Abstract
Atomic Force Microscopy (AFM) has become an invaluable tool for studying the micro- and nanoworlds. As a stand-alone, high-resolution imaging technique and force transducer, it defies most other surface instrumentation in ease of use, sensitivity and versatility. The main strength of AFM relies on the possibility to operate in an aqueous environment on a wide variety of biological samples, from single molecules – DNA or proteins – to macromolecular assemblies like biological membranes. Understanding the effect of mechanical stress on membranes is of primary importance in biophysics, since cells are known to perform their function under a complex combination of forces. In the later years, AFM-based Force-Spectroscopy (AFM-FS) has provided a new vista on membrane mechanics in a confined area within the nanometer realm, where most of the specific molecular interactions take place. Lipid membranes are electrostatically charged entities that physiologically coexist with electrolyte solutions. Thus, specific interactions with ions are a matter of considerable interest. The distribution of ions in the solution and their interaction with the membranes are factors that substantially modify the structure and dynamics of the cell membranes. Furthermore, signaling processes are modified by the membrane capability of retaining ions. Supported Lipid Bilayers (SLBs) are a versatile tool to investigate phospholipid membranes mimicking biological surfaces. In the present contribution, we review selected experiments on the mechanical stability of SLBs as models of lipid membranes by means of AFM-FS, with special focus on the effect of cations and ionic strength in the overall nanomechanical stability.
Introduction
Cells can be thermodynamically defined as open systems in constant exchange of mass, energy and information with the environment. To this end, the cell membrane is a key structure that defines the confines of the cell and some of its internal compartments. Besides, it is not only essential as a structural part of the cell, but it also provides a support matrix for many types of proteins that are inserted on it (Dowhan Citation1997). In the early 1970s, Singer and Nicolson proposed the fluid-mosaic-model (Singer and Nicolson Citation1972), depicturing cell membranes as two-dimensional liquids where all lipid and protein molecules diffuse easily. Concerning the composition, besides all the protein and carbohydrates, lipids are the main components in molar fraction of the cell membrane (van Meer et al. Citation2008). Lipids are a broad family which covers many different chemical structures: Sphingolipids (ceramides, sphyngomyelin, gangliosides, sphingosines); sterols (cholesterol and vitamins); or phospholipids. Biological membranes are electrostatically charged entities that physiologically coexist with electrolyte solutions. Thus, specific interactions with ions are a matter of considerable interest (Bockmann et al. Citation2003, Pandit et al. Citation2003, Bockmann and Grubmuller Citation2004, Gurtovenko et al. Citation2005). The distribution of ions in the solution and their interaction with the membrane are factors that substantially modify the structure and dynamics of the cell membranes (Aroti et al. Citation2007, Miettinen et al. Citation2009). Furthermore, signaling processes are modified by the membrane capability of retaining ions (Berkowitz et al. Citation2006).
Supported lipid bilayers (SLBs) are a versatile platform to investigate phospholipid membranes mimicking biological systems. These lipid bilayers are planar, well-organized, reproducible, composition-tunable and easy to obtain. They have shown to be viable model systems of the plasma membrane.
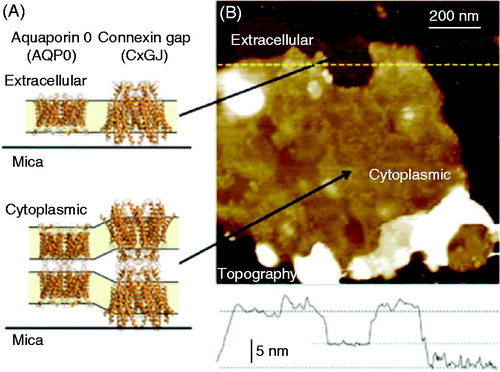
Since it was born about 30 years ago, Atomic Force Microscopy (AFM), a scanning probe microscopy technique (Binnig et al. Citation1986), has become an invaluable tool to study micro- and nanoworlds. Regarding the study of biological systems, the main strength of AFM relies on the possibility to operate in a liquid environment on a wide variety of biological samples, from single molecules such as DNA or proteins to macromolecular assemblies or even whole cells (Parot et al. Citation2007). AFM emerged as a well-established technique for imaging the lateral organization of SLBs. The main advantage in respect to other techniques is that the structure of biological samples, such as cell or lipid membranes can be visualized, not only in liquid media, but in real time with (sub)nanometer resolution (Dufrêne Citation2008, Mueller and Dufrêne Citation2008). illustrates an example of high-resolution AFM imaging used to study native plasma membranes isolated from the eye lens. For a review about the most outstanding analysis in SLBs using AFM imaging see El Kirat et al. (Citation2010). Moreover, due to the ability of AFM to sense and apply forces with pN sensitivity, it is an excellent tool to gather information about molecular interactions at the single molecule level through what is known as force spectroscopy (FS) (Lee et al. Citation1994, Corcoran et al. Citation1997, Fisher et al. Citation1999, Giannotti and Vancso Citation2007). Nanomechanical studies of lipid bilayers contribute to the comprehension of fundamental aspects concerning the structural, mechanical, and biological properties of membranes.
Figure 1. AFM imaging allows SLBs observations. Isolated plasma membranes from the eye lens maintain the native double-layered architecture. (A) Crystal structure representation of the lens membrane proteins connexin and aquaporin 0, showing single layer configuration (exposing the extracellular side) and the native, double layer configuration (exposing the cytoplasmic side) (PDB codes 2ZW3 and 2B6O.40,41 Structural images rendered using Pymol, http://www.pymol.org). (B) Overview topography of an isolated lens membrane revealing a darker, single layer region and a brighter double layer region. The line graph shows the cross-section along the dotted line in the image and reveals the thickness of the single and double layers. The false color scale is 25 nm. Reprinted by permission from the Royal Society of Chemistry (Rico et al. Citation2013).
Herein, we review selected mechanical experiments on SLBs using AFM-FS. Focus is placed on analyzing the influence of different cations and ionic strength on the mechanical stability of membranes.
Supported lipid bilayers
Lipid bilayers are among the most important self-assembled structures in nature. In particular, phospholipid bilayers resemble cell membranes in key aspects; for instance, they retain two-dimensional fluidity and suppose an excellent environment for the insertion of membrane proteins. Therefore, model bilayer systems are very manageable platforms to investigate biological processes that occur at the cellular or subcellular level. Such model membranes have proved to be appropriate for studies of cell signaling (Heldin Citation1995, Kasahara and Sanai Citation2001, Qi et al. Citation2001, Stoddart et al. Citation2002), pathogen attack (Ono and Freed Citation2001, Xu et al. Citation2002) and ligand-receptor interactions (Plant et al. Citation1995, Yang et al. Citation2003), among others. The first time a planar bilayer system was reported to be used for research purposes was in the 1960s by Mueller et al. (Citation1962), consisting of two solution chambers with a 1 mm hole painted by lipid molecules, the so-called black lipid membranes (Ottovaleitmannova and Tien Citation1992). Since then, several supported systems have emerged in order to develop more sophisticated approaches for both biophysical studies and sensor design: Self-assembled monolayer-monolayer systems, polymer-cushioned phospholipid bilayers, arrays of supported lipid phospholipid membranes, bilayer coated microfluidics and solid-supported lipid bilayers (SLBs).
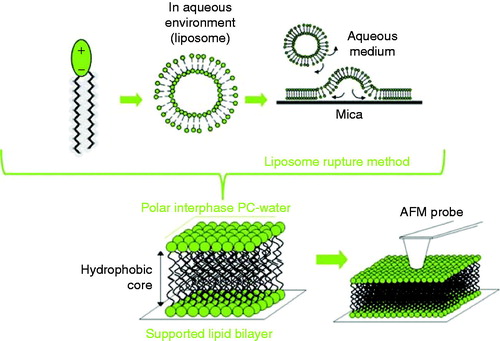
SLBs are robust and stable systems suitable to be studied by AFM. The most used SLB preparation methods are Langmuir-Blodgett (LB) and the liposome rupture method. The LB technique consists on a lipid monolayer disposed in a water-air interface, with lateral mobile barriers by which the monolayer is compressed (Talham et al. Citation2008). Then, the phospholipid monolayer may be transferred to a solid substrate at a controlled surface pressure and speed (called LB when the substrate is disposed perpendicular in respect to the monolayer and Langmuir-Schaefer when they are parallel) (Vie et al. Citation1998). In order to obtain a lipid bilayer, a second transference can be made on top of the previous monolayer, if it is of different composition, it would form an asymmetric SLB (Dufrêne et al. Citation1997, Rinia et al. Citation1999). On the other hand, the liposome rupture method is simple and a very popular method to prepare SLBs (Brian and McConnell Citation1984, Jass et al. Citation2000, Richter Citation2006, Attwood et al. Citation2013). As shown in , the method consists of the deposition of a suspension of small unilamellar vesicles (SUVs) onto the substrate, in order for the SUVs to adsorb from the bulk onto the surfaces. Despite the mechanism not being fully understood, it is generally acknowledged that SUVs in the substrate may fuse with one another, deform, flatten and finally rupture to form a continuous SLB (Mingeot-Leclercq et al. Citation2008). The final structure has been reported to be strongly influenced by factors such as SUVs’ size, lipid composition and concentration, pH, temperature, ionic strength, the presence of divalent cations (especially Ca2+ and Mg2+), surface roughness and surface charge density (Stelzle et al. Citation1993, Reimhult et al. Citation2003). Suitable substrates for SLBs formation should be clean, smooth and hydrophilic; mica substrate is the most popular one.
Nanomechanical characterization of lipid membranes
Lipid bilayers are closely related to biomembrane function, not only because of their structural role under a complex combination of forces (Vogel and Sheetz Citation2006), but also due to their contribution to the function of several membrane proteins (Ursell et al. Citation2008, Phillips et al. Citation2009). Specifically, the chemical composition of lipid bilayers has been shown to take a part in the gating process of ion and mechanosensitive channels (Perozo et al. Citation2002, Oliver et al. Citation2004, Suchyna et al. Citation2004, Ramu et al. Citation2006, Schmidt et al. Citation2006, Schmidt and MacKinnon Citation2008). The concrete mechanochemical change of the membrane is also significantly related to the correct unfolding (Hong and Tamm Citation2004) and aggregation processes (Reynwar et al. Citation2007) of membrane proteins. Therefore, there has been increasing interest in understanding the interplay between the lateral lipid organization and the overall membrane function.
The mechanical properties of lipid bilayers have been assessed by means of different techniques. Probably the most remarkable one is the micropipette aspiration technique (Evans and Rawicz Citation1990, Rawicz et al. Citation2000), which has provided a great deal of information regarding quantitative values for membrane elastic moduli for shear and bending, and also for interbilayer friction (Evans et al. Citation2003, Heinrich and Rawicz Citation2005). However, this technique is limited to the use of giant vesicles, thus providing a microscopic insight on bilayer mechanical stability. At the nanometer scale, the surface force apparatus (SFA) has provided valuable information regarding lipid bilayer adhesion, fusion and healing, as well as interaction forces between lipid bilayers (Marra and Israelachvili Citation1985, Helm et al. Citation1989, Benz et al. Citation2004). Within the last decades, AFM-FS has emerged as an essential tool to quantitatively characterize the mechanical properties of lipid membranes, with the advantage of high spatial range sensitivity and versatility, as well as the possibility of locating and probing confined areas of membranes under environmentally controlled conditions (Garcia-Manyes and Sanz Citation2010, Picas et al. Citation2012, Redondo-Morata et al. Citation2012c).
Breakthrough force as a molecular fingerprint
AFM-based Force Spectroscopy (FS) has proven to be a suitable technique to assess the mechanical properties of a wide variety of systems at the nanoscale, like the indentation of hard materials during the approach of the AFM tip to the surface (Corcoran et al. Citation1997) and the stretching of discrete macromolecular structures such as polysaccharides (Giannotti and Vancso Citation2007), proteins (Fisher et al. Citation1999) and DNA (Lee et al. Citation1994) while the tip retracts away from the surface. This technique directly measures the interaction forces when a small number of molecules take part. In the case of lipid membranes, FS is especially valuable in terms of spatial accuracy and force resolution. Classically, FS experiments are conducted under constant velocity conditions, this is, the deflection of the cantilever is measured while the AFM tip is approaching and retracting from a surface. When the spring constant of the cantilever is known, the measured deflection is converted into force. To illustrate this scenario, a schematic representation of a typical AFM-FS force curve for the indentation of an SLB is shown in .
Figure 3. Probing the mechanical stability of a lipid bilayer with AFM-FS. (Left) typical plot of the vertical force versus the vertical piezo movement towards the surface. Three different parts can be distinguished in the curve associated to the progression described in the cartoons: (a) the AFM tip and sample are not yet in mechanical contact, (b) the tip elastically deforms the lipid bilayer, and (c) the tip ruptures the bilayer and becomes in contact with the mica substrate. In the right graph, force is represented versus tip-sample separation, where the force at which the bilayer ruptures, the breakthrough force Fb, is highlighted. With this kind of plot, bilayer thickness can be measured.
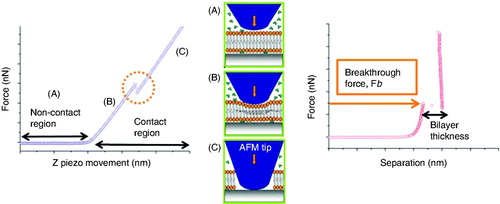
Experimentally, the lipid bilayer spread onto the mica substrate is identified by means of AFM imaging. Then, a set of force curves is performed in the center of the bilayer domains by approaching and retracting the AFM cantilever (ended in a sharp tip) at constant velocity. When the cantilever tip is away from the bilayer sample, there is no mechanical interaction between the tip and the bilayer (). On further approaching the cantilever tip to the bilayer sample, they become in mechanical contact. As the tip approaches the sample, the short-range interactions between tip and sample start arising. These interaction forces have different origins, the most remarkable being Derjaguin-Landau-Verwey-Overbeek (DLVO) forces (corresponding to the charge of the so-called diffuse layer and van der Waals forces), hydration forces and steric forces (Butt et al. Citation2005). The origin and magnitude of these forces are decidedly dependent on the bilayer chemistry, the AFM tip chemistry and the physicochemical properties of the liquid environment. Frequently, the combination of these forces gives rise to a few hundred piconewtons. Then, the bilayer is elastically deformed by the AFM probe () until the tip suddenly ruptures the membrane and becomes in contact with the substrate (). As depicted in , a discontinuity can be observed on the approaching force curve; this is interpreted as the penetration of the AFM tip through the SLB (Franz et al. Citation2002). The vertical force at which this discontinuity happens is the maximum force the bilayer is able to withstand before breaking, and is the so-called breakthrough force (Fb) – or yield threshold force. This discontinuity measures ca. 4 nm in separation, which correlates well with the height of a lipid bilayer. Such a breakthrough event typically occurs at several nN of force. Noticeably, these force measurements are highly reproducible in sequential experiments – performed at the same velocity; even for different tips and samples, the distribution of Fb values is consistent, and the error is almost entirely derived from the calibration of the spring constant of the cantilever (when equipartition theorem is used, error in calibration is around 10–15% [Proksch et al. Citation2004]). Analogous to the force required to indent hard materials as single crystals (Fraxedas et al. Citation2002), the deshybridization force of DNA (Clausen-Schaumann et al. Citation2000, Krautbauer et al. Citation2000), the chair-boat transition force in polysaccharides (Marszalek et al. Citation1998) or the unfolding force of single proteins (Carrion-Vazquez et al. Citation1999), the breakthrough force during indentation of lipid bilayers clearly fingerprints the nanomechanical stability of this system.
These kind of breakthrough events have been detected during the nanomechanical study of a wide variety of thin films. It was first reported in the case of a surfactant bilayer (Ducker and Clarke Citation1994), but it has also been observed probing the ordering mechanisms of supported monolayers (Oncins et al. Citation2008a, Citation2008b, Torrent-Burgues et al. Citation2006, Citation2008) and characterization of squeezed liquid films (Hofbauer et al. Citation2009, O’Shea et al. Citation2010). In the case of the lipid bilayers, the first report of a breakthrough event was performed by Dufrêne et al. (Citation1997, Citation1998) when analyzing DSPE (1,2-distearoyl-sn-glycero-3-phosphoethanolamine), DGDG (digalactosyldiglyceride) and DOPE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine) bilayer systems. Since then, Fb value can be considered as the fingerprint of the bilayer stability under the experimental conditions in which the measurements are performed. So far, different variables were reported to affect the mechanical stability of lipid bilayers and, as a consequence, the Fb value: temperature, lipid chemistry, bilayer composition, pH, ionic strength and electrolyte composition (Garcia-Manyes et al. Citation2005a, Citation2005b, Citation2006, Garcia-Manyes and Sanz Citation2010, Redondo-Morata et al. Citation2012b, Citation2012c).
A theoretical model that relates the force of the film rupture to microscopic properties of the bilayer has been described by Butt et al. (Butt and Franz Citation2002, Loi et al. Citation2002). According to this model, the rupture of the membrane is an activated process, with an associated energy barrier that follows the Arrhenius law, a fact supported by the exponential dependence of the Fb to the tip velocity (Sisquella et al. Citation2010). The distribution of forces necessary to create a hole under the AFM tip is closely related to the line tension (Γ), which represents the free energy associated with the unsaturated bonds of the molecules at the edge of the hole, and with the effective spreading pressure (S), which is used to quantify the tendency of the film to spread into the gap between the tip and the substrate. This model has some limitations since, for instance, it simplifies the molecular nature of the membrane, as it is considered a fluid in the lateral dimension; however, it has been shown to provide realistic quantitative numbers to explain film indentation on supported substrates under a wide variety of conditions (Chiantia et al. Citation2006, Garcia-Saez et al. Citation2007b, Garcia-Manyes et al. Citation2010, Redondo-Morata et al. Citation2012a).
SLBs mechanics is a combination of forces
In order to understand the mechanical interaction between the AFM probe and the lipid bilayer, the nature of the different forces that arise in an FS experiment must be taken into account. As previously mentioned, at this length-scale (below 100 nm), and prior to the contact between the tip and the sample, the main interactions are electrostatic and van der Waals forces, i.e. DLVO interactions. Furthermore, hydration and steric forces have also been described as being responsible for short range interactions in the membranes (Nabika et al. Citation2008). In order to assess and compare experimentally the effect of different cations or different ionic strength in the mechanical response of the membrane it is necessary to independently measure the DLVO forces arising between the studied samples and the AFM probes.
On the one hand, to calculate the interaction forces between the sample and the tip, the surface charge density (σ) value of the probe has to be experimentally calculated. To do that, force curves can be performed on the back of a silicon nitride chip coming from the same batch as the tested AFM probe. Considering that the σ value of both surfaces in contact is the same, the application of the DLVO theory is straightforward. Experimental details of this method can be found in Yin and Drelich (Citation2008). The effective σ value was calculated in Redondo-Morata et al. (Citation2012d), resulting in a value of −0.040 cm−2.
On the other hand, in order to calculate the σ value of the lipid membranes, a first approach can be performed by measuring the liposomes mobility in an electrophoretic field and the ξ-potential value. When a particle like a liposome is dispersed in a liquid medium, ξ-potential corresponds to the potential difference between the dispersion medium and the stationary layer of fluid attached to this dispersed particle.
DLPC (1,2-dilauroyl-sn-glycero-3-phosphocholine) and DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine) bilayers are interesting model membranes as they only differ in the number of -CH2- groups in the fatty acid chain. DPPC, with 16 -CH2- groups per chain, has a liquid-gel transition temperature (Tm) of 42 °C, and is tested in its gel phase at room temperature, whereas DLPC, with 12 -CH2- groups per chain, has a Tm = −2 °C, so at room temperature it is tested in its liquid phase. shows the experimental ξ-potential values for both DPPC and DLPC liposome suspensions in the presence of the different cations in the medium. Electrolytes increase the net ξ-potential values of liposomes in respect to water, reflecting that indeed positive cations may adsorb on the surface of the polar head of the phospholipid molecules in a greater extent than negative ions. Phosphatidylcholine (PC) phospholipid heads are zwitterionic, so they are theoretically uncharged at neutral pH, but actually they are negatively charged when they are in solution. This is mainly due to the orientation of the phospholipid headgroups and the hydration layers formed around the headgroup-water interface (Domingo et al. Citation1994, McIntosh Citation1996). ξ-potential values experimentally measured can be then used to calculate the σ of the lipid bilayer (Butt Citation1992).
Table 1. ξ-potential values of the DPPC and DLPC liposomes depending on the cation present in the measuring solution. Buffer solutions correspond to 150 mM of the electrolyte and blank experiment corresponds to measurements in pure water.
DLVO forces have a slight contribution in the total AFM tip-sample interaction
According to the DLVO theory and using the calculated σ values for the AFM tip (σtip) and the σ value of the SLB (σlipid), DLVO interaction between the two surfaces as the tip approaches the bilayer can be calculated by adding van der Waals forces to electrostatic forces. The theoretical model was originally developed by Drelich et al. (Drelich et al. Citation2007, Yin and Drelich Citation2008) and allows calculation of electrostatic and van der Waals forces for a conical tip-flat substrate system.
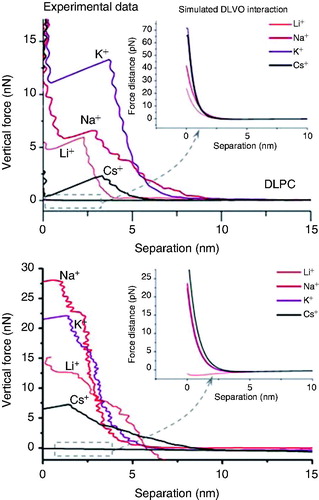
shows a set of data corresponding to the force curves obtained for DPPC and DLPC. In the inset, it is shown the calculated DLVO interaction, considering the experimental data for σtip and σlipid values obtained in Redondo-Morata et al. (Citation2012d). As it can be seen, while the experimental force values reach up to tens of nN, the calculated interaction is a slight repulsion in the pN range both for DLPC and DPPC for all the studied electrolyte solutions. At these high ionic strength conditions, the surface charges of both SLB and AFM tip are highly screened, so tip-sample interaction is mostly mechanical. Interestingly, it seems that experimentally measured breakthrough force values and tip-sample interactions in the contact region should not be discussed in terms of electrostatic interaction but in terms of mechanical deformation.
Figure 4. Fb vs. separation curves performed on DLPC bilayers (upper graph) and DPPC bilayers (lower graph) immersed in different buffer solutions. The insets correspond to the simulated DLVO interactions considering the experimentally measured σtip and σsample values. Reprinted by permission from the Biophysical Society (Redondo-Morata et al. Citation2012d).
Effect of different cations on the mechanical stability of lipid bilayers
The specific chemical environment strongly modifies both the structural and thermodynamic behavior of SLBs. Regarding the influence of the electrolyte solution on the membrane, it is found that ‘specific’ effects for different ions of the same charge, in many cases, follow a trend as a function of the ion size (Petrache et al. Citation2006b, Aroti et al. Citation2007, Garcia-Celma et al. Citation2007, Leontidis et al. Citation2007, Citation2009, Leontidis and Aroti Citation2009). Detailed information about the location of ions with respect to the phospholipid membranes can be obtained from Molecular Dynamics (MD) simulations (Bockmann and Grubmuller Citation2004, Pandit et al. Citation2004, Gurtovenko Citation2005, Gurtovenko et al. Citation2005, Petrache et al. Citation2006a, Cordomi et al. Citation2008, Citation2009, Yi et al. Citation2008), which show that ion binding modifies the area per lipid, lipid ordering, orientation of the lipid head dipole and the charge distribution along the system, among others. One of the most studied ions, partly because of its biological relevance, is K+, which has been the subject of controversy in literature. MD simulations showed that Na+ ions have a strong effect on PC bilayers, increasing the lateral interaction between phospholipid molecules. Although the binding of K+ was found to be much weaker, mainly due to the larger size of K+ compared to Na+ (Cordomi et al. Citation2008), these results were not conclusive due to the uncertainty in the estimation of the strength of the different force-fields (Gurtovenko and Vattulainen Citation2008, Cordomi et al. Citation2009). In addition, in some simulations it was pointed out that the size of K+ ion could have been exaggerated (Gurtovenko and Vattulainen Citation2008). Despite the current computational resources, the dynamics simulations of ions within a lipid bilayer cannot be as long as they should in order to fully understand the interaction between the ion and the surrounding phospholipids. Nevertheless, differences in the residence times and binding behavior between different ion species were observed (Cordomi et al. Citation2008, Citation2009, Miettinen et al. Citation2009).
AFM-FS has shown to be a very suitable technique to shed light in this particular topic. In a recent work, Redondo-Morata et al. (2010) systematically probed SLBs of distinct composition immersed in electrolytes composed of a variety of monovalent and divalent metal cations, providing information which clearly demonstrates that there is an independent and important contribution of each ion to the gross mechanical resistance of the lipid membranes. In the later subsections we are going to illustrate the effect of the metallic cations in the resilience of SLBs.
Alkali cations
Redondo-Morata et al. (Citation2012d) quantified the Fb values for DPPC and DLPC model SLBs for different alkali cations in the solution, which are thought to change the lateral interactions between molecules via cohesive electrostatic interactions. DPPC and DLPC bilayers were tested in the presence of different electrolyte solutions: NaCl, KCl, LiCl, CsCl, TEA (150 mM) and a blank (pure water). The mean Fb values obtained are shown in . As it can be seen, Fb values are always higher in the case of DPPC; solid-like phases are known to show higher Fb values than liquid phases (Leontidis and Aroti Citation2009) as they show a smaller intermolecular distance. This fact increases the van der Waals interactions between hydrocarbon chains and it also draws polar headgroups closer, greatly increasing the electrostatic attractive interactions.
Figure 5. Fb values vs. ionic composition for DPPC and DLPC bilayers immersed in different electrolyte solutions. Reprinted by permission from the Biophysical Society (Redondo-Morata et al. Citation2012d).
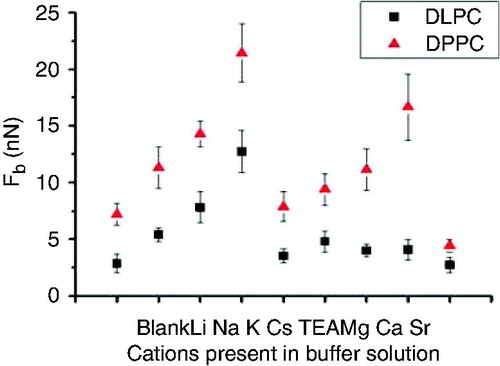
Fb value, as a hallmark of the bilayer mechanical stability, is considerably sensitive to the nature of the cations present in the electrolyte solution, as they have a strong effect on the mechanical stability of the membranes due to a reduction in the intermolecular distances in the bilayer promoted by polar headgroup screening enhancement (Garcia-Manyes et al. Citation2005a). For both SLBs, DPPC and DLPC, the maximum Fb value observed corresponds to the presence of K+ while it is minimized in the presence of Cs+. These results show that monovalent ions have an important role in the bilayer ordering, modifying its mechanical properties by affecting the bilayer packing. It must be pointed out that in the case of a gel phase, Fb value can be more than 3-fold increased depending on the cation present in the solution, and such is the remarkable effect of different monovalent cations in the overall structure of the membranes. From these results, it was proposed that cation size plays a key role in their adsorption between the phospholipid polar headgroups and, consequently, in the membrane mechanics. In fact, small ions as Li+ increase the SLB Fb values in respect to pure water but higher Fb values are obtained for bigger ions. From these results, it seems clear that cation size and, consequently, its ability to enter the membrane and interact with the polar moieties, plays an important role in the mechanical strength of the membrane.
These results reported in Redondo-Morata et al. (Citation2012d) quantitatively agree with previous data that show how Na+ cations adsorb more efficiently into PC membranes than other alkali cations (Gurtovenko and Vattulainen Citation2008), being preferentially located at the phosphate region and enabling the adsorption of Cl− ions at the choline region. The influence of the cations has often been explained by means of the classical Gouy-Chapman theory, which predicts the same behavior for ions with the same valence (McLaughlin Citation1989). The FS approach undoubtedly shows that same-valence cations have a remarkably different effect on the structure of the SLBs and this fact is discussed taking into account both the ionic radius and the compactness of the SLB. Therefore K+, being bigger than Na+, would accommodate better between the polar headgroups when a larger area per molecule is available, as in the case of DLPC.
The case of K+
The specific adsorption of K+ in membranes has been widely discussed in the literature; while some MD simulations proposed that K+ cations do not bind to the lipid headgroup oxygen atoms (Cordomi et al. Citation2008), other MD simulations showed that K+ cations bind less to PC membranes than Na+ due to their larger radius (Cordomi et al. Citation2009), with Cs+ (the larger among the studied alkali cations) exhibiting the lowest interaction with the membrane (Vacha et al. Citation2009). However, it has also been reported that the K+ binding behavior is dependent on the forcefield parameters chosen in MD simulations (Gurtovenko and Vattulainen Citation2008, Vacha et al. Citation2009). Gurtovenko et al. reported a study where the effect of NaCl and KCl was compared in PC and phosphatidylethanolamine (PE) membranes by means of MD simulations, concluding that the binding of KCl was much weaker than that of NaCl for PC membranes and negligible for PE membranes (Gurtovenko and Vattulainen Citation2008). Furthermore, they also noted that the effect of K+ was forcefield-dependent, and pointed out that Gromacs forcefield parameter seems to exaggerate the size of K+ ion. FS experiments help to open up a new perspective about the effect of K+ in zwitterionic membranes. In Redondo-Morata et al. (Citation2012d), the mechanical stability of DPPC and DLPC membranes in the presence of K+ was evaluated and they concluded that K+ provokes a variation in the lateral interactions at the molecular level, as experimentally proved by an increase of Fb values for both gel and liquid phase membranes.
Alkaline earth cations: Ca2+ and Mg2+
Concerning Ca2+ and Mg2+ cations, specific effects may be noteworthy because of their physiological relevance. Interestingly, Redondo-Morata et al. found that Ca2+ has a higher contribution in the lateral cohesive forces involved in lipid membranes than Mg2+ in the case of gel phases (Redondo-Morata et al. Citation2012d). In the case of liquid-like phases, both cations have virtually the same contribution to the mechanics of the SLB. With regard to cations having an optimum radius to sterically fit in each lipid membrane, Ca2+ cations, with an intermediate size between that of Na+ and K+, properly fit both in DPPC and DLPC intermolecular distances. Nevertheless, Mg2+, being smaller than Ca2+, provides a weak improvement to the lateral cohesion in DPPC-solid-like-bilayers. It is worth noting that Mg2+ has an ionic radius even smaller than that of Li+. We have seen above that Li+ contributes weakly to the stability of studied lipid membrane, demonstrating that the effect of the cations is not only dependent on their specific radius but also on their electric charge.
Effect of cations in the activation energy of the rupture process
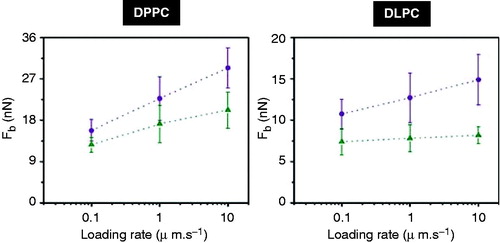
It is well established that Fb value depends on the loading rate (Butt and Franz Citation2002, Franz et al. Citation2002, Loi et al. Citation2002, Sullan et al. Citation2010). As different cations give rise to different Fb values at a fixed AFM tip velocity, it is conceivable to think that the presence of different ions will result in a different kinetic rate for the rupture process. In a recent work, Sullan et al. (Citation2010) used the loading rate dependence of the Fb value to calculate differences in the activation energy for the rupture process as a function of the cholesterol content. For DPPC and DLPC in the presence of Na+ and K+, the rate dependence of the Fb value is shown in for three different loading rates (0.1, 1 and 10 μm·s−1). In both cases, the Fb rate dependence is flatter for Na+ than for K+. These results confirm the fact that both Na+ and K+ modify the kinetics of the rupture process, leading to different activation energy. This is in accordance with models described in the literature, where the lipid bilayer rupture is considered as a two-state process with a single energy barrier (Butt and Franz Citation2002, Loi et al. Citation2002).
Effect of ionic strength on the mechanical stability of lipid bilayers
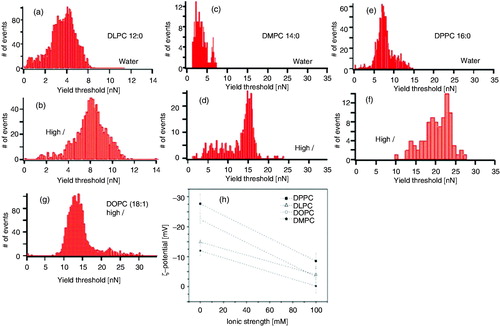
As previously discussed, the role of the physicochemical environment is susceptible to be studied by means of AFM-FS. It is known that ionic strength increases the electrostatic interaction between phospholipid headgroups due to a charge screening effect and this fact leads to a higher proximity of the hydrophobic phospholipid tails and the consequent increase of van der Waals interactions (Butt Citation1992, Pandit et al. Citation2003, Petrache et al. Citation2004, Citation2006a, Citation2006b, Gurtovenko Citation2005, Kandasamy and Larson Citation2006, Aroti et al. Citation2007, Leontidis et al. Citation2007, Gurtovenko and Vattulainen Citation2008, Vacha et al. Citation2009, Citation2010). A pioneer study about the effect of ionic strength in the mechanical stability of lipids was performed by Garcia-Manyes et al. (Citation2005a), showing not only that high ionic strength induces a better and faster deposition of PC bilayers onto mica substrates (Garcia-Manyes et al. Citation2006), but also an increase of the Fb value, upon Na+ and Mg2+ addition. In this work, DPPC, DMPC (1,2-dimyristoyl-sn-glycero-3-phosphocholine), DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine) and DLPC model membranes, as well as well as natural Escherichia coli membranes, were investigated to probe the effect of ionic strength on the mechanical stability of lipid bilayers. As shown in , the force required to puncture the lipid bilayer in all cases is significantly inferior at low ionic strength conditions. Furthermore, upon the addition of divalent cations such as Mg2+, the mechanical stability of SLBs is further increased.
Figure 7. Histograms of the Fb (yield threshold) values for: (a and b) DLPC, (c and d) DMPC and (e and f) DPPC, in water and 150 mM NaCl + 20 mM MgCl2; (g) DOPC in 150 mM NaCl + 20 mM MgCl2. (h) ζ-Potential (zeta potential) values of the PC liposomes as the ionic strength increases. Every point in the graph is the average of 15 independent measurements. Error bars stand for standard deviation value within the measurement. Lines are a guide to the eye. Reproduced from Garcia-Manyes et al. (Citation2005a) with permission. Copyright 2005 Elsevier.
It is well established that electrostatic interactions have a pivotal role in the structural and dynamic properties of lipid bilayers (Garcia-Manyes et al. Citation2005a, Citation2006, Pedersen et al. Citation2006, Sinn et al. Citation2006, Pabst et al. Citation2007, Porasso and Cascales Citation2009, Vernier et al. Citation2009). This is the case of Na+ or Ca2+, which strongly interact with the carbonyl oxygen groups of PC polar moieties while changing the orientation of the phospholipids and their molecular packing (Bockmann et al. Citation2003, Pandit et al. Citation2003, Bockmann and Grubmuller Citation2004, Vacha et al. Citation2009, Citation2010).
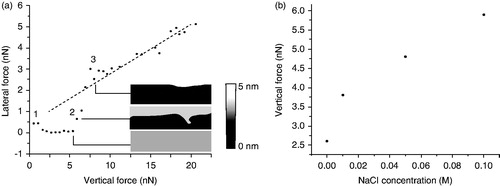
Interestingly, this increment in the lipid order also influences the brittleness of the lipid bilayer. This is known as a result of friction studies on PC bilayers. By means of lateral force microscopy technique, it is possible to study tribological properties of the lipid bilayers. Trunfio-Sfarghiu et al. (Citation2008) combined an AFM-FS mode with a tribometer to correlate the mechanical resistance and the friction forces of a DOPC bilayer. They showed how the friction coefficient is related to the Fb value, since for higher the Fb values, the friction coefficients were lower and more stable. In addition, Oncins et al. (Citation2005) studied the structural changes caused by Na+ on a DMPC bilayer. They observed three different friction regimes as the vertical force exerted by the AFM probe on the SLB increased, as shown in . In this work it was concluded that the cohesion of the film and the Fb value are reduced in the absence of NaCl.
Figure 8. (a) Lateral force vs. vertical force curves on DMPC bilayers in an aqueous environment. NaCl concentration is 0.1 M. Graphs is accompanied by bilayer topographic image obtained simultaneously with the friction data. The error bars represent the confidence intervals (at 95%, number of samples 256). A reference lateral force vs. vertical force curve obtained on mica is included in the panel (dashed line). Curves were divided into three regions, the beginning of each of which is labeled 1, 2, and 3. The first region shows an extremely low friction force because of the repulsive electrostatic interactions due to DMPC polar heads and the tip. During the second region, the tip creates defects or begins to break the bilayer and the lateral force increases steeply. In the third region the tip contacts the mica beneath the bilayer. (b) Vertical forces at which the second region begins as a function of NaCl concentration. Adapted from Oncins et al. (Citation2005) with permission, Copyright 2005 American Chemical Society.
Conclusions
Despite the high complexity of the cell membranes, synthetic lipid membranes and, specifically, SLBs have been shown to be simple models that are appropriate to study the influence of environmental variations, like ionic strength and the different cations, on the mechanical stability of membranes. The AFM-FS technique represents a robust platform to experimentally assess the quantitative characterization of the mechanical properties of these membranes, with the advantage of high spatial range sensitivity and versatility, as well as the possibility of locating and probing confined areas of membranes under environmentally controlled conditions and with nano- to piconewton sensitivity.
A detailed experimental quantitative AFM-FS study about the effect of monovalent and divalent cations on the nanomechanics of model PC membranes reviewed here deals with the effect of these cations in the internal structure of phospholipid membranes, opening up new avenues for characterizing the interplay between ordering and (nano)mechanical stability. It has been experimentally shown that the presence of different alkali and alkaline earth cations have a noticeable effect on the vertical force needed to puncture PC SLBs with the AFM probe. In the context of DLVO theory, interaction forces arising between the SLB surface and the AFM tip have been experimentally proven to be mainly due to the mechanical resistance of the lipid bilayer, while the contribution of the electrostatic interaction between both interfaces is almost negligible.
The penetration of the cations on the polar moiety of the membrane and the consequent decrease in intermolecular distances is controlled by the cations’ size, charge density and hydration but also by the initial distance between the SLB phospholipids. Notwithstanding, it was experimentally proved that K+ has a contribution to the overall mechanical stability in both DPPC and DLPC bilayers, an issue which aroused certain controversy in the literature. In the case of divalent cations, Ca2+, with an ionic radius between Na+ and K+, greatly increases the Fb value for solid-like phase membranes, while Mg2+ proves to be too small to effectively reduce the intermolecular distances between individual phospholipids. In the case of DLPC-liquid-like-SLBs, all tested divalent cations proved to be too small to modify the resilience of the membrane for the same ionic strength than monovalent ions. These exposed facts lead to confirm the idea that there are several physical-chemical mechanisms which contribute to determine ion-specific adsorption to SLBs. The nature of the metallic cation, size and charge density directly determine the penetration of these ions into the polar region of PC membranes. Besides, the intermolecular distance of phospholipids in the bilayer contributes to a preferential ionic adsorption according to the real size of the cations in solution.
Up-to-date investigations reveal that an extension of these studies to more biological relevant membranes has a promising future. For instance, among the ongoing studies on more sophisticated systems, it is worth mentioning the analysis of asymmetric bilayers (Manno et al. Citation2002), the insertion of membrane proteins (Domenech et al. Citation2007, Garcia-Saez et al. Citation2007a), or mixtures with different sterols (Tierney et al. Citation2005) or surfactants (Custers et al. Citation2005, Leonenko et al. Citation2006, Finot et al. Citation2010). The ultimate goal is to fully understand the high mechanochemical complexity of biological membranes.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
Support from Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR) through 2009SGR00277 is acknowledged.
References
- Aroti A, Leontidis E, Dubois M, Zemb T. 2007. Effects of monovalent anions of the Hofmeister series on DPPC lipid bilayers part I: swelling and in-plane equations of state. Biophys J 93:1580–1590
- Attwood SJ, Choi Y, Leonenko Z. 2013. Preparation of DOPC and DPPC supported planar lipid bilayers for Atomic Force Microscopy and Atomic Force Spectroscopy. Int J Mol Sci 14:3514–3539
- Benz M, Gutsmann T, Chen NH, Tadmor R, Israelachvili J. 2004. Correlation of AFM and SFA measurements concerning the stability of supported lipid bilayers. Biophys J 86:870–879
- Berkowitz ML, Bostick DL, Pandit S. 2006. Aqueous solutions next to phospholipid membrane surfaces: insights from simulations. Chem Rev 106:1527–1539
- Binnig G, Quate CF, Gerber C. 1986. Atomic force microscope. Phys Rev Lett 56:930–933
- Bockmann RA, Grubmuller H. 2004. Binding of monovalent and divalent cations to phospholipid bilayers: a molecular dynamics study. Biophys J 86:370A
- Bockmann RA, Hac A, Heimburg T, Grubmuller H. 2003. Effect of sodium chloride on a lipid bilayer. Biophys J 85:1647–1655
- Brian AA, McConnell HM. 1984. Allogeneic stimulation of cyto-toxic t-cells by supported planar membranes. Proc Natl Acad Sci USA – Biolog Sci 81:6159–6163
- Butt HJ. 1992. Electrostatic interaction in scanning probe microscopy when imaging in electrolyte solutions. Nanotechnology 3:60
- Butt HJ, Cappella B, Kappl M. 2005. Force measurements with the atomic force microscope: technique, interpretation and applications. Surf Sci Rep 59:1–152
- Butt HJ, Franz V. 2002. Rupture of molecular thin films observed in atomic force microscopy. I. Theory. Phys Rev E Stat Nonlin Soft Matter Phys 66:031601
- Carrion-Vazquez M, Oberhauser AF, Fowler SB, Marszalek PE, Broedel SE, Clarke J, et al. 1999. AFM and chemical unfolding of a single protein follow the same pathway. Biophys J 76:A173
- Clausen-Schaumann H, Rief M, Tolksdorf C, Gaub HE. 2000. Mechanical stability of single DNA molecules. Biophys J 78:1997–2007
- Corcoran SG, Colton RJ, Lilleodden ET, Gerberich WW. 1997. Anomalous plastic deformation at surfaces: nanoindentation of gold single crystals. Phys Rev B 55:16057–16060
- Cordomi A, Edholm O, Perez JJ. 2008. Effect of ions on a dipal-mitoyl phosphatidylcholine bilayer. A molecular dynamics simulation study. J Phys Chem B 112:1397–1408
- Cordomi A, Edholm O, Perez JJ. 2009. Effect of force field parameters on sodium and potassium ion binding to dipalmitoyl phosphati-dylcholine bilayers. J Chem Theory Comput 5:2125–2134
- Custers JPA, Kelemen P, van den Broeke LJP, Stuart MAC, Keurentjes JTF. 2005. Reversible binding of multivalent ions by surfactant self-assembly. J Am Chem Soc 127:1594–1595
- Chiantia S, Ries J, Kahya N, Schwille P. 2006. Combined AFM and two-focus SFCS study of raft-exhibiting model membranes. ChemPhysChem 7:2409–2418
- Domenech O, Redondo L, Picas L, Morros A, Montero MT, Hernandez-Borrell J. 2007. Atomic force microscopy characterization of supported planar bilayers that mimic the mitochon-drial inner membrane. J Mol Recognit 20:546–553
- Domingo JC, Mora M, Demadariaga MA. 1994. Role of headgroup structure in the phase-behavior of n-acylethanolamine phospho-lipids-hydrogen-bonding ability and headgroup size. Chem Phys Lipids 69:229–240
- Dowhan W. 1997. Molecular basis for membrane phospholipid diversity: why are there so many lipids? Annu Rev Biochem 66:199–232
- Drelich J, Long J, Yeung A. 2007. Determining surface potential of the bitumen-water interface at nanoscale resolution using atomic force microscopy. Can J Chem Eng 85:625–634
- Ducker WA, Clarke DR. 1994. Controlled modification of silicon-nitride interactions in water via zwitterionic surfactant adsorption. Colloid Surfaces A 93:275–292
- Dufrêne YF. 2008. Towards nanomicrobiology using atomic force microscopy. Nat Rev Microbiol 6:674–680
- Dufrêne YF, Barger WR, Green JBD, Lee GU. 1997. Nanometer-scale surface properties of mixed phospholipid monolayers and bilayers. Langmuir 13:4779–4784
- Dufrêne YF, Boland T, Schneider JW, Barger WR, Lee GU. 1998. Characterization of the physical properties of model biomem-branes at the nanometer scale with the atomic force microscope. Faraday Discuss 111:79–94
- El Kirat K, Morandat S, Dufrene YF. 2010. Nanoscale analysis of supported lipid bilayers using atomic force microscopy. Biochim Biophys Acta 1798:750–765
- Evans E, Heinrich V, Ludwig F, Rawicz W. 2003. Dynamic tension spectroscopy and strength of biomembranes. Biophys J 85:2342–2350
- Evans E, Rawicz W. 1990. Entropy-driven tension and bending elasticity in condensed-fluid membranes. Phys Rev Lett 64:2094–2097
- Finot E, Leonenko Y, Moores B, Eng L, Amrein M, Leonenko Z. 2010. Effect of cholesterol on electrostatics in lipid-protein films of a pulmonary surfactant. Langmuir 26:1929–1935
- Fisher TE, Oberhauser AF, Carrion-Vazquez M, Marszalek PE, Fernandez JM. 1999. The study of protein mechanics with the atomic force microscope. Trends Biochem Sci 24:379–384
- Franz V, Loi S, Muller H, Bamberg E, Butt HH. 2002. Tip penetration through lipid bilayers in atomic force microscopy. Colloid Surface B 23:191–200
- Fraxedas J, Garcia-Manyes S, Gorostiza P, Sanz F. 2002. Nanoin-dentation: Toward the sensing ofatomic interactions. Proc Natl Acad Sci USA 99:5228–5232
- Garcia-Celma JJ, Hatahet L, Kunz W, Fendler K. 2007. Specific anion and cation binding to lipid membranes investigated on a solid supported membrane. Langmuir 23:10074–10080
- Garcia-Manyes S, Oncins G, Sanz F. 2005a. Effect of ion-binding and chemical phospholipid structure on the nanomechanics of lipid bilayers studied by force spectroscopy. Biophys J 89:1812–1826
- Garcia-Manyes S, Oncins G, Sanz F. 2005b. Effect of temperature on the nanomechanics of lipid bilayers studied by force spec-troscopy. Biophys J 89:4261–4274
- Garcia-Manyes S, Oncins G, Sanz F. 2006. Effect of pH and ionic strength on phospholipid nanomechanics and on deposition process onto hydrophilic surfaces measured by AFM. Electro-chim Acta 51:5029–5036
- Garcia-Manyes S, Redondo-Morata L, Oncins G, Sanz F. 2010. Nanomechanics of lipid bilayers: heads or tails? J Am Chem Soc 132:12874–12886
- Garcia-Manyes S, Sanz F. 2010. Nanomechanics oflipid bilayers by force spectroscopy with AFM: a perspective. Biochim Biophys Acta 1798:741–749
- Garcia-Saez AJ, Chiantia S, Salgado J, Schwille P. 2007a. Mechanism of pore formation studied by AFM: Effect of Bax-derived peptide on the line tension. Biophys J 612A–612A
- Garcia-Saez AJ, Chiantia S, Salgado J, Schwille P. 2007b. Pore formation by a bax-derived peptide: effect on the line tension of the membrane probed by AFM. Biophys J 93:103–112
- Giannotti MI, Vancso GJ. 2007. Interrogation of single synthetic polymer chains and polysaccharides by AFM-based force spectroscopy. Chem Phys Chem 8:2290–22307
- Gurtovenko AA. 2005. Asymmetry of lipid bilayers induced by monovalent salt: atomistic molecular-dynamics study. J Chem Phys 122:244902
- Gurtovenko AA, Miettinen M, Karttunen M, Vattulainen I. 2005. Effect of monovalent salt on cationic lipid membranes as revealed by molecular dynamics simulations. J Phys Chem B 109:21126–21134
- Gurtovenko AA, Vattulainen I. 2008. Effect of NaCl and KCl on phosphatidylcholine and phosphatidylethanolamine lipid membranes: insight from atomic-scale simulations for understanding salt-induced effects in the plasma membrane. J Phys Chem B 112:1953–1962
- Heinrich V, Rawicz W. 2005. Automated, high-resolution micro-pipet aspiration reveals new insight into the physical properties of fluid membranes. Langmuir 21:1962–1971
- Heldin CH. 1995. Dimerization of cell-surface receptors in signal-transduction. Cell 80:213–223
- Helm CA, Israelachvili JN, McGuiggan PM. 1989. Molecular mechanisms and forces involved in the adhesion and fusion of amphiphilic bilayers. Science 246:919–922
- Hofbauer W, Ho RJ, Hairulnizam R, Gosvami NN, O'Shea SJ. 2009. Crystalline structure and squeeze-out dissipation of liquid solvation layers observed by small-amplitude dynamic AFM. Phys Rev B 80:134104
- Hong HD, Tamm LK. 2004. Elastic coupling of integral membrane protein stability to lipid bilayer forces. Proc Natl Acad Sci USA 101:4065-4070
- Jass J, Tjarnhage T, Puu G. 2000. From liposomes to supported, planar bilayer structures on hydrophilic and hydrophobic surfaces: an atomic force microscopy study. Biophys J 79:3153–3163
- Kandasamy SK, Larson RG. 2006. Effect of salt on the interactions of antimicrobial peptides with zwitterionic lipid bilayers. Bio-chim Biophys Acta 1758:1274–1284
- Kasahara K, Sanai Y. 2001. Involvement of lipid raft signaling in ganglioside-mediated neural function. Trends Glycosci Glyco-technol 13:587–594
- Krautbauer R, Clausen-Schaumann H, Gaub HE. 2000. Cisplatin changes the mechanics of single DNA molecules. Angew Chem Int Edit 39:3912
- Lee GU, Chrisey LA, Colton RJ. 1994. Direct measurement of the forces between complementary strands of DNA. Science 266:771–773
- Leonenko Z, Finot E, Vassiliev V, Amrein M. 2006. Effect of cholesterol on the physical properties of pulmonary surfactant films: atomic force measurements study. Ultramicroscopy 106:687–694
- Leontidis E, Aroti A. 2009. Liquid expanded monolayers of lipids as model systems to understand the Anionic Hofmeister Series: 2. Ion partitioning is mostly a matter of size. J Phys Chem B 113:1460–1467
- Leontidis E, Aroti A, Belloni L. 2009. Liquid expanded mono-layers of lipids as model systems to understand the Anionic Hofmeister Series: 1. A tale of models. J Phys Chem B 113:1447–1459
- Leontidis E, Aroti A, Belloni L, Dubois M, Zemb T. 2007. Effects of monovalent anions of the Hofmeister series on DPPC lipid Bilayers part II: modeling the perpendicular and lateral equation of state. Biophys J 93:1591–1607
- Loi S, Sun G, Franz V, Butt H-J. 2002. Rupture of molecular thin films observed in atomic force microscopy. II. Experiment. Phys Rev E Stat Nonlin Soft Matter Phys 66:031602
- Manno S, Takakuwa Y, Mohandas N. 2002. Identification of a functional role for lipid asymmetry in biological membranes: phosphatidylserine-skeletal protein interactions modulate membrane stability. Proc Natl Acad Sci USA 99:1943–1948
- Marra J, Israelachvili J. 1985. Direct measurements of forces between phosphatidylcholine and phosphatidylethanolamine bilayers in aqueous-electrolyte solutions. Biochemistry 24:4608–4618
- Marszalek PE, Oberhauser AF, Pang YP, Fernandez JM. 1998. Polysaccharide elasticity governed by chair-boat transitions of the glucopyranose ring. Nature 396:661–664
- McIntosh TJ. 1996. Hydration properties of lamellar and non-lamellar phases of phosphatidylcholine and phosphatidyletha-nolamine. Chem Phys Lipids 81:117–131
- McLaughlin S. 1989. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem 18:113–136
- Miettinen MS, Gurtovenko AA, Vattulainen I, Karttunen M. 2009. Ion dynamics in cationic lipid bilayer systems in saline solutions. J Phys Chem B 113:9226–9234
- Mingeot-Leclercq MP, Deleu M, Brasseur R, Dufrêne YF. 2008. Atomic force microscopy of supported lipid bilayers. Nat Protoc 3:1654–1659
- Mueller DJ, Dufrêne YF. 2008. Atomic force microscopy as a multifunctional molecular toolbox in nanobiotechnology. Nat Nanotechnol 3:261–269
- Mueller P, Rudin DO, Tien HT, Wescott WC. 1962. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature 194:979
- Nabika H, Fukasawa A, Murakoshi K. 2008. Tuning the dynamics and molecular distribution of the self-spreading lipid bilayer. Phys Chem Chem Phys 10:2243–2248
- O'Shea SJ, Gosvami NN, Lim LTW, Hofbauer W. 2010. Liquid atomic force microscopy: solvation forces, molecular order, and squeeze-out. Jpn J Appl Phys 49
- Oliver D, Lien CC, Soom M, Baukrowitz T, Jonas P, Fakler B. 2004. Functional conversion between A-type and delayed rectifier K+ channels by membrane lipids. Science 304:265–270
- Oncins G, Garcia-Manyes S, Sanz F. 2005. Study of frictional properties of a phospholipid bilayer in a liquid environment with lateral force microscopy as a function of NaCl concentration. Langmuir 21:7373–7379
- Oncins G, Torrent-Burgues J, Sanz F. 2008a. Nanomechanical properties of arachidic acid Langmuir-Blodgett films. J Phys Chem C 112:1967–1974
- Oncins G, Vericat C, Sanz F. 2008b. Mechanical properties of alkanethiol monolayers studied by force spectroscopy. J Chem Phys 128:044701
- Ono A, Freed EO. 2001. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc Natl Acad Sci USA 98:13925–13930
- Ottovaleitmannova A, Tien HT. 1992. Bilayer lipid-membranes – an experimental system for biomolecular electronic devices development. Prog Surf Sci 41:337–445
- Pabst G, Hodzic A, Strancar J, Danner S, Rappolt M, Laggner P. 2007. Rigidification of neutral lipid bilayers in the presence of salts. Biophys J 93:2688696
- Pandit S, Bostick D, Berkowitz M. 2004. Mixed bilayer containing DPPC and DPPS: Lipid complexation, ion binding, and electrostatics. Biophys J 86:368A–368A
- Pandit SA, Bostick D, Berkowitz ML. 2003. Molecular dynamics simulation of a dipalmitoylphosphatidylcholine bilayer with NaCl. Biophys J 84:3743–3750
- Parot P, Dufrêne YF, Hinterdorfer P, Le Grimellec C, Navajas D, Pellequer JL, et al. 2007. Past, present and future of atomic force microscopy in life sciences and medicine. J Mol Recognit 20:418–431
- Pedersen UR, Leidy C, Westh P, Peters GH. 2006. The effect of calcium on the properties of charged phospholipid bilayers. Biochim Biophys Acta 1758:573–582
- Perozo E, Kloda A, Cortes DM, Martinac B. 2002. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat Struct Biol 9:696–703
- Petrache HI, Kimchi I, Harries D, Tristram-Nagle S, Podgornik R, Parsegian VA. 2004. Forces measured between neutral lipid bilayers swollen by mononvalent salt. Biophys J 86:379A–379A
- Petrache HI, Tristram-Nagle S, Harries D, Kucerka N, Nagle JF, Parsegian VA. 2006a. Swelling of phospholipids by monovalent salt. J Lipid Res 47:302–309
- Petrache HI, Zemb T, Belloni L, Parsegian VA. 2006b. Salt screening and specific ion adsorption determine neutral-lipid membrane interactions. Proc Natl Acad Sci USA 103:7982–7987
- Phillips R, Ursell T, Wiggins P, Sens P. 2009. Emerging roles for lipids in shaping membrane-protein function. Nature 459:379–385
- Picas L, Rico F, Scheuring S. 2012. Direct measurement of the mechanical properties of lipid phases in supported bilayers. Biophys J 102:L1–L3
- Plant AL, Brighamburke M, Petrella EC, Oshannessy DJ. 1995. Phospholipid alkanethiol bilayers for cell-surface receptor studies by surface-plasmon resonance. Anal Biochem 226:342–348
- Porasso RD, Cascales JJL. 2009. Study of the effect of Na+ and Ca2 + ion concentration on the structure of an asymmetric DPPC/DPPC plus DPPS lipid bilayer by molecular dynamics simulation. Colloids Surf B-Biointerfaces 73:42–50
- Proksch R, Schaffer TE, Cleveland JP, Callahan RC, Viani MB. 2004. Finite optical spot size and position corrections in thermal spring constant calibration. Nanotechnology 15:1344–1350
- Qi SY, Groves JT, Chakraborty AK. 2001. Synaptic pattern formation during cellular recognition. Proc Natl Acad Sci USA 98:6548–6553
- Ramu Y, Xu Y, Lu Z. 2006. Enzymatic activation of voltage-gated potassium channels. Nature 442:696–699
- Rawicz W, Olbrich KC, McIntosh T, Needham D, Evans E. 2000. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys J 79:328–339
- Redondo-Morata L, Giannotti MI, Sanz F. 2012a. AFM-based force-clamp monitors lipid bilayer failure kinetics. Langmuir 28:6403–6410
- Redondo-Morata L, Giannotti MI, Sanz F. 2012b. Influence of cholesterol on the phase transition of lipid bilayers: A -temperature-controlled force spectroscopy study. Langmuir 28:12851–12860
- Redondo-Morata L, Giannotti MI, Sanz F. 2012c. Stability of lipid bilayers as model membranes: atomic force microscopy and spec-troscopy approach. In: Baró AM, Reifenberger RG, editors. Atomic force microscopy in liquid: biological applications. Wein-heim, Germany: Wiley-VCH Verlag & Co. KGaA. pp 259–284
- Redondo-Morata L, Oncins G, Sanz F. 2012d. Force spectroscopy reveals the effect of different ions in the nanomechanical behavior of phospholipid model membranes: the case of potassium cation. Biophys J 102:66–74
- Reimhult E, Hook F, Kasemo B. 2003. Intact vesicle adsorption and supported biomembrane formation from vesicles in solution: influence of surface chemistry, vesicle size, temperature, and osmotic pressure. Langmuir 19:1681–1691
- Reynwar BJ, Illya G, Harmandaris VA, Mueller MM, Kremer K, Deserno M. 2007. Aggregation and vesiculation of membrane proteins by curvature-mediated interactions. Nature 447:461–464
- Rico F, Picas L, Colom A, Buzhynskyy N, Scheuring S. 2013. The mechanics of membrane proteins is a signature of biological function. Soft Matter 9:7866–7873
- Richter RP. 2006. Formation of solid-supported lipid bilayers: an integrated view. Langmuir 22:3497–3505
- Rinia HA, Demel RA, van der Eerden J, de Kruijff B. 1999. Blistering of Langmuir-Blodgett bilayers containing anionic phospholipids as observed by atomic force microscopy. Biophys J 77:1683–1693
- Schmidt D, Jiang Q-X, MacKinnon R. 2006. Phospholipids and the origin of cationic gating charges in voltage sensors. Nature 444:775–779
- Schmidt D, MacKinnon R. 2008. Voltage-dependent K(+) channel gating and voltage sensor toxin sensitivity depend on the mechanical state of the lipid membrane. Proc Natl Acad Sci USA 105:19276–19281
- Singer SJ, Nicolson GL. 1972. Fluid mosaic model of structure of cell-membranes. Science 175:720
- Sinn CG, Antonietti M, Dimova R. 2006. Binding of calcium to phosphatidylcholine-phosphatidylserine membranes. Colloid Surf A 282:410–419
- Sisquella X, de Pourcq K, Alguacil J, Robles J, Sanz F, Anselmetti D, et al. 2010. A single-molecule force spectroscopy nanosensor for the identification of new antibiotics and antimalarials. FASEB J 24:4203–4217
- Stelzle M, Weissmuller G, Sackmann E. 1993. On the application of supported bilayers as receptive layers for biosensors with electrical detection. J Phys Chem 97:2974–2981
- Stoddart A, Dykstra ML, Brown BK, Song WX, Pierce SK, Brodsky FM. 2002. Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity 17:451–462
- Suchyna TM, Tape SE, Koeppe RE, Andersen OS, Sachs F, Gottlieb PA. 2004. Bilayer-dependent inhibition of mechano-sensitive channels by neuroactive peptide enantiomers. Nature 430:235–240
- Sullan RMA, Li JK, Hao CC, Walker GC, Zou S. 2010. Cholesterol-dependent nanomechanical stability of phase-segregated multicomponent lipid bilayers. Biophys J 99:507–516
- Talham DR, Yamamoto T, Meisel MW. 2008. Langmuir-Blodgett films of molecular organic materials. J Phys: Condens Matter 20:184006
- Tierney KJ, Block DE, Longo ML. 2005. Elasticity and phase behavior of DPPC membrane modulated by cholesterol, ergos-terol, and ethanol. Biophys J 89:2481–2493
- Torrent-Burgues J, Oncins G, Sanz F. 2008. Study of mixed Langmuir and Langmuir-Blodgett films of dissimilar components by AFM and force spectroscopy. Colloid Surface A 321:70–75
- Torrent-Burgues J, Pla M, Escriche L, Casabo J, Errachid A, Sanz F. 2006. Characterization of Langmuir and Langmuir-Blodgett films of a thiomacrocyclic ionophore by surface pressure and AFM. J Colloid Interface Sci 301:585–593
- Trunfio-Sfarghiu A-M, Berthier Y, Meurisse M-H, Rieu J-P. 2008. Role of nanomechanical properties in the tribological performance of phospholipid biomimetic surfaces. Langmuir 24:8765–8771
- Ursell T, Kondev J, Reeves D, Wiggins PA, Phillips R. 2008. The role of lipid bilayer mechanics in mechanosensation. In: Kamkin A, Kiseleva I, editors. Mechanosensitivity in cells and tissues 1: mechanosensitive ion channels. New York: Springer Press. pp 37–70
- Vacha R, Jurkiewicz P, Petrov M, Berkowitz ML, Bockmann RA, Barucha-Kraszewska J, et al. 2010. Mechanism of interaction of monovalent ions with phosphatidylcholine lipid membranes. J Phys Chem B 114:9504–9509
- Vacha R, Siu SWI, Petrov M, Bockmann RA, Barucha-Kraszewska J, Jurkiewicz P, et al. 2009. Effects of alkali cations and halide anions on the DOPC lipid membrane. J Phys Chem A 113:7235–7243
- van Meer G, Voelker DR, Feigenson GW. 2008. Membrane lipids: Where they are and how they behave. Nat Rev Mol Cell Biol 9:112–124
- Vernier PT, Ziegler MJ, Dimova R. 2009. Calcium binding and head group dipole angle in phosphatidylserine-phosphatidylcholine bilayers. Langmuir 25:1020–1027
- Vie V, Van Mau N, Lesniewska E, Goudonnet JP, Heitz F, Le Grimellec C. 1998. Distribution of ganglioside G(M1) between two-component, two-phase phosphatidylcholine monolayers. Langmuir 14:4574–4583
- Vogel V, Sheetz M. 2006. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol 7:265–275
- Xu L, Frederik P, Pirollo KF, Tang WH, Rait A, Xiang LM, et al. 2002. Self-assembly of a virus-mimicking nanostructure system for efficient tumor-targeted gene delivery. Hum Gene Ther 13:469–481
- Yang TL, Baryshnikova OK, Mao HB, Holden MA, Cremer PS. 2003. Investigations of bivalent antibody binding on fluid-supported phospholipid membranes: the effect of hapten density. J Am Chem Soc 125:4779–4784
- Yi M, Nymeyer H, Zhou HX. 2008. Test of the Gouy-Chapman theory for a charged lipid membrane against explicit-solvent molecular dynamics simulations. Phys Rev Lett 101:038103
- Yin XH, Drelich J. 2008. Surface charge microscopy: novel technique for mapping charge-mosaic surfaces in electrolyte solutions. Langmuir 24:8013–8020