA, Splinter PL, Huang BQ, Larusso NF. 2006. Cytoskeletal and motor proteins facilitate trafficking of AQP1-containing vesicles in cholangiocytes. Biol Cell 98:43–52.
- Tornroth-Horsefield S, Hedfalk K, Fischer G, Lindkvist-Petersson K, Neutze R. 2010. Structural insights into eukaryotic aquaporin regulation. FEBS Lett 584:2580–2588.
- Tornroth-Horsefield S, Wang Y, Hedfalk K, Johanson U, Karlsson M, Tajkhorshid E, 2006. Structural mechanism of plant aquaporin gating. Nature 439:688–694.
- Valenti G, Procino G, Tamma G, Carmosino M, Svelto M. 2005. Minireview: Aquaporin 2 trafficking. Endocrinology 146:5063–5070.
- Verkman AS. 2003. Role of aquaporin water channels in eye function. Exp Eye Res 76:137–143.
- Walz T, Fujiyoshi Y, Engel A. 2009. The AQP structure and functional implications. Handb Exp Pharmacol 31–56.
- Wang F, Feng XC, Li YM, Yang H, MA TH. 2006. Aquaporins as potential drug targets. Acta Pharmacol Sin 27(4), 395–401.
- Wang Y, Tajkhorshid E. 2007. Molecular mechanisms of conduction and selectivity in aquaporin water channels. J Nutr 137:1509S–1515S; discussion 1516S–1517S.
- Woo J, Chae YK, Jang SJ, Kim MS, Baek JH, Park JC, 2008. Membrane trafficking of AQP5 and cAMP dependent phosphorylation in bronchial epithelium. Biochem Biophys Res Commun 366:321–327.
- Wu B, Steinbronn C, Alsterfjord M, Zeuthen T, Beitz E. 2009. Concerted action of two cation filters in the aquaporin water channel. Embo J 28:2188–2194.
- Yang F, Kawedia JD, Menon AG. 2003. Cyclic AMP regulates aquaporin 5 expression at both transcriptional and post-transcriptional levels through a protein kinase A pathway. J Biol Chem 278:32173–32180.
- Yasui H, Kubota M, Iguchi K, Usui S, Kiho T, Hirano K. 2008. Membrane trafficking of aquaporin 3 induced by epinephrine. Biochem Biophys Res Commun 373:613–617.
- Yin TJ, Yu SY, Xiao L, Zhang J, Liu C, Lu YP, 2008. [Correlation of aquaporin 1 with hypoxia-inducible factor 1 in breast cancer]. Zhonghua Yi Xue Za Zhi 88:258–260.
- Zador Z, Stiver S, Wang V, Manley GT. 2009. Role of aquaporin-4 in cerebral edema and stroke. Handb Exp Pharmacol 159–170.
- Zelenina M. 2010. Regulation of brain aquaporins. Neurochem Int 57:468–488.
- Zhang W, Xu Y, Chen Z, Xu Z, Xu H. 2011. Knockdown of aquaporin 3 is involved in intestinal barrier integrity impairment. FEBS Lett 585:3113–3119.
- Zhang W, Zitron E, Homme M, Kihm L, Morath C, Scherer D, 2007. Aquaporin-1 channel function is positively regulated by protein kinase C. J Biol Chem 282:20933–20940.
- Christensen BM Zelenina M, Aperia A Nielsen S. 2000. Localization and regulation of PKA-phosphorylated AQP2 in response to V(2)-receptor agonist/antagonist treatment. Am J Physiol Renal Physiol 278 F29–42.
NOTICE OF CORRECTION
The early online version of this article published online ahead of print on 21 November 2012 contained an error on page 3. The figure legend for Figure 1 should have read “Schematic diagram of the shared structural architecture of aquaporins. Six transmembrane α-helices are connected by alternating extracellular and intracellular loops with the carboxyl and amino termini orientated towards the cytoplasm. Two of these connecting loops (B and E) fold into the transmembrane pore so that the protein resembles an hour-glass. Loops B and E each form a helical region containing highly conserved Asn-Pro-Ala (NPA) motifs that facilitate selectivity of the pore. The approximate positions of serine or threonine protein kinase phosphorylation sites involved in AQP translocation are indicated as follows; ▴ = Ser235 in AQP0; ⧫ = Thr157 and ○ = Thr239 in AQP1; □ = Ser256 in AQP2; = Ser111 and
= Ser180 in AQP4;
= Ser152 in AQP5;
= Ser11 and ▵ = Ser222 in AQP9”. This has been corrected for the current version.
