Abstract
Transmembrane b-type cytochromes, which are crucially involved in electron transfer chains, bind one or more heme (Fe-protoporphyrin IX) molecules non-covalently. Similarly, chlorophylls are typically also non-covalently bound by several membrane integral polypeptides involved in photosynthesis. While both, chlorophyll and heme, are tetrapyrrole macrocycles, they have different substituents at the tetrapyrrole ring moiety. Furthermore, the central metal ion is Mg2+ in chlorophyll and Fe2+/3+ in heme. As heme and chlorophyll a have similar structures and might both be ligated by two histidine residues of a polypeptide chain, and as the local concentration of chlorophyll a might be up to 100-times higher than the concentration of heme, the question arises, as to how an organism ensures specific binding of heme, but not of chlorophyll, to transmembrane apo-cytochromes involved in photosynthetic electron transfer reactions. As shown here, Fe-protoporphyrin IX derivatives with modified substituents at the tetrapyrrole ring moiety still bind to an apo-cytochrome; however, association appears to be reduced. This indicates that hydrophobic and polar interactions of the ring substituents with the protein moiety stabilize the protein/heme-complex but are not essential per se. However, removal or replacement of the central Fe-ion completely abolishes formation of a holo-protein complex, and thus the central iron ion appears to determine heme binding to apo-cytochrome b6.
Introduction
In photosynthetic organelles and organisms, such as chloroplasts and cyanobacteria, several membrane integral proteins are involved in charge transfer reactions across membranes, and many of these proteins contain chlorophyll or heme cofactors (Cramer and Whitmarsh Citation1977, Gray and Winkler Citation1996, Schaefer et al. Citation1996).
The cytochrome b6f complex is one of the three integral membrane protein complexes of the photosynthetic electron transport chain (Bernát and Rögner Citation2011, Cramer et al. Citation2011, Hasan et al. Citation2013). Heme binding is important for the stability of this complex, since in the absence of heme, the polypeptides of two of the major components of the complex, cytochrome b6 and cytochrome f, are sensitive to proteolytical degradation (Kuras et al. Citation1995, Citation1997, Malnoe et al. Citation2011). Based on the cytochrome b6f crystal structures, binding of the two b-type hemes ofcytochrome b6 via His ligation promotes formation of a closely packed four helix bundle (Kurisu et al. Citation2003, Stroebel et al. Citation2003, Hasan et al. Citation2013), whereas in case of cytochrome f, the free α-amino group of Tyr1 serves as the sixth heme iron ligand and permits close packing of cytochrome f (Martinez et al. Citation1994). Furthermore, the time point of heme insertion plays an important role during the assembly of the cytochrome b6f complex dimer and for stability of individual subunits, as reviewed in detail recently (Hasan et al. Citation2013). Covalent attachment of hemes to c-type cytochromes has been analyzed in detail and four protein systems, catalyzing covalent attachment of hemes to c-type cytochromes, have been identified in pro- and eukaryotes (Thöny-Meyer Citation1997, de Vitry Citation2011). In contrast, in case of b-type cytochrome assembly, individual steps involved in heme delivery and/or hemebinding are not understood (Thöny-Meyer Citation1997). Although sequential binding of hemes to apo-cytochromes has been analyzed in some detail in vitro (as further discussed below), a comprehensive view of principles guiding heme binding to b-type cytochromes has not emerged. As in vitro and in vivo studies indicate spontaneous binding of tetrapyrrole molecules to natural or designed transmembrane (TM) b-type cytochromes (Francke et al. Citation1999, Cordova et al. Citation2007, Dreher et al. Citation2007, Korendovych et al. Citation2010, Lujan et al. Citation2012), and as diverse tetrapyrrole macro-cycles might be present at a time in chloroplasts, the question arises how specificity in heme binding is maintained.
In TM b-type cytochromes, which are, e.g. crucially involved in photosynthetic and respiratory electron transfer chains, the heme cofactor is typically ligated by two His residues of the polypeptide chain, which serve as the fifth and sixth ligands of the central iron atom, respectively (von Jagow and Sebald Citation1980, Yun et al. Citation1991). However, other residues of the polypeptide chain are also involved in formation of a heme binding pocket and favorable interactions between the heme and the protein drive protein assembly and stabilize the holo-cytochrome (Koch and Schneider Citation2007). Especially, interactions of the negatively charged heme propionyl groups with the positively charged side-chains of Arg or Lys residues appear to be crucial for heme binding (Cutler et al. Citation1989, Cross et al. Citation1995, Prodöhl et al. Citation2005), as these residues provide positive charges in close proximity to the negatively charged heme propionyl groups (Stroebel et al. Citation2003, Cramer et al. Citation2005). Just as hemes in b-type cytochromes, chlorophylls are typically also non-covalently bound by membrane integral polypeptides and the holo-proteins are typically involved in photosynthetic light harvesting and energy transfer. Although more diverse, chlorophyll molecules might also be axially ligated by His residues, as e.g. observed in photosystem II, similar to the axial ligation of hemes in TM b-type cytochromes (Zouni et al. Citation2001, Kamiya and Shen Citation2003). In vitro studies have indicated that, similar to TM b-type cytochromes, also chlorophyll containing TM proteins can assemble spontaneously, without the help of additional factors (Paulsen et al. Citation1990, Cammarata and Schmidt Citation1992, Horn et al. Citation2007).
Both, chlorophyll and heme molecules are tetra-pyrrole macrocycles, and both share a common bio-synthetic pathway. However, the two tetrapyrroles have different substituents at the tetrapyrrole ring moiety and, in contrast to heme, chlorophyll a has an additional fifth ring (E-ring) (Willows Citation2003, Tanaka and Tanaka Citation2007). Furthermore, the central metal ion is Mg2+ in chlorophyll and Fe2+/3+ in heme. In plants, the biosynthesis pathway of all tetrapyrroles is localized in chloroplasts and only the last steps of heme synthesis are localized in both, mitochondria and chloroplasts (Tanaka and Tanaka Citation2007). Thus, after their synthesis, both, hemes and chlorophylls, are present in thylakoids and might be incorporated into an appropriate apo-protein. However, as heme and chlorophyll a have similar structures and as the concentration of chlorophyll a might be up to 100-times higher than the concentration of heme (Moulin and Smith Citation2005), the question arises, as to how an organism ensures specific binding of heme, but not of chlorophyll or chlorophyll precursors, to a TM apo-cytochrome.
In the past few years, progress has been made towards understanding the influence of the heme cofactor on folding and assembly of TM b-type cytochromes. Details of TM b-type cytochrome assembly have been elucidated mainly in vitro by using cytochrome b6 and the cytochrome b559-like protein, cytochrome , as models (Prodöhl et al. Citation2005, Citation2007, Dreher et al. Citation2008). Cytochrome
is a homo-dimer of the 44 amino-acid long β subunit (PsbF) of a cyanobacterial cytochrome b559 protein (Shinopoulos and Brudvig Citation2012), which spans a membrane with only a single TM helix. This TM homo-dimer binds one heme cofactor non-covalently, resulting in formation of the holo-cytochrome (Francke et al. Citation1999, Prodöhl et al. Citation2005). In the case of the PsbF homo-dimer, integration of the individual TM helices into the membrane and their subsequent interaction is independent of and precedes heme binding (Prodöhl et al. Citation2005, Volkmer et al. Citation2006, Akdogan et al. Citation2012, Weber and Schneider Citation2013). Additionally, heme binding to a TM b-type cytochrome has been studied using the TM cytochrome b6 protein, a core subunit of the cytochrome b6f complex. This four-helix bundle protein binds two heme cofactors non-covalently via four His residues conserved in the TM helices B and D (de Vitry et al. Citation2004, Hasan et al. Citation2013). In vivo, a third heme is covalently attached at the Qi site of apo-cytochrome b6 via a single cysteine residue (Kurisu et al. Citation2003, Stroebel et al. Citation2003, Cramer et al. Citation2005), but this heme is not of general importance for holo-cytochrome assembly and stability in vivo and in vitro (Dreher et al. Citation2008, Saint-Marcoux et al. Citation2009). Apo-cytochrome b6 integrates into a membrane independent of the cofactor and thereafter appears to adopt a conformation that allows subsequent heme binding in multiple steps (Kuras et al. Citation1997, Dreher et al. Citation2007, Dreher et al. Citation2008, Saint-Marcoux et al. Citation2009). Mutations of the heme-ligating His residues conserved in helices B and D have indicated that binding of the low-potential heme bL is a prerequisite for binding of the second, high-potential heme bH (Kuras et al. Citation1997, Dreher et al. Citation2008). Noteworthy, a similar assembly pathway has been suggested before for the cytochrome b subunit of Rhodobacter sphaeroides (Yun et al. Citation1991).
Based on these findings, a cytochrome b6 assembly has been proposed, in which at first heme bL binds in between His86 and His187 located on helix B and D, respectively, whereby His86 appears to have a predominate function. Only after binding of heme bL the second heme is bound by His100 and His202 (Yun et al. Citation1991, Kuras et al. Citation1997, Dreher et al. Citation2008). Although some details of heme binding to apo-cytochrome b6 are described, it still is an open question how binding of specifically heme is achieved, whereas unspecific binding of structurally similar tetrapyrroles, such as chlorophyll a, is avoided.
To address this question, we have tested binding of heme, chlorophyll as well as several protoporphyrin IX derivatives, varying in their central ion, to purified apo-cytochrome b6. While apo-cytochrome b6 binds heme with high affinity, chlorophyll a is not bound. As heme contains a central Fe-ion, whereas chlorophyll contains Mg2+, we tested a potential impact of the central ion on holo-protein formation. In contrast to Fe-pro-toporphyrin IX (heme), the protoporphyrin IX moiety alone (without a central metal ion) as well as Mg-protoporphyrin IX does not bind to apo-cytochrome b6, and thus the central Fe-ion appears to determine heme binding to apo-cytochrome b6. Furthermore, Fe-protoporphyrin IX derivatives with modified substituents at the tetrapyrrole ring moiety still bind to the apo-protein. However, association appears to be reduced, indicating that hydrophobic and polar interactions of the ring substituents with the apo-protein stabilize the protein/heme-complex. Interactions of the two heme propionyl groups with the apo-cytochrome appear to be of special importance.
Materials and methods
Expression and purification of cytochrome b6
Construction of the plasmid pRHMb6, which codes for the spinach apo-cytochrome b6 protein N-terminally fused to the maltose-binding protein (MBP) of Escherichia coli, is described in detail elsewhere (Prodöhl et al. Citation2007). For protein expression, the plasmid pRHMb6 was transformed into E. coli BL21 (DE3) cells. Het-erologous expression was performed as described in detail recently (Prodöhl et al. Citation2007). After induction of protein expression, cells were harvested after 3 h and broken by sonication. Subsequently, inclusion bodies were isolated and solubilized in buffer containing 50 mM SDS, prior to protein purification via Ni-NTA affinity chromatography (Prodöhl et al. Citation2007). The protein was eluted from the Ni-NTA column by decreasing the buffer pH to 4.5. Folding of the protein was initiated by exchanging SDS to the milder, non-ionic detergent n-dodecyl-β-D-maltoside (DDM) (Prodöhl et al. Citation2007).
Reconstitution of the holo-protein
Heme solutions were prepared exactly as described in Prodöhl et al. (Citation2007). The protoporphyrin IX derivatives Fe(III) protoporphyrin IX dimethylester chloride, Fe(III) deuteroporphyrin IX chloride, Mn (III) protoporphyrin IX chloride, Mg(II) protopor-phyrin IX disodium salt, protoporphyrin IX disodium salt (all from Frontier Scientific, Logan, Utah, USA), Zn(II) Protoporphyrin IX and chlorophyll a (Sigma-Aldrich) were dissolved in 50 mM Tris pH 8, 50 mM NaCl and 5 mM DDM to a final concentration of 0.5 mM. For analyzing binding of the respective porphyrins, these were mixed with apo-cytochrome b6 in a protein:porphyrin ratio of 1:2. Visible absor-bance spectra were recorded from 350–700 nm with a spectral bandwidth of 0.5 nm on a Perkin Elmer Lambda 35 instrument. Spectra were either taken under air-oxidizing conditions or porphyrins were reduced by addition of sodium dithionite to a final concentration of 5 mM. To quantitatively determine binding of porphyrins to apo-cytochrome b6, increasing amounts of apo-cytochrome b6 were titrated into a solution containing 5 μM of the respective porphyrin.
Results and discussion
Apo-cytochrome b6 binds heme but not chlorophyll a
Although heme and chlorophyll a share a common biosynthesis pathway and have similar structures, their respective affinities to bind to cytochrome b6 differ significantly. In , purification of the heterologously expressed cytochrome b6 protein is shown, and in , absorbance spectra of in vitro reconstituted apo-cytochrome b6 are shown under both oxidizing and reducing conditions. Compared to the spectrum of free heme, addition of apo-cytochrome b6 leads to a clear red shift of 9 and 10 nm of the Soret band under oxidizing and reducing conditions, respectively, and the band width at half maximum is drastically reduced (). Furthermore, in the redox difference spectrum (inset in ) a maximum at 562 nm is pronounced, which is absent in case of free heme. The maxima of the absorbance and redox difference spectra obtained with cytochrome b6 are prototypical and demonstrate heme binding to the apo-cytochrome and holo-cytochrome b6 formation, as previously discussed (Prodöhl et al. Citation2007). While this and previous analyses clearly indicate spontaneous binding of heme to apo-cytochrome b6, the spectral characteristics of chlorophyll a were not altered at all after addition of apo-cytochrome b6 (, ). Since the spectroscopic properties of chlorophyll a were not affected, and thus the chlorophyll environment was not altered, chlorophyll a does not appear to bind to apo-cytochrome b6.
Figure 1. Purification and in vitro reconstitution of holo-cytochrome b6. (A) SDS-PAGE analysis (10% SDS-gel) of the apo-cytochrome b6 expression and purification. 1: Cell extract before induction; 2: total cellular extract; 3: soluble proteins; 4: inclusion bodies; 5: affinity purified apo-cytochrome b6 fused to MBP. (B, C) Absorbance spectra show specific binding of heme to apo-cytochrome b6 under oxidizing (B) and reducing (C) conditions. Vis spectra of free heme (dark grey) and 150 μg in vitro reconstituted apo-cytochrome b6 (grey) are shown. The inset in Figure (C) shows the redox difference spectrum.
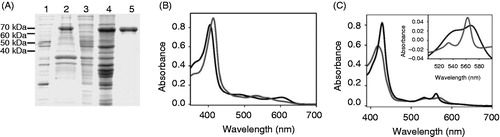
Figure 2. Chlorophyll a does not associate with apo-cytochrome b6. Absorbance spectra of free chlorophyll a (dark grey) and chlorophyll a after addition of 150 μg cytochrome b6 (grey) recorded under oxidizing conditions.
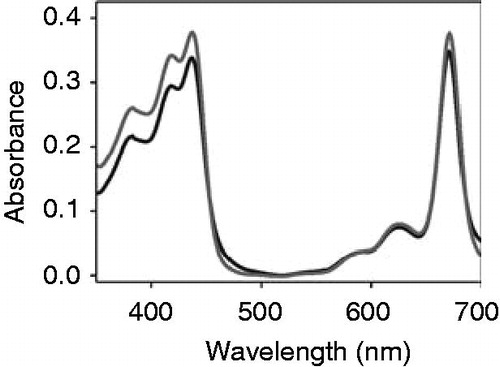
Table 1. Absorbance maxima offree protoporphyrin IX derivatives (without cyt b6) and in presence ofcytochrome b6 (with cyt b6) are listed. The bandwidth at half maximum (fwhm) is given for the Soret band (γ-band) of each spectrum. Absorbance maxima and the fwhm values were obtained after Gaussian deconvolution of the respective absorbance curve using the program PeakFit (Systat Software, Inc.).
To further test binding of heme vs. chlorophyll a to apo-cytochrome b6, we purified the protein a second time after addition of the two different cofactors and monitored whether the cofactors co-eluted with the protein (). Solely upon addition of heme, a spectrum of a cofactor containing holo-cytochrome was monitored, whereas in the case of chlorophyll the cofactor did not co-elute with the protein, which further demonstrates that chlorophyll a does not bind to the apo-protein. However, this observation begs the question, which structural features of Fe-protoporphyrin IX determine its selective binding to apo-cytochrome b6.
Figure 3. Heme but not chlorophyll co-elutes with cytochrome b6 after in vitro reconstitution. Free heme and chlorophyll a, respectively, were added to isolated apo-cytochrome b6 and the protein was purified via Ni-NTA chromatography. After elution of the protein, absorbance spectra were recorded under oxidizing (dark grey) and reducing conditions (grey) in case of heme. Chlorophyll a did not elute from the column together with the protein, therefore a chlorophyll a spectrum is not visible (light grey).
The central iron ion is essential for porphyrin binding to apo-cytochrome b6
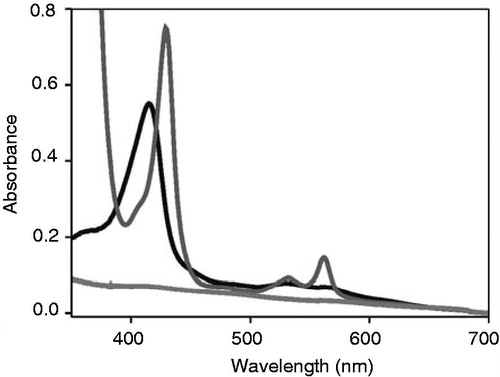
In order to identify structural determinants driving specific binding of heme, but not of chlorophyll a to apo-cytochrome b6, binding of commercially available protoporphyrin IX derivatives, having different central metal ions, to apo-cytochrome b6 was analyzed ( and Supplementary Figure S1, available online). The absorbance spectrum of free protopor-phyrin IX (without a central metal ion) has characteristic maxima at 409, 506, 541, 578 and 632 nm. Addition of apo-cytochrome b6 to protoporphyrin IX did neither influence the maxima of protoporphyrin IX absorbance spectrum nor the band widths at half maximum (). Thus, the protoporphyrin IX environment is not altered (), and hence apo-cytochrome b6 does not bind protoporphyrin IX. As the heme central ion interacts with the heme-ligating His residues of cytochrome b6, this observation assorts well with the recent observation that heme binding to apo-cytochrome b6 can be disturbed when the heme ligating His residues are mutated to Ala (Kuras et al. Citation1997, Dreher et al. Citation2008). Thus, these data indicate that binding of a metal center by the cytochrome b6 His residues is crucial for porphyrin binding to apo-cytochrome b6. To subsequently monitor interactions of the protein with a metal center in greater detail, we next analyzed binding of the chlorophyll synthesis intermediate Mg-protoporphyrin IX, which differs from heme only in its metal center.
Figure 4. Apo-cytochrome b6 does not bind porphyrins with central and porphyrins in presence of 145 μg apo-cytochrome b6 (grey) and porphyrins in presence of 145 mg apo-cytochrome b6 (grey) are show nunder oxidizing conditions. (A) ProtoporphyrinIX, (B) Mg-protoporphyrin IX.
As shown in , the absorbance maxima and the band width at half maximum of the free porphyrin were not influenced by addition of apo-cytochrome b6 (), and thus replacement of Fe2+/3+ by Mg2+ already eliminates binding of the protoporphyrin IX molecule. In addition, we have also analyzed binding of protoporphyrin IX derivatives, which have zinc or manganese as central metal ions (Supplementary Figure S1) and also these analyzed protoporphyrins do not appear to interact with the apo-cytochrome, which further highlights the special role of the Fe2+/3+-center. While the central Fe2+/3+ ions of cytochromes are typically hexa-coordinated in vivo, the Mg2+ ions of most chlorophylls are only penta-coordinated (Cogdell et al. Citation2003, Smith et al. Citation2010). Because of this, the position of the Mg2+ ion is slightly out of the equatorial plane, and it has been argued that a sixth axial ligand would be energetically less favorable (Storm Citation1970). Thus, the preferred penta- vs. hexa-coordination state might explain the observed differences in chlorophyll vs. heme binding described here. However, as the Mg2+ ions of a certain fraction of chlorophylls are also hexa-coordinated in vivo, other factors are most likely as well crucial for binding of defined tetrapyrroles in vivo. These factors might include, but are not limited to, the His ligand side-chain rotamers (Braun et al. Citation2011) and the different tetrapyrrole ring substituents (as further addressed below).
Taken together, these data indicate that specifically Fe-protoporphyrin IX interacts with the apo-cyto-chrome, and replacement by a different metal ion or its complete removal prevents distinct binding to apo-cytochrome b6. This leads to the assumption that it is the central iron ion, which is crucial for specific binding of Fe-protoporphyrin IX to apo-cytochrome b6, which already nicely elucidates why cytochrome b6 specifically binds Fe-protoporphyrin IX in vivo but not chlorophyll or chlorophyll precursors, as further discussed below.
The propionyl and ethylene side chains of protoporphyrin IX affect binding of Fe-protoporphyrin IX to apo-cytochrome b6
While the above presented results suggest that the central metal ion is crucial for binding of Fe-protoporphyrin IX to the TM apo-cytochrome, a potential impact of the tetrapyrrole side chains on the stability of cytochrome b6 remains open. Based on previous analyses, apo-cytochrome b6 folds into a structure, which allows binding of the heme cofactors, resulting in structural rearrangements and formation of the holo-cytochrome (Dreher et al. Citation2007, Citation2008, Prodöhl et al. Citation2007). Many forces guide formation and stability of the holo-cytochrome, and the entropy loss, which occurs when the protein interacts with the cofactor, is minimized by formation of favorable interactions between the protoporphyrin cofactor and the protein, resulting in an enthalpy gain and finally in formation of the holo-cytochrome. Such favorable interactions might include hydrophobic and van der Waals interactions, as well as formation of hydrogen bonds or salt bridges.
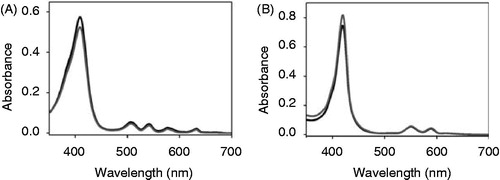
To identify the impact of individual tetrapyrrole ring substituents on binding to the apo-cytochrome, we analyzed binding of two Fe-protoporphyrin IX derivatives ( and ). In case of deuteropor-phyrin IX, the two ethylene groups at rings A and B, which might establish hydrophobic contacts and stabilize van der Waals interactions, are replaced by hydrogens. When apo-cytochrome b6 was added to a solution containing Fe-deuteroporphyrin IX, differences in the absorbance spectra were observed (, ). Under oxidizing conditions the g-maximum of Fe-deuteroporphyrin IX in presence of apo-cytochrome b6 is clearly red-shifted by 5 nm compared to the free porphyrin, and the peak intensity increases in presence of the protein. Furthermore, the pronounced α-band maximum of free Fe-deuteroporphyrin IX at 595 nm disappeared after addition of the apo-cytochrome b6. Under reducing conditions, a 4 nm red shift of the g-maximum was observed after addition of the apo-cytochrome b6 together with a rise in the absorbance intensity, when compared to the free porphyrin. Thus, the observed spectral changes (summarized in ) clearly indicate that this porphyrin interacts with the protein moiety. In the redox difference spectra (inset in ), free Fe-deuteroporphyrin IX has a broad absorbance maximum between 500 and 580 nm without any clearly defined maxima. In contrast, after addition of apo-cytochrome b6 to Fe-deuteroporphyrin IX, a maximum at 547 nm appeared, demonstrating specific binding of Fe-deuteroporphyrin IX to apo-cytochrome b6 and holo-cytochrome formation. These results lead to the conclusion that the ethylene substituents at the tetrapyrrole ring moiety are not essential per se for heme binding. However, compared to the spectra obtained after in vitro reconstitution of cytochrome b6 with heme (), the spectra were less well defined and the width at half maximum of the Soret band was not reduced, which might indicate destabilization of the holo-cytochrome due to reduced cofactor binding, as further analyzed below.
Figure 5. Chemical structures ofheme, chlorophyll a and protoporphyrin IX derivatives used in this study. Arrows indicate side chains varied compared to heme. In Fe-deuteroporphyrin IX ethylene side chains 2 and 4 are substituted for hydrogen atoms whereas in Fe-protoporphyrin IX dimethylester the propionyl groups 6 and 7 are esterified with methyl groups.
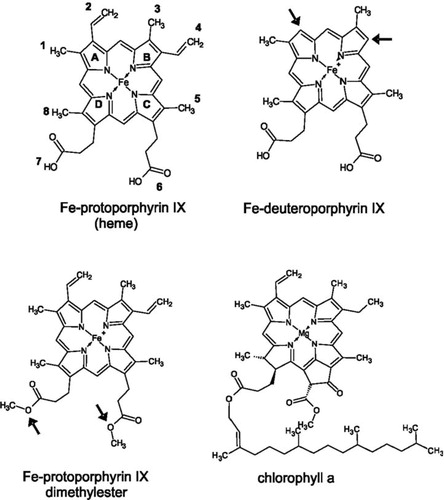
Figure 6. Apo-cytochrome b6 binds porphyrins with modified side chains. Absorbance spectra of Fe-deuteroporphyrin IX (A, B) as well as of Fe-protoporphyrin IX dimethylester (C, D) were recorded under oxidizing (A, C) and reducing (B, D) conditions. The spectrum of free porphyrin (dark grey) is compared to the spectrum of the porphyrin after addition of 150 μg apo-cytochrome b6 (grey). The insets in (B) and (D) are redox difference spectra.
Interaction of the heme propionyl side chains with positively charged amino acid side chains of the protein are discussed to be of special importance for heme binding, and thus for assembly of the holo-cytochrome. Therefore, the second analyzed protoporphyrin IX derivative, the Fe-protoporphyrin IX dimethylester, possesses esterified propionyl side chains at rings C and D ( and ). Under oxidizing conditions, the γ-maximum of the free porphyrin is shifted by 8 nm after the addition of apo-cytochrome b6 (). Under reducing conditions, the free porphyrin shows a broad γ-band at around 400 nm and β- and α-band maximum at 569 nm and 601 nm, respectively. The α-maximum disappeared upon addition of apo-cytochrome b6, and a well-defined γ-maximum at 425 nm, a β-band at 530 nm and an α-band maximum at 559 nm was pronounced. Under both, oxidizing and reducing conditions the absorbance intensity of the γ-maximum rose after addition of the apo-cytochrome b6, which was however more obvious under reducing conditions. Thus, after addition of apo-cytochrome b6, a well defined spectrum was observed under reducing conditions, which is rather different from the spectrum of free Fe-protoporphyrin IX dimethylester. Furthermore, the redox difference spectrum shows two distinct maxima at 530 nm and 560 nm (inset, ), in contrast to the free porphyrin. These differences between the spectral properties demonstrate a specific interaction between Fe-protoporphyrin IX dimethylester and apo-cytochrome b6. However, binding of Fe-protoporphyrin IX dimethylester seems to be weaker compared to Fe-protoporphyrin IX, and the band widths at half maximum do not change that significantly ().
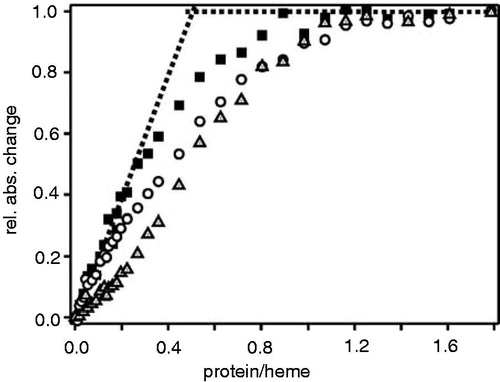
Therefore, to identify differences in binding of these two heme derivatives to the apo-protein in greater detail, we performed titration experiments and measured porphyrin binding to apo-cytochrome b6 by absorbance spectroscopy at different porphyrin-to-protein ratios (). At a ratio of about 2 hemes per protein (i.e. a protein-to-heme ratio of 0.5) the absorbance does not change drastically anymore, indicating that one apo-cytochrome b6 protein binds 2 heme molecules with high affinity, as already analyzed in detail previously (Prodöhl et al. Citation2007, Dreher et al. Citation2008). Furthermore, the titrations also show that apo-cytochrome b6 associates more weakly with the heme derivatives Fe-deuteroporphyrin IX and Fe-protoporphyrin IX dimethylester. In addition, the sigmoidal curve observed in case of protoporphy-rin IX dimethylester suggests that binding of (at least) protoporphyrin IX dimethylester to apo-cytochrome b6 is highly cooperative, i.e. binding of the first heme significantly promotes binding of the second heme. These findings are supported by previous mutational analyses, which have revealed that binding of the low potential heme bL has to occur prior to binding of the high potential heme bH, and thus binding of the first heme is a prerequisite for binding of the second heme (Kuras et al. Citation1997, Dreher et al. Citation2008).
Figure 7. Porphyrin binding isotherms for cytochrome b6. Increasing amounts ofapo-cytochrome b6 were titrated into buffer containing either 5 μM heme (squares), Fe-deuteroporphyrin IX (circles) or Fe-protoporphyrin IX dimethylester (triangles). The protein-to-heme ratio was plotted against the normalized absorbance change at 413 nm. Each data point represents an individual measurement.
Together, the results indicate that interactions of the heme propionyl group with positively charged residues of the polypeptide chain are crucial for high affinity binding of the heme cofactor. Noteworthy, while ionic interactions between the positively charged amino acid side chain and the negatively charged heme propionyl groups are abolished in the case of the analyzed ester, formation of stabilizing hydrogen bonds to the ester carbonyl oxygen is still possible, which might ensure binding of the dimethylester derivative. Furthermore, replacement of the two ethylene groups affects the stability of the protein:heme complex, most likely since favorable van der Waals interactions between the two heme ethylene groups and the protein moiety are eliminated, resulting in less enthalpy gain after binding of the protoporphyrin.
Conclusions
In plants, all tetrapyrroles share a common biosynthesis pathway, which is localized in plastids, and only the last two steps of heme synthesis are localized in mitochondria as well (Cornah et al. Citation2003). The first precursor, 5-aminolaevulinic acid, is generated from glutamate. Two molecules are condensed to porpho-bilinogen, which thereafter polymerizes to form the linear tetrapyrrole 1-hydroxymethylbillane. Ring closure is followed by several decarboxylation and oxidation reactions at the tetrapyrrole macrocycle, finally leading to the formation of protoporphyrin IX (Beale Citation1999, Tanaka and Tanaka Citation2007). At this point chlorophyll and heme synthesis pathways split up. Insertion of Mg2+ by the Mg-chelatase generates Mg-protoporphyrin IX, which is further converted in multiple steps to chlorophyll. Heme is directly synthesized from protoporphyrin IX by insertion of Fe2+ catalyzed by the ferrochelatase. The data presented here indicate that the substituents at the tetrapyrrole ring influence association with a TM apo-cyto-chrome. Typically, heme and chlorophyll cofactors bind at pre-defined binding cavities to the protein moiety. Thus, as chlorophyll a contains a long phytyl side chain, this large ring substituent might already abolish binding of chlorophyll to apo-cytochromes in vivo due to steric clashes. However, binding of the chlorophyll synthesis intermediate Mg-protoporphyrin IX would then not be abolished. Based on the presented results, incorporation of por-phyrins into apo-cytochrome b6 is only possible after the last step of heme synthesis, i.e. after insertion of the central iron ion, is completed. Free protoporphy-rin IX, as well as the chlorophyll synthesis intermediate Mg-protoporphyrin IX, are not able to bind to the cytochrome b6 heme binding sites. Noteworthy, the heme iron center is also crucial for functional assembly of the E. coli TM cytochrome b556 protein (Nihei et al. Citation2001), and while E. coli does not synthesize chlorophyll, in this case the iron-center appears to be also crucial for binding of a protopor-phyrin molecule. Thus, the Fe-chelatase is the key enzyme in the heme biosynthesis pathway, which in the end determines the fate of the protoporphyrin IX moiety and thereby might control assembly of TM b-type cytochromes.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Supplementary Figure S1
Download PDF (119.8 KB)Supplementary Figure S1
Download MS Word (139 KB)Acknowledgements
This work was supported by grants from the Ministry of Science, Research and Arts of Baden-Württem-berg, from the Centre of Complex Matter and from the University of Mainz. We thank Daniel Otzen for helpful discussions and Hildegard Pearson for critically reading the manuscript.
References
- Akdogan Y, Anbazhagan V, Hinderberger D, Schneider D. 2012. Heme binding constricts the conformational dynamics of the cytochrome heme binding cavity. Biochemistry 51:7149–7156
- Beale SI. 1999. Enzymes of chlorophyll biosynthesis. Photosynth Res 60:43–73
- Bernát G, Rogner M. 2011. Center of the cyanobacterial electron transport network: The cytochrome b6f complex. In: Peschek GA, et al. editors. Bioenergetic processes of cyanobacteria. Dordrecht, Heidelberg, London, New York: Springer Science and Business Media B.V. pp 573–605
- Braun P, Goldberg E, Negron C, von Jan M, Xu F, Nanda V, et al. 2011. Design principles for chlorophyll-binding sites in helical proteins. Proteins 79:463–476
- Cammarata KV, Schmidt GW. 1992. In vitro reconstitution of a light-harvesting gene product: Deletion mutagenesis and analyses of pigment binding. Biochemistry 31:2779–2789
- Cogdell RJ, Isaacs NW, Freer AA, Howard TD, Gardiner AT, Prince SM, et al. 2003. The structural basis of light-harvesting in purple bacteria. FEBS Lett 555:35–39
- Cordova JM, Noack PL, Hilcove SA, Lear JD, Ghirlanda G. 2007. Design of a functional membrane protein by engineering a heme-binding site in glycophorin A. J Am Chem Soc 129:512–518
- Cornah JE, Terry MJ, Smith AG. 2003. Green or red: what stops the traffic in the tetrapyrrole pathway? Trends Plant Sci 8:224–230
- Cramer WA, Hasan SS, Yamashita E. 2011. The Q cycle of cytochrome bc complexes: a structure perspective. Biochim Biophys Acta 1807:788–802
- Cramer WA, Whitmarsh J. 1977. Photosynthetic cytochromes. Annu Rev Plant Physiol Plant Mol Biol 28:133–172
- Cramer WA, Yan J, Zhang H, Kurisu G, Smith JL. 2005. Structure of the cytochrome b6f complex: new prosthetic groups, Q-space, and the ‘hors d’oeuvres hypothesis’ for assembly of the complex. Photosynth Res 85:133–143
- Cross AR, Rae J, Curnutte JT. 1995. Cytochrome b-245 of the neutrophil superoxide-generating system contains two noniden-tical hemes. J Biol Chem 270:17075–17077
- Cutler RL, Davies AM, Creighton S, Warshel A, Moore GR, Smith M, et al. 1989. Role of arginine-38 in regulation of the cytochrome-C oxidation-reduction equilibrium. Biochemistry 28:3188–3197
- de Vitry C. 2011. Cytochrome c maturation system on the negative side of bioenergetic membranes: CCB or System IV. FEBS J 278:4189–4197
- de Vitry C, Desbois A, Redeker V, Zito F, Wollman FA. 2004. Biochemical and spectroscopic characterization of the covalent binding of heme to cytochrome b6. Biochemistry 43:3956–3968
- Dreher C, Prodohl A, Hielscher R, Hellwig P, Schneider D. 2008. Multiple step assembly of the transmembrane cytochrome b6. J Mol Biol 382:1057–1065
- Dreher C, Prodöhl A, Weber M, Schneider D. 2007. Heme binding properties of heterologously expressed spinach cytochrome b (6): Implications for transmembrane b-type cytochrome formation. FEBS Lett 581:2647–2651
- Francke C, Loyal R, Ohad I, Haehnel W. 1999. In vitro assembly of a beta2 cytochrome b559-like complex from the chemically synthesised beta-subunit encoded by the Synechocystis sp. 6803 psbF gene. FEBS Lett 442:75–78
- Gray HB, Winkler JR. 1996. Electron transfer in proteins. Annu Rev Biochem 65:537–561
- Hasan SS, Yamashita E, Cramer WA. 2013. Transmembrane signaling and assembly of the cytochrome bf-lipidic charge transfer complex. Biochim Biophys Acta 1827:1295–1308
- Horn R, Grundmann G, Paulsen H. 2007. Consecutive binding of chlorophylls a and b during the assembly in vitro of light-harvesting chlorophyll-a/b protein (LHCIIb). J Mol Biol 366:1045–1054
- Kamiya N, Shen JR. 2003. Crystal structure of oxygen-evolving photosystem II from Thermosynechococcus vulcanus at 3.7-A resolution. Proc Natl Acad Sci USA 100:98–103
- Koch HG, Schneider D. 2007. Folding, assembly and stability of transmembrane cytochromes. Curr Chem Biol 1:59–74
- Korendovych IV, Senes A, Kim YH, Lear JD, Fry HC, Therien MJ, et al. 2010. De novo design and molecular assembly of a transmembrane diporphyrin-binding protein complex. J Am Chem Soc 132:15516–15518
- Kuras R, Buschlen S, Wollman FA. 1995. Maturation of pre-apocytochrome f in vivo. A site-directed mutagenesis study in Chlamydomonas reinhardtii. J Biol Chem 270:27797–27803
- Kuras R, de Vitry C, Choquet Y, Girard-Bascou J, Culler D, Buschlen S, et al. 1997. Molecular genetic identification of a pathway for heme binding to cytochrome b6. J Biol Chem 272:32427–32435
- Kurisu G, Zhang H, Smith JL, Cramer WA. 2003. Structure ofthe cytochrome b6f complex of oxygenic photosynthesis: tuning the cavity. Science 302:1009–1014
- Lujan MA, Martinez JI, Alonso PJ, Guerrero F, Roncel M, Ortega JM, et al. 2012. Reconstitution, spectroscopy, and redox properties of the photosynthetic recombinant cytochrome b (559) from higher plants. Photosynth Res 112:193–204
- Malnoe A, Wollman FA, de Vitry C, Rappaport F. 2011. Photo-synthetic growth despite a broken Q-cycle. Nat Commun 2:301
- Martinez SE, Huang D, Szczepaniak A, Cramer WA, Smith JL. 1994. Crystal structure of chloroplast cytochrome f reveals a novel cyto-chrome fold and unexpected heme ligation. Structure 2:95–105
- Moulin M, Smith AG. 2005. Regulation of tetrapyrrole biosynthesis in higher plants. Biochem Soc Trans 33(Pt 4):737–742
- Nihei C, Nakayashiki T, Nakamura K, Inokuchi H, Gennis RB, Kojima S, et al. 2001. Abortive assembly of succinate-ubiquinone reductase (complex II) in a ferrochelatase-deficient mutant of Escherichia coli. Mol Genet Genomics 265:394–404
- Paulsen H, Rümler U, Rudiger W. 1990. Reconstitution of pigment-containing complexes from light-harvesting chloro-phyll-a/b-binding protein overexpressed in Escherichia coli. Planta 181:204–211
- Prodöhl A, Dreher C, Hielscher R, Hellwig P, Schneider D. 2007. Heterologous expression and in vitro assembly of the trans-membrane cytochrome b6. Protein Expr Purif 56:279–285
- Prodöhl A, Volkmer T, Finger C, Schneider D. 2005. Defining the structural basis for assembly of a transmembrane cytochrome. J Mol Biol 350:744–756
- Saint-Marcoux D, Wollman FA, de Vitry C. 2009. Biogenesis of cytochrome b6 in photosynthetic membranes. J Cell Biol 185:1195–1207
- Schaefer G, Purschke W, Schmidt CL. 1996. On the origin of respiration: Electron transport proteins from archaea to man. FEMS Microbiol Rev 18:173–188
- Shinopoulos KE, Brudvig GW. 2012. Cytochrome b559 and cyclic electron transfer within photosystem II. Biochim Biophys Acta 1817:66–75
- Smith LJ, Kahraman A, Thornton JM. 2010. Heme proteins – diversity in structural characteristics, function and folding. Proteins 78:2349–2368
- Stroebel D, Choquet Y, Popot JL, Picot D. 2003. An atypical haem in the cytochrome b(6)f complex. Nature 426:413–418
- Storm CB. 1970. Conformation of metalloporphyrins in solution. J Am Chem Soc 92:1423
- Tanaka R, Tanaka A. 2007. Tetrapyrrole biosynthesis in higher plants. Annu Rev Plant Biol 58:321–346
- Thöny-Meyer L. 1997. Biogenesis of respiratory cytochromes in bacteria. Microbiol Mol Biol Rev 61:337–376
- Volkmer T, Becker C, Prodöhl A, Finger C, Schneider D. 2006. Assembly of a transmembrane b-type cytochrome is mainly driven by transmembrane helix interactions. Biochim Biophys Acta 1758:1815–1822
- von Jagow G, Sebald W. 1980. b-Type cytochromes. Annu Rev Biochem 49:281–314
- Weber M, Schneider D. 2013. Six amino acids define a minimal dimerization sequence and stabilize a transmembrane helix dimer by close packing and hydrogen bonding. FEBS Lett 587:1592–1596
- Willows RD. 2003. Biosynthesis of chlorophylls from protoporphyrin IX. Nat Prod Rep 20:327–341
- Yun CH, Crofts AR, Gennis RB. 1991. Assignment of the histi-dine axial ligands to the cytochrome bH and cytochrome bL components of the bc1 complex from Rhodobacter sphaer-oides by site-directed mutagenesis. Biochemistry 30:6747–6754
- Zouni A, Witt HT, Kern J, Fromme P, Krauss N, Saenger W, et al. 2001. Crystal structure of photosystem II from Synechococcus elongatus at 3.8 A resolution. Nature 409:739–743
