Abstract
Absorption of dietary fat in the small intestine involves epithelial exposure to potentially harmful molecules such as bile salts and free fatty acids. We used organ culture of porcine jejunal explants incubated with a pre-digested mixture of fat (plant oil), bile and pancreatin to mimick the physiological process of dietary fat absorption, and short exposures to the fat mixture caused fat droplet accumulation within villus enterocytes. Lucifer yellow (LY), a fluorescent membrane-impermeable polar tracer was included to monitor epithelial integrity. Both in controls and during fat absorption LY penetrated the epithelium and accumulated in the basal lamina and the lamina propria. LY was also seen in the paracellular space, whereas villus enterocytes were generally only weakly labeled except for small amounts taken up by apical endocytosis. In the crypts, however, fat absorption induced cell permeabilization with LY accumulating in the cytosol and nucleus. Morphologically, both apical and basolateral membranes appeared intact, indicating that the leakiness was caused by minor lesions in the membrane. Albeit to a lesser extent, bile alone was capable of permeabilizing crypt cells, implying that the surfactant properties of bile salts are involved in the process. In addition to LY, crypt enterocytes also became permeable for albumin, ovalbumin and insulin. In conclusion, during fat absorption the permeability of the gut epithelium is increased mainly in the crypts. A possible explanation is that cell membranes of immature crypt cells, lacking detergent-resistant lipid raft microdomains, are less resistant to the deleterious effects of bile salts and free fatty acids.
Introduction
Fat, mostly as triacylglycerols, constitutes a sizeable portion of the human diet with adults consuming about 100 g each day, and a finely tuned collaboration among the organs of the gastrointestinal tract ensures that virtually all dietary fat is absorbed. Given its nutritional importance in health and disease, the complex biology of fat digestion, absorption, and transport has been intensively studied for a number of decades so that its molecular mechanisms today are known in great detail (Friedman and Nylund Citation1980, Mansbach and Siddiqi Citation2010, Pan and Hussain Citation2012). Briefly, the uptake of dietary fat is a sequential process mainly taking part in the duodenum and proximal jejunum. An essential step involves luminal emulsification of the water insoluble fat with hepatic bile, generating nm-sized mixed micelles with surfactant bile salts and phospholipids (Martinez-Augustin and Sanchez de Citation2008, Maldonado-Valderrama et al. Citation2011). This solubilization is a prerequisite for efficient intraluminal hydrolysis carried out by pancreatic lipases, yielding free fatty acids and monoacylglycerols as the main products of digestion. These are subsequently taken up across the epithelial brush border, either by diffusion or with the help of transport proteins (Pan and Hussain Citation2012), whereas the uptake of bile salts awaits passage of the chyme into the ileum (Dawson Citation2011). Once inside the enterocytes, dietary fatty acids and monoacylglycerols are utilized for triacylglycerol synthesis in the endoplasmic reticulum. The triacylglycerols are then either incorporated into nascent chylomicrons or, if formed on the cytosolic side of the ER, enter a cytosolic storage pool of fat droplets (Mansbach and Siddiqi Citation2010). Finally, after passage through the secretory pathway, chylomicrons are discharged by exocytosis from the basolateral cell surface into the lamina propria.
Albeit a daily recurring physiological process, absorption of dietary fat is currently suspected of having potentially adverse consequences for the gut. Thus, aside their caloric contributions, high-fat diets in conjunction with the gut microbiota reportedly cause alterations in epithelial integrity that may lead to increased gut permeability and ultimately help trigger chronic inflammatory, and other diseases (Frazier et al. Citation2011, Tilg and Kaser Citation2011, Moreira et al. Citation2012, Ulluwishewa et al. Citation2011). The molecular mechanisms underlying these pathological changes are complex and far from fully understood, but bile salts in particular have been claimed to exert specific pathological effects on the epithelium (Martinez-Augustin and Sanchez de Citation2008, Maillette de Buy and Beuers Citation2010). Thus, in a scanning electron microscope study, bile salts were observed to form cracks in the jejunal mucosa (Oumi and Yamamoto Citation2000). Unconjugated bile salts were those most potent in action, suggesting that deconjugation caused by bacterial overgrowth may induce breakdown of the epithelial integrity. In another electron microscopic study on bile salt cytotoxicity against the biliary epithelium, a marked damage of intracellular organelles was observed, whereas cell membranes and tight junctions appeared intact (Benedetti et al. Citation1997). In addition to bile salts, free fatty acids generated during fat digestion are a potential hazard threatening the epithelial integrity (Mansbach and Siddiqi Citation2010). Thus, when transported into the cytosol, they have the propensity to perturb the cellular membrane, potentially leading to cell death (Stralfors Citation1990). Conceivably, this would occur in cells with an inadequate ability to dispose of absorbed free fatty acids, typically by re-esterification to triacylglycerol (Listenberger et al. Citation2003).
Epithelial changes occurring under conditions mimicking the physiological absorption of dietary fat have previously been studied using the human intestinal Caco-2 cells (Ho and Storch Citation2001, Murota and Storch Citation2005) or a mucosal explant organ culture model (Hansen et al. Citation2007). In the latter system, fat absorption induced a transient, clathrin-dependent selective endocytosis of the brush border enzyme alkaline phosphatase (AP) (Hansen et al. Citation2007), a GPI-anchored protein that hydrolyzes monophosphate esters and resides in lipid raft microdomains (Engle et al. Citation1995, Danielsen and Hansen Citation2006, Citation2008). More recently, we observed that free fatty acids are not only taken up into the enterocyte cytosol but also, to some extent, inserted into the brush border membrane where they generated separate low-density microdomains of detergent resistant membranes highly enriched in AP (Hansen et al. Citation2011). Taken together, these observations imply that products of intraluminal digestion of dietary fat alter the membrane composition of the intestinal brush border and thereby also its functional properties.
In the present work, the non-toxic fluorescent polar tracer Lucifer yellow (LY) (Hanani Citation2012) was used to investigate further the possible effects of fat absorption on the intestinal epithelial integrity. We observed that short exposures (0.5–1 h) to a fat mixture mimicking that generated during intraluminal emulsification and digestion of triacylglycerols caused permeabilization of enterocytes. Surprisingly, cells in the crypts that do not engage in fat absorption were those most frequently permeabilized, indicating that this phenomenon is not directly linked to the absorptive process. A possible explanation could be that the immature cells of the crypts lack the detergent-resistant lipid raft microdomains characteristic of the mature brush border of villus enterocytes.
Materials and methods
Materials
Lucifer yellow CH (ammonium salt), a fluorescein isothiocyanate (FITC) protein labeling kit, mouse monoclonal anti-fluorescein antibodies, Alexa Fluor-conjugated secondary antibodies for immunofluorescence microscopy, and ProLong antifade reagent with DAPI were obtained from Invitrogen (California, USA), methyl-β-cyclodextrin, unfractionated bovine bile, pancreatin from porcine pancreas, Nile red, Ruthenium red, ovalbumin, porcine insulin, and porcine albumin from Sigma-Aldrich (Missouri, USA), and a monoclonal antibody to the α-chain of Na+/K+-ATPase from Affinity Bioreagents (now Thermo Scientific, Massachusetts, USA). A rabbit antibody to porcine aminopeptidase N was prepared as previously described (Hansen et al. Citation1987). Rapeseed oil (manufactured by Nordic Food Partners A/S, Copenhagen) was purchased from a local supermarket. 100 ml of oil had a nutritional value of 3400 kJ and contained 92 g of total fat (triacylglycerol) of which 7 g was saturated fatty acids, 58 g monounsaturated fatty acids, and 27 g polyunsaturated fatty acids. Segments of jejunum, taken 1–2 m from the pylorus of overnight fasted, post-weaned pigs, were surgically removed from the anaesthetized animals by licensed staff at the Department of Experimental Medicine, the Panum Institute, University of Copenhagen.
Organ culture of intestinal mucosal explants
Small intestines from a total of eight pigs were used in separate experiments of the study. Freshly obtained jejunal segments of about 20 cm in length were quickly rinsed in ice-cold RPMI medium, and mucosal explants weighing about 0.1 g were excised with a scalpel and cultured in RPMI medium at 37°C for periods of 0.5–1 h, essentially as described previously (Danielsen et al. Citation1982). In some experiments, whole segments of intestine, including the muscularis and serosal layers were excised and cultured. For each animal, control and fat absorption explants (3–4 explants for each condition) were cultured in parallel. For fat absorption experiments, a mixture containing rapeseed oil (10%), bile (1%) and pancreatin (1%) was made up in RPMI medium and incubated overnight at room temperature or for 5 h at 30°C. This mixture was diluted 4× with fresh RPMI medium immediately before use. Lucifer yellow (LY) was dissolved in medium (10 mg/ml) just before use and used at a concentration of 1 mg/ml. Methyl-β-cyclodextrin, freshly dissolved in medium (10%), was used at a final concentration of 1%. When used, freshly dissolved Ruthenium red was present at a concentration of 0.5 mg/ml, and in experiments with FITC-conjugated albumin, ovalbumin and insulin, these proteins were added to the medium to obtain a final concentration of 10 μg/ml.
FITC-conjugation of proteins
FITC-conjugated albumin, ovalbumin and insulin were prepared using a labeling kit according to the protocol supplied by the manufacturer. Briefly, 200 μl (50–100 μg of albumin, ovalbumin or insulin) dissolved in 50 mM HEPES-HCl, 150 mM NaCl, pH 7.1, was mixed with 20 μl 1 M sodium bicarbonate, pH 9.0, in a reaction tube before 10–20 μl freshly prepared dye stock solution was added. The mixture was incubated at room temperature with magnetic stirring for 1 h protected from light. After incubation, unconjugated FITC was removed either by centrifugation in a spin column or by extensive dialysis against PBS.
Fluorescence microscopy
Immediately after organ culture mucosal explants were rinsed in fresh RPMI medium and fixed in 4% paraformaldehyde in 0.1 M sodium phosphate, pH 7.2 (buffer A) for 2 h at 4°C. After fixation, the explants were rinsed three times in buffer A before immersion in 25% sucrose in buffer A overnight and subsequent mounting and sectioning at −19°C in a cryostat (Leica CM1850). For immunolabeling of aminopeptidase N and Na+/K+-ATPase, sections were incubated for 1 h at room temperature with anti-aminopeptidase N or anti-Na+/K+-ATPase, respectively (1:100 dilution) in 50 mM Tris-HCl, 150 mM NaCl, 0.5% ovalbumin, 0.1% gelatin, 0.2% teleostean gelatin, 0.05% Tween 20, pH 7.2 (buffer B), followed by incubation for 1 h at room temperature with Alexa-conjugated secondary antibodies (1:200 dilution in buffer B). For visualization of fat droplets/chylomicrons, sections were incubated for 5 min with Nile red (0.5 mg/ml dissolved in acetone diluted 1:100 with 75% glycerol). A control with omission of the primary antibody was routinely included in immunolabeling experiments. Sections were mounted in antifade medium with DAPI (except for those stained with Nile red) and examined in a fluorescence microscope (Leica DM 4000B) fitted with a digital camera (Leica DC 300FX).
Electron microscopy
Immediately after organ culture and rinse in RPMI medium mucosal explants were fixed overnight at 4°C in 2.5% glutaraldehyde in buffer A in the absence or presence of 0.5 mg/ml Ruthenium red. After a wash in buffer A the tissue was postfixed in 1% osmium tetroxide in buffer A in the absence or presence of 0.5 mg/ml Ruthenium red for 1 h on ice and subsequently dehydrated in ethanol and embedded in Epon as previously described (Danielsen et al. Citation1995). Ultrathin Epon sections were cut on an LKB Ultrotome III ultramicrotome and collected on grids previously rinsed in 20% acetic acid, 99% ethanol and H2O. The sections were stained in 1% uranyl acetate in H2O and in lead citrate, and finally examined in a Zeiss EM 900 electron microscope equipped with a Mega View II camera.
Results
Fat absorption and deposition in intestinal mucosal explants
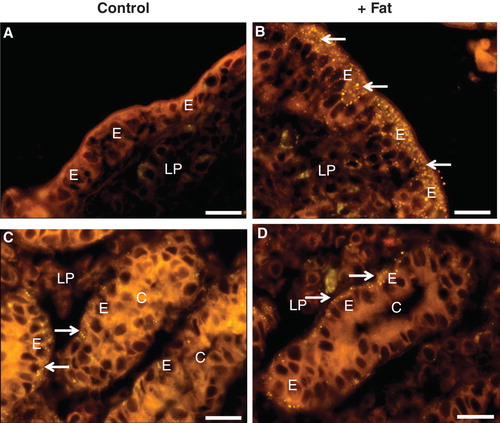
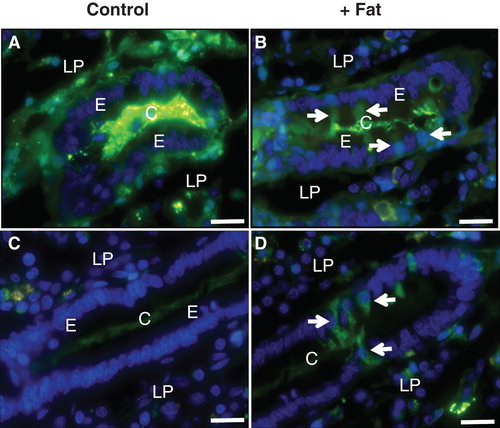
Organ culture of intestinal mucosal explants is a long established in vivo-like model system that maintains the tissue viability for periods up to 24 h (Danielsen et al. Citation1982). With this system, we have previously used a pre-incubated mixture of plant oil, bile, and pancreatin to mimick the physiological processes occurring in the gut epithelium during fat absorption (Hansen et al. 2003a, Citation2007, Citation2011). In comparison with ‘fasting' controls cultured in parallel, exposure to this fat absorption mixture did not cause observable changes in the gross morphology of the mucosal explants, neither in the villus- nor the crypt regions. But as can be seen in , a brief exposure to such a mixture resulted in the generation of numerous lipid droplets scattered in the apical cytoplasm of villus enterocytes, as evidenced by staining with the lipophilic dye Nile red. By 1 h, some lipid particles were also seen in the underlying lamina propria, indicating that enterocytic export of chylomicrons from the basolateral cell surface had commenced. In the crypts, no lipid droplets were detected in the apical cytoplasm of the enterocytes, showing that immature crypt cells do not engage directly in the absorptive process. Some lipid droplets were seen close to the basal surface of the cells (). However, a similar labeling pattern was also observed for crypt cells of control explants, showing that these particles are not of immediate dietary origin.
Figure 1. Absorption and deposition of fat visualized by staining with Nile red after culture for 1 h in the absence (Control) or presence (+ Fat) of a physiological mixture of digested fat. (A) In a section of villus from a control explant no or few fat droplets were visible in enterocytes (E) or the lamina propria (LP). (B) After fat absorption numerous punctae representing fat droplets or nascent chylomicrons were seen in the apical cytoplasm of enterocytes. Some were also seen in the basal part of the cells and in the underlying lamina propria, indicative of enterocytic export of chylomicrons. (C) & (D) In the crypts (C), distinct fat droplets (marked by arrows) were seen lining the basal membrane of the enterocytes regardless whether fat absorption had taken place or not. Bars, 20 μm. This Figure is reproduced in color in Molecular Membrane Biology online.
Intestinal permeability probed with Lucifer yellow
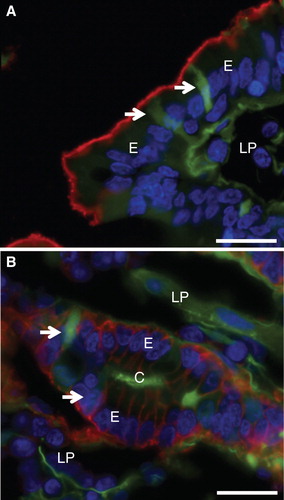
Lucifer yellow (LY) is a small (molecular mass 444), fixable, non-toxic and membrane-impermeable polar tracer commonly used for studying cell structure and communication (Hanani Citation2012). In mucosal explants labeled with LY for 1 h, the fluorescent dye accumulated most intensively along the basal lamina of the epithelium and, to a lesser extent, within the extracellular space of the lamina propria (). At higher magnification (, insert), a distinct labeling along the lateral sides of the villus enterocytes was revealed, whereas the cytoplasm was generally only weakly labeled. However, small, bright punctae lining the apical cell surface were indicative of an endocytic uptake, as previously visualized with FM dye, a lipophilic membrane marker (Hansen et al. Citation2009).
Figure 2. Permeabilization of enterocytes by a fat mixture after culture for 1 h in the presence of LY. (A) In the villus region of a control explant LY was mainly seen along the basal lamina and in the lamina propria. Enterocytes generally appeared LY-negative, but at higher magnification (insert) small punctae, indicative of endocytosis, were visible. In addition, LY was seen along the lateral sides of the enterocytes (marked by arrows), indicating a paracellular passage through the epithelium. (B) Fat absorption did not change the general LY labeling of the villus, but occasionally permeabilized enterocytes were seen (arrow). (C) & (E) In the crypts of control explants LY was mainly seen in the lumen and along the basal lamina. (D) & (F) After fat absorption several enterocytes in the crypts accumulated LY in the cytosol and nucleus (some marked by arrows), whereas their neighboring cells were seemingly unaffected. Bars, 20 μm. This Figure is reproduced in color in Molecular Membrane Biology online.
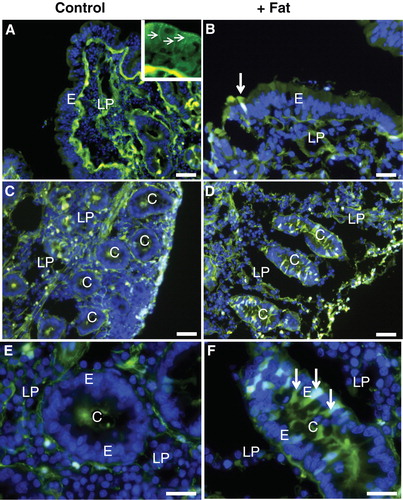
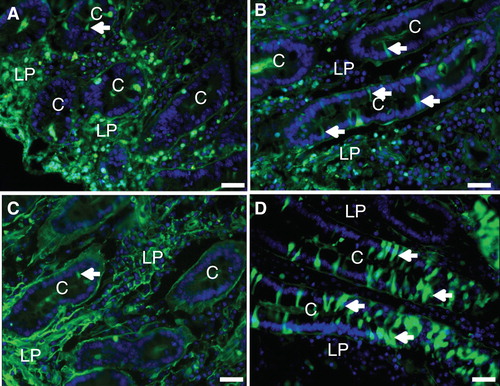
Fat absorption did not affect the overall LY labeling pattern of the villi. Single enterocytes that had taken up large amounts of the tracer into the cytoplasm and nucleus were sporadically observed (), as they were also in controls. In the crypts, however, such permeabilized cells occurred much more frequently after fat absorption than in controls (). The staining intensity displayed by individual crypt cells varied, indicating different degrees or stages of permeabilization. LY-permeable cells were also occasionally detectable in crypts of control explants, but unlike the situation with fat absorption they were absent from most cross sections of crypts (). Culture in the presence of 0.25% bile alone (without pre-digested fat and pancreatin) also caused an increase in the number of LY-positive cells in the crypts, albeit to a lesser extent than the full pre-digested fat mixture (). To assess the possibility of the fat mixture reaching the crypt cells from the serosal side of the mucosal explants, experiments using whole segments of jejunum, including the muscularis and serosal layers, were performed. As shown in , numerous cells in the crypts were permeabilized to LY by exposure to the fat mixture for 1 h also when access from the serosal side was thus prevented. Therefore, although access ‘from behind' cannot be excluded, we conclude that exposure to the fat mixture from the luminal side is sufficient to cause crypt cell permeabilization.
Figure 3. (A) & (B) Mucosal explants cultured for 1 h with LY in the absence or presence of bile. LY-positive cells were only rarely observed in the control (A), but frequently seen in the crypts after exposure to bile (some marked by arrows) (B). (C) & (D) Whole segments of jejunum, including the muscularis- and serosal layers, cultured for 1 h with LY in the absence (C) or presence (D) of the fat mixture. As seen with mucosal explants, numerous LY-positive cells were seen after fat absorption (some marked by arrows). Bars, 20 μm. This Figure is reproduced in color in Molecular Membrane Biology online.
A morphometric analysis of the permeabilization of crypt cells occurring during fat absorption is given in . The Table confirms that only a minor fraction of the crypts in the controls contained LY-positive cells, whereas almost all crypts after fat absorption contained permeabilized cells. In addition, it can be seen that the permeabilization effect of fat absorption was pronounced already by 30 min of culture.
Table I. Permeabilization of crypt cells during fat absorption. Mucosal explants were cultured in the presence of LY for 30 min or 1 h in the absence (Control) or presence of a digested fat mixture (+Fat) as described in Methods. After culture, images of paraformaldehyde-fixed sections of the cultured explants were examined by immunofluorescence microscopy and the total number of identifiable crypts, the number of LY-positive crypts (i.e., crypts with at least one LY-positive cell), and the total number of LY-positive crypt cells were counted. Experiments were performed with mucosal explants obtained from two pigs, and a total number of 24 images, covering eight tissue sections (4 Controls, 4 +Fat), were analyzed.
shows mucosal explant sections double labeled for 30 min with Nile red and LY to assess the relationship between the enterocytic fat uptake/deposition and permeabilization. At the villi, the few LY-permeable cells had similar high contents of apical lipid droplets as the abundant LY-negative enterocytes (). Likewise in the crypts, the many LY-permeable cells seen after fat exposure were not morphologically distinct from neighboring cells (). This was also the case for the few LY-permeable cells seen in control explants ().
Figure 4. Double labeling with LY and Nile red after culture for 30 min in the absence or presence of fat absorption. (A), (B), (C), (D) Both in the villus region and in the crypts, the LY-permeable enterocytes (marked by arrows) appeared morphologically similar to their LY-negative neighboring cells, both in the control and after fat absorption. In (B), the high-magnification insert shows that two LY-permeable cells had also accumulated fat droplets/chylomicrons. Bars, 20 μm. This Figure is reproduced in color in Molecular Membrane Biology online.
Taken together, the above results show that conditions mimicking the physiological absorption of dietary fat induced a permeabilization of enterocytes to a polar tracer of low molecular weight. The permeabilization occurred seemingly random among neighboring cells, and indicates a loss of membrane integrity of those cells affected and thereby a potential breach of the intestinal barrier. However, immature crypt cells, where lipid deposition is minimal, were far more susceptible to permeabilization than mature villus enterocytes, indicating that permeabilization is not simply a consequence of a massive fat absorption.
Conceivably, the permeabilization observed could be reflected in changes in the overall cell membrane or cytoskeleton organization. However, as probed with both an apical (aminopeptidase N, ) and a basolateral (Na+/K+-ATPase, ) membrane marker, the two surface domains of the enterocytes appeared intact following incubation with the fat mixture.
Figure 5. Cell integrity probed by membrane- and cytoskeletal markers after culture for 1 h with fat absorption in the presence of LY. After culture, sections were immunolabeled with antibodies to aminopeptidase N (a) or Na+/K+-ATPase (b). (A) Intense red labeling for aminopeptidase N was confined to the apical enterocyte brush border of the villus. (B) In the crypts, red labeling showed Na+/K+-ATPase distinctly along the basolateral membrane of enterocytes. (In both images, cells permeabilized for LY are marked by arrows). Bars, 20 μm. This Figure is reproduced in color in Molecular Membrane Biology online.
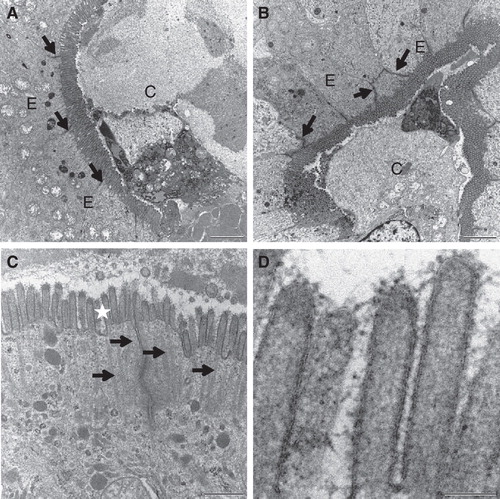
Ruthenium red is an electron-dense membrane marker suitable for electron microscopy that we have previously used for studying the ultrastructure of the brush border (Hansen et al. 2003b). As shown in , no difference in the staining of brush border was detected after fat absorption. The microvillus organization appeared regular, and at higher magnification an intact membrane bilayer was apparent.
Figure 6. Cell membrane integrity probed with Ruthenium red. Mucosal explants were cultured in the absence (A) or presence (B–D) of the fat mixture for 1 h. During the last 15 min of culture, Ruthenium red was added to the medium in (A) & (B). (A) & (B) A dark staining of Ruthenium red can be seen at the enterocyte brush border and in the paracellular space (marked by arrows) in the crypts of control explants and after fat absorption. (C) Short (∼ 0.5 μm) microvilli with intact actin rootlets (marked by arrows) of immature enterocytes in the crypt. (D) Microvilli marked by asterisk in (C) shown at higher magnification. An intact membrane bilayer structure can be seen. Bars, 2 μm (A, B); 0.5 μm (C); 0.1 μm (D).
Collectively, the experiments at the light microscopy and electron microscopy levels indicate that the general epithelial cell membrane architecture is maintained during fat absorption. Permeabilization is therefore most likely caused by minor lesions of the membrane that do not compromise the overall cell integrity. The observation that villus enterocytes are less affected by fat absorption than crypt cells may well rely on the atypical, stable lipid raft organization that renders the mature brush border resistant to the harsh conditions often prevailing in the gut lumen (Danielsen and Hansen Citation2003).
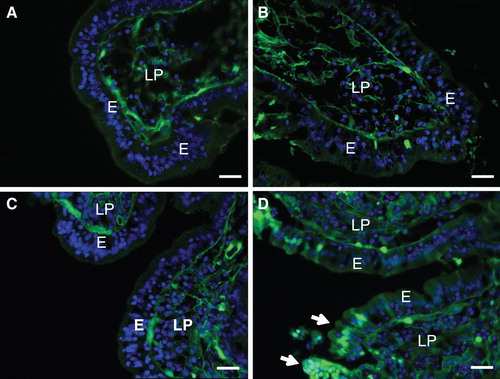
To assess this possibility we used two different treatments to disrupt the lipid rafts of the brush border. In one experiment, a wash with 10 mM lactose was used to release galactosyl-binding proteins such as galectin-4 (Braccia et al. Citation2003) and anti-glycosyl antibodies (Hansen et al. Citation2005) from the membrane. But as shown in this treatment did not cause an increased permeability of villus enterocytes to LY, suggesting that the lectin-mediated cross-linking of membrane glycolipids- and proteins is not important for protecting the membrane during fat absorption. In a second experiment methyl-β-cyclodextrin was used for removal of cholesterol, a membrane lipid associated with lipid rafts. Methyl-β-cyclodextrin is an agent commonly used for extracting cholesterol from cell membranes (Kilsdonk et al. Citation1995, Neufeld et al. Citation1996), and we have previously used it to remove up to 50–70% of the cholesterol from the brush border (Hansen et al. Citation2000, Citation2001). Treatment with methyl-β-cyclodextrin had no or little effect on the LY-permeability of villus cells in control explants (). After fat absorption, however, clusters of permeabilized cells were occasionally observed along the villi, indicating lesions in the brush border membrane of the affected enterocytes (). This experiment thus suggests that the cholesterol of the brush border membrane helps protecting it from the deleterious effects of fat absorption.
Figure 7. Effect of lipid raft disruption by a lactose wash or cholesterol removal. (A) & (B) Mucosal explants were cultured for 1 h in the presence of LY and 10 mM lactose in the absence (A) or presence (B) of the fat mixture. Lactose, which releases galectin-4 and anti-glycosyl antibodies from the brush border, did not increase the permeability of villus enterocytes to LY. (C) & (D) Mucosal explants cultured for 30 min with LY in the presence of 1% methyl-β-cyclodextrin, followed by continued culture for 30 min in the absence (C) or presence (D) of the fat mixture. Without fat, cholesterol depletion did not markedly increase the permeability of LY, but with the fat mixture, more lesions consisting of clusters of permeabilized enterocytes were observed (marked by arrows). Bars, 20 μm. This Figure is reproduced in color in Molecular Membrane Biology online.
Uptake of proteins induced by dietary fat absorption
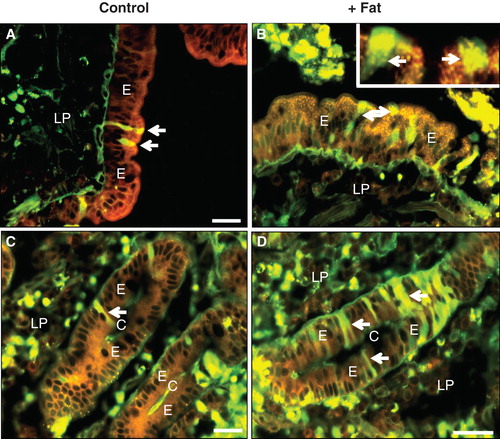
With the exception of the neonatal stage, undigested dietary proteins within the lumen of the gut are not normally absorbed. To assess the extent of the permeabilization induced by the fat absorption, we next studied the binding and uptake of FITC-labeled bovine albumin and ovalbumin. As shown in , after incubation for 1 h albumin bound to the villus enterocyte brush border, but little or no endocytic uptake or paracellular passage was observed. Similarly in the crypts, albumin was only detected in the lumen, indicating that the epithelial barrier prevented any uptake (). During fat absorption, albumin binding to the villus brush border was very patchy compared with the control situation and no uptake was observed (). However, in the crypts albumin was taken up by several cells. As with LY, the uptake was not confined to punctae, indicative of endocytosis, but to the entire cytosol (). Fat absorption induced a similar uptake of ovalbumin () as well as insulin () into several crypt cells. Taken together, these results indicate that the membrane permeabilization detected with LY was of sufficient magnitude also to allow entry of native proteins into the cytosol.
Figure 8. Fat absorption-induced uptake of FITC-albumin during culture for 1 h. (A) In the villus region of the control, albumin bound to the luminal brush border of enterocytes (marked by arrows), but no cellular uptake was detected. (B) After fat absorption, binding of albumin along the villi was patchy and scarce. (C) & (E) In the crypts of the control explant albumin was only seen in the lumen. (D) & (F) After fat absorption, albumin uptake into the cytosol and nucleus was frequently observed in enterocytes of the crypts (marked by arrows). Bars, 20 μm. This Figure is reproduced in color in Molecular Membrane Biology online.
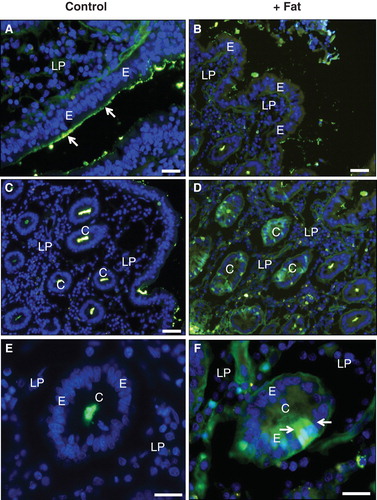
Figure 9. Uptake of FITC-ovalbumin and FITC-insulin in the crypts induced by fat absorption. (A) & (C) No uptake of ovalbumin (A) or insulin (C) occurred in the crypts of the control explant. (B) & (D) After fat absorption, both ovalbumin (B) and insulin (D) had been taken up by several enterocytes in the crypts (marked by arrows). Bars, 20 μm. This Figure is reproduced in color in Molecular Membrane Biology online.
Discussion
Dietary fat absorption has long been known to affect the small intestinal epithelium. Thus, fat absorption is accompanied by the appearance of intestinal AP in the blood (Keiding Citation1966, Inglis et al. Citation1967), as well as epithelial secretion of surfactant-like particles, so called because of their similarity to pulmonary surfactant (DeSchryver-Kecskemeti et al. Citation1989, Eliakim et al. Citation1991). These observations are likely to be related to the fat-induced changes in the brush border membrane more recently observed (Hansen et al. Citation2007, Citation2011). Collectively, they may reflect the existence of a complex regulatory relationship involving AP and the diet (Lalles Citation2010, Lynes and Widmaier Citation2011), although it has also been reported that no direct association between AP release and chylomicron formation seems to exist (Nauli et al. Citation2003). In a more general sense it has also been recognized that the physiological process of dietary fat absorption takes its toll on the small intestinal epithelium and that this basically reflects the inherent danger to the gut posed by the need for a system enabling the organism to absorb water-insoluble nutrients from the diet (Martinez-Augustin and Sanchez de Citation2008, Mansbach and Siddiqi Citation2010). Here, interest has mainly focused on the detrimental effects of bile salts, in particular deconjugated bile salts, owing to their surfactant properties. Collectively, previous works indicate that the extent of the epithelial tissue damage and increased gut permeability is dependent on concentration and type of bile salt (Low-Beer et al. Citation1970, Fagundes-Neto et al. Citation1981, Oumi and Yamamoto Citation2000).
The molecular interactions between surfactants/detergents and biomembranes have been the focus of numerous studies for decades and the subject of many reviews (Lichtenberg et al. Citation1983, Silvius Citation1992, Almgren Citation2000, le Maire et al. Citation2000). Briefly, at low concentrations the surfactant, for instance a bile salt, associates with the membrane without much loss in the general structure of the bilayer. At higher surfactant concentrations an increase in membrane permeability may occur prior to any effect on the general structure, and finally, when the concentration is increased beyond a critical lamellar/micellar transition concentration, mixed micelles will form and membrane solubilization occur (Lichtenberg et al. Citation1983). The concentration of bile used in this work (0.25%) was well within the physiological range and did not cause microscopically observable changes in the overall epithelial cell membrane architecture, either when used as part of the fat mixture or used alone (without fat and pancreatin). It therefore seems likely that bile salts caused minor lesions by disturbing the membrane bilayer without solubilization of its lipids. The results obtained indicate that although bile alone to some extent caused crypt cell permeabilization, it was a less effective agent than the full fat mixture. This suggests that other components, most likely the free fatty acids generated by digestion, also contribute to the overall effect. In this context, we recently observed that fatty acids become incorporated into the brush border during fat absorption and in the process change the microdomain organization of the membrane (Hansen et al. Citation2011). It is tempting to speculate that such membrane rearrangements and possible changes in fluidity may generate transient leaks in the bilayer.
It should be noted that lacking intestinal motility, the explant culture system probably displays a diminished luminal fluid flow compared to the situation in vivo. Likewise, differences in the mucosal barrier protection, caused for instance by possible changes in the mucus layer, should also be borne in mind. Regarding the crypt area, this implies that the degree of exposure to bile salts and free fatty acids may differ accordingly from the in vivo situation. Nevertheless, the main finding of the present work was that immature enterocytes in the crypts are those most prone to become leaky during digestion and absorption of dietary fat although crypt cells do not actively participate in the absorptive process and may seem less exposed to the harsh luminal environment than villus cells. A possible explanation why the latter are more resistant is that their mature brush border is exceptionally robust due to its lipid raft organization (Danielsen and Hansen Citation2003, Citation2006). That cholesterol removal with methyl-β-cyclodextrin to some extent rendered villus enterocytes LY-permeable after fat absorption underscores the importance of this particular lipid raft-associated membrane component. Due to its relative small size, cholesterol is generally thought to act as a spacer, filling out the void spaces between the more bulky sphingolipid molecules (Simons and Ikonen Citation1997, Simons and Gerl Citation2010). This property may therefore be of importance for protection against small leaks caused by the components of the fat mixture. In contrast, the apical membrane of immature crypt enterocytes resembles a ‘normal' cell membrane that is more prone to the deleterious effects of surfactant molecules (Alessandri et al. Citation1990).
Conceivably, a breach of the gut epithelial barrier induced by fat absorption may constitute a health hazard by providing luminal pathogens with a portal for invasion of the organism. Thus, in addition to LY, FITC-conjugated albumin, ovalbumin and insulin also leaked into the cytosol of crypt enterocytes during fat absorption, showing that passage through the cell membrane is not entirely restricted to small molecules. However, using 51Cr-EDTA as a marker for intestinal permeability in the rat, it has previously been reported that the intestinal epithelium undergoes injury and restitution during the normal course of digestion and absorption of a meal (Kvietys et al. Citation1991). Similarly, using phenol red as a probe, it was concluded that surfactant-induced acute intestinal wall damage is rapidly repaired (Swenson et al. Citation1994). Most likely, the permeabilization of crypt cells visualized by LY therefore represents the natural ‘wear and tear' of the small intestine that occurs during absorption of dietary fat.
Conclusions
Although the small intestine is suitably designed to act both as an absorptive/digestive surface for dietary nutrients as well as a permeability barrier for pathogens, the membrane integrity of the epithelium is challenged daily by exposure to the surfactant activity of bile salts and fatty acids during the physiological process of fat absorption. The results of the present work indicate that the cells of the crypts are those most susceptible to these potentially harmful agents, possibly owing to an immature brush border lacking a lipid raft organization. Interestingly, the idea that the intestinal epithelium consists of a tight (villus) and a leaky (crypt) epithelium arranged in parallel has previously been proposed (Madara and Marcial Citation1984), but evidently our results obtained by the use of a mucosal organ culture system should be translated to the in vivo situation in future work. Finally, we propose that LY could be a useful diagnostic tool in future studies of epithelial damage, for instance for assessing long-term effects of high-fat diets.
Acknowledgements
The study was supported by grants from Augustinus Fonden (Grant number 11-0253), Aase og Ejnar Danielsens Fond (Grant number 1´0-000128), Brødrene Hartmanns Fond (Grant number A11481), Fonden til Lægevidenskabens Fremme (Grant number 10-58), and Hørslev Fonden (Grant number 203866-MIA). E.M.D. planned and supervised the study and wrote the manuscript. G.H.H. participated in designing the experiments, discussion of results and drafting of the manuscript. K.R. and L.-L. N.-C. performed the major part of the experimental work and participated in the discussion of the results.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alessandri JM, Arfi TS, Thieulin C. 1990. [The mucosa of the small intestine: Development of the cellular lipid composition during enterocyte differentiation and postnatal maturation]. Reprod Nutr Dev 30:551–576.
- Almgren M. 2000. Mixed micelles and other structures in the solubilization of bilayer lipid membranes by surfactants. Biochim Biophys Acta 1508:146–163.
- Benedetti A, Alvaro D, Bassotti C, Gigliozzi A, Ferretti G, La RT, 1997. Cytotoxicity of bile salts against biliary epithelium: A study in isolated bile ductule fragments and isolated perfused rat liver. Hepatology 26:9–21.
- Braccia A, Villani M, Immerdal L, Niels-Christiansen LL, Nystrom BT, Hansen GH, 2003. Microvillar membrane microdomains exist at physiological temperature – role of galectin-4 as lipid raft stabilizer revealed by ‘superrafts'. J Biol Chem 278:15679–15684.
- Danielsen EM, Hansen GH. 2003. Lipid rafts in epithelial brush borders: Atypical membrane microdomains with specialized functions. Biochim Biophys Acta 1617:1–9.
- Danielsen EM, Hansen GH. 2006. Lipid raft organization and function in brush borders of epithelial cells. Mol Membr Biol 23:71–79.
- Danielsen EM, Hansen GH. 2008. Lipid raft organization and function in the small intestinal brush border. J Physiol Biochem 64:377–382.
- Danielsen EM, Hansen GH, Niels-Christiansen LL. 1995. Localization and biosynthesis of aminopeptidase N in pig fetal small intestine. Gastroenterology 109:1039–1050.
- Danielsen EM, Sjostrom H, Noren O, Bro B, Dabelsteen E. 1982. Biosynthesis of intestinal microvillar proteins. Characterization of intestinal explants in organ culture and evidence for the existence of pro-forms of the microvillar enzymes. Biochem J 202:647–654.
- Dawson PA. 2011. Role of the intestinal bile acid transporters in bile acid and drug disposition. Handb Exp Pharmacol 169–203.
- DeSchryver-Kecskemeti K, Eliakim R, Carroll S, Stenson WF, Moxley MA, Alpers DH. 1989. Intestinal surfactant-like material. A novel secretory product of the rat enterocyte. J Clin Invest 84:1355–1361.
- Eliakim R, Mahmood A, Alpers DH. 1991. Rat intestinal alkaline phosphatase secretion into lumen and serum is coordinately regulated. Biochim Biophys Acta 1091:1–8.
- Engle MJ, Mahmood A, Alpers DH. 1995. Two rat intestinal alkaline phosphatase isoforms with different carboxyl-terminal peptides are both membrane-bound by a glycan phosphatidylinositol linkage. J Biol Chem 270:11935–11940.
- Fagundes-Neto U, Teichberg S, Bayne MA, Morton B, Lifshitz F. 1981. Bile salt-enhanced rat jejunal absorption of a macromolecular tracer. Lab Invest 44:18–26.
- Frazier TH, DiBaise JK, McClain CJ. 2011. Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury. J Parenter Enteral Nutr 35:14S–20S.
- Friedman HI, Nylund B. 1980. Intestinal fat digestion, absorption, and transport. A review. Am J Clin Nutr 33:1108–1139.
- Hanani M. 2012. Lucifer yellow – an angel rather than the devil. J Cell Mol Med 16:22–31.
- Hansen GH, Immerdal L, Thorsen E, Niels-Christiansen LL, Nystrom BT, Demant EJ, 2001. Lipid rafts exist as stable cholesterol-independent microdomains in the brush border membrane of enterocytes. J Biol Chem 276:32338–32344.
- Hansen GH, Niels-Christiansen LL, Immerdal L, Danielsen EM. 2003a. Scavenger receptor class B type I (SR-BI) in pig enterocytes: Trafficking from the brush border to lipid droplets during fat absorption. Gut 52:1424–1431.
- Hansen GH, Niels-Christiansen LL, Immerdal L, Nystrom BT, Danielsen EM. 2007. Intestinal alkaline phosphatase: Selective endocytosis from the enterocyte brush border during fat absorption. Am J Physiol Gastrointest Liver Physiol 293:G1325–G1332.
- Hansen GH, Niels-Christiansen LL, Thorsen E, Immerdal L, Danielsen EM. 2000. Cholesterol depletion of enterocytes. Effect on the Golgi complex and apical membrane trafficking. J Biol Chem 275:5136–5142.
- Hansen GH, Pedersen ED, Immerdal L, Niels-Christiansen LL, Danielsen EM. 2005. Anti-glycosyl antibodies in lipid rafts of the enterocyte brush border: A possible host defense against pathogens. Am J Physiol Gastrointest Liver Physiol 289:G1100–G1107.
- Hansen GH, Pedersen J, Niels-Christiansen LL, Immerdal L, Danielsen EM. 2003b. Deep-apical tubules: Dynamic lipid-raft microdomains in the brush-border region of enterocytes. Biochem J 373:125–132.
- Hansen GH, Rasmussen K, Niels-Christiansen LL, Danielsen EM. 2009. Endocytic trafficking from the small intestinal brush border probed with FM dye. Am J Physiol Gastrointest Liver Physiol 297:G708–G715.
- Hansen GH, Rasmussen K, Niels-Christiansen LL, Danielsen EM. 2011. Dietary free fatty acids form alkaline phosphatase-enriched microdomains in the intestinal brush border membrane. Mol Membr Biol 28:136–144.
- Hansen GH, Sjostrom H, Noren O, Dabelsteen E. 1987. Immunomicroscopic localization of aminopeptidase N in the pig enterocyte. Implications for the route of intracellular transport. Eur J Cell Biol 43:253–259.
- Ho SY, Storch J. 2001. Common mechanisms of monoacylglycerol and fatty acid uptake by human intestinal Caco-2 cells. Am J Physiol Cell Physiol 281:C1106–C1117.
- Inglis NI, Krant MJ, Fishman WH. 1967. Influence of a fat-enriched meal on human serum (L-phenylalanine-sensitive) ‘intestinal' alkaline phosphatase. Proc Soc Exp Biol Med 124:699–702.
- Keiding NR. 1966. Intestinal alkaline phosphatase in human lymph and serum. Scand J Clin Lab Invest 18:134–140.
- Kilsdonk EP, Yancey PG, Stoudt GW, Bangerter FW, Johnson WJ, Phillips MC, 1995. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem 270:17250–17256.
- Kvietys PR, Specian RD, Grisham MB, Tso P. 1991. Jejunal mucosal injury and restitution: Role of hydrolytic products of food digestion. Am J Physiol 261:G384–G391.
- Lalles JP. 2010. Intestinal alkaline phosphatase: Multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr Rev 68:323–332.
- le Maire M, Champeil P, Moller JV. 2000. Interaction of membrane proteins and lipids with solubilizing detergents. Biochim Biophys Acta 1508:86–111.
- Lichtenberg D, Robson RJ, Dennis EA. 1983. Solubilization of phospholipids by detergents. Structural and kinetic aspects. Biochim Biophys Acta 737:285–304.
- Listenberger LL, Han X, Lewis SE, Cases S, Farese RV Jr, Ory DS, 2003. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA 100:3077–3082.
- Low-Beer TS, Schneider RE, Dobbins WO. 1970. Morphological changes of the small-intestinal mucosa of guinea pig and hamster following incubation in vitro and perfusion in vivo with unconjugated bile salts. Gut 11:486–492.
- Lynes MD, Widmaier EP. 2011. Involvement of CD36 and intestinal alkaline phosphatases in fatty acid transport in enterocytes, and the response to a high-fat diet. Life Sci 88:384–391.
- Madara JL, Marcial MA. 1984. Structural correlates of intestinal tight-junction permeability. Kroc Found Ser 17:77–100.
- Maillette de Buy WL, Beuers U. 2010. Bile salts and cholestasis. Dig Liver Dis 42:409–418.
- Maldonado-Valderrama J, Wilde P, Macierzanka A, Mackie A. 2011. The role of bile salts in digestion. Adv Colloid Interface Sci 165:36–46.
- Mansbach CM, Siddiqi SA. 2010. The biogenesis of chylomicrons. Annu Rev Physiol 72:315–333.
- Martinez-Augustin O, Sanchez de MF. 2008. Intestinal bile acid physiology and pathophysiology. World J Gastroenterol 14: 5630–5640.
- Moreira AP, Texeira TF, Ferreira AB, do Carmo Gouveia PM, de Cassia Goncalves AR. 2012. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr 1–9.
- Murota K, Storch J. 2005. Uptake of micellar long-chain fatty acid and sn-2-monoacylglycerol into human intestinal Caco-2 cells exhibits characteristics of protein-mediated transport. J Nutr 135:1626–1630.
- Nauli AM, Zheng S, Yang Q, Li R, Jandacek R, Tso P. 2003. Intestinal alkaline phosphatase release is not associated with chylomicron formation. Am J Physiol Gastrointest Liver Physiol 284:G583–G587.
- Neufeld EB, Cooney AM, Pitha J, Dawidowicz EA, Dwyer NK, Pentchev PG, 1996. Intracellular trafficking of cholesterol monitored with a cyclodextrin. J Biol Chem 271:21604–21613.
- Oumi M, Yamamoto T. 2000. A scanning electron microscope study on the effects of different bile salts on the epithelial lining of jejunal mucosa. Med Electron Microsc 33:11–15.
- Pan X, Hussain MM. 2012. Gut triglyceride production. Biochim Biophys Acta 1821:727–735.
- Silvius JR. 1992. Solubilization and functional reconstitution of biomembrane components. Annu Rev Biophys Biomol Struct 21:323–348.
- Simons K, Gerl MJ. 2010. Revitalizing membrane rafts: New tools and insights. Nat Rev Mol Cell Biol 11:688–699.
- Simons K, Ikonen E. 1997. Functional rafts in cell membranes. Nature 387:569–572.
- Stralfors P. 1990. Autolysis of isolated adipocytes by endogenously produced fatty acids. FEBS Lett 263:153–154.
- Swenson ES, Milisen WB, Curatolo W. 1994. Intestinal permeability enhancement: efficacy, acute local toxicity, and reversibility. Pharm Res 11:1132–1142.
- Tilg H, Kaser A. 2011. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest 121:2126–2132.
- Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. 2011. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr 141:769–776.