Abstract
Förster resonance energy transfer (FRET) is a photophysical process by which a donor (D) molecule in an electronic excited state transfers its excitation energy to a second species, the acceptor (A). Since FRET efficiency depends on D-A separation, the measurement of donor fluorescence in presence and absence of the acceptor allows determination of this distance, and therefore FRET has been extensively used as a “spectroscopic ruler”. In membranes, interpretation of FRET is more complex, since one D may be surrounded by many A molecules. Such is the case encountered with membrane proteins and lipids in the bilayer. This paper reviews the application of a model built to analyze FRET data between a single tryptophan mutant of the transmembrane protein lactose permease (W151/C154G of LacY), the sugar/H+ symporter from Escherichia coli, and different pyrene-labeled phospholipids. Several variables of the system with biological implication have been investigated: The selectivity of LacY for different species of phospholipids, the enhancement of the sensitivity of the FRET modeling, and the mutation of a particular aminoacid (D68C) of the protein. The results obtained support: (i) Preference of LacY for phosphatidylethanolamine (PE) over phosphatidylglycerol (PG); (ii) affinity of LacY for fluid (Lα) phases; and (iii) importance of the aspartic acid in position 68 in the sequence of LacY regarding the interaction with the phospholipid environment. Besides, by exploring the enhancement of the sensitivity by using pure lipid matrices with higher mole fractions of labelled-phospholipid, the dependence on acyl chain composition is unveiled.
Introduction
In physical terms, the plasma membrane is a highly universal biological structure, which has been regarded as a boundary region that separates the discrete mass of the cytoplasm from the cellular environment. Presently, the membrane is recognized as a heterogeneous and dynamic structure that provides the basis not only for cell compartmentalization, but also for specific metabolic processes to take place. The membrane has two major components, lipids and proteins. The intricate coordination between these two components is a fundamental determinant in membrane organization and function. A central aspect in lipid–protein interaction is protein preference for selected lipid species or classes, which may act as the driving force for enrichment of these components in the bilayer region surrounding the transmembrane proteins (annular layer), at the expense of other lipids (Lee, Citation2003).
Traditionally, characterization of lipid–protein selectivity has been mostly carried out using electron spin resonance (ESR) spectroscopy, which differentiates between lipids in the annular region that exchange more slowly than those within the bulk lipid pool, and are thus often referred to as immobilized (Marsh & Horváth, Citation1998). Fluorescence static or collisional quenching methods (Everett et al., Citation1986; O’Keefe et al., Citation2000; Williamson et al., Citation2002) can also provide a similar structural type of information. These techniques are able to probe the lipid environment in direct contact with the protein, but are insensitive to the presence of lipids displaced from the protein/lipid interface. During the last decade, Förster resonance energy transfer (FRET) has emerged as a viable alternative for the study of lipid composition surrounding membrane proteins (Cabré et al., Citation2012; Fernandes et al., Citation2004). In this article, we present a model for FRET between a transmembrane protein and fluorescently labelled phospholipids, which may distribute unequally between the annular and bulk membrane regions. To illustrate both the potential and limitations of this method, we review its application to Escherichia coli lactose permease (LacY).
FRET from a protein to annular and bulk lipid probes
FRET is a photophysical process by which a donor (D) molecule in an electronic excited state transfers its excitation energy to a second species, named the acceptor (A). Because the donor returns to the electronic ground state following FRET, its fluorescence is effectively quenched, and therefore the extent of FRET is easily assessed by measuring the degree of quenching of D fluorescence, either in steady state (reduction of fluorescence intensity) or in time resolved (faster fluorescence decay) conditions. Alternatively, if A is fluorescent, the appearance or enhancement of A fluorescence following donor excitation can also be used to detect and quantify FRET. The rate constant of FRET between a D molecule, with fluorescence lifetime τ0, and an A molecule, separated by a distance R, is given by (Förster, Citation1949):
where R0 is the critical distance, which can be calculated from:
where in turn κ2 is the orientation factor (see van der Meer et al., Citation2013 for a detailed discussion), ΦD is the D quantum yield in the absence of A, n is the refractive index, λ is the wavelength, I(λ) is the normalized D emission spectrum, and ε(λ) is the A molar absorption spectrum. As is clear from Equation (2), R0 can be calculated from spectroscopic data. If the λ units used in Equation (2) are nm, then the calculated R0 has Å units. R0 can be conveniently calculated by pasting the spectral data and carrying out numerical integration in a spreadsheet, such as that made available by Visser et al. (Citation2011). One common source of uncertainty is the value assumed for κ2. For a given donor-acceptor pair, κ2 can take values between 0 and 4, and the average corresponding to the dynamic isotropic limit (<κ2 >= 2/3) is often used. While the dynamic (fast reorientation) hypothesis is likely to hold reasonably well for fluorophores located in fluid membranes, they commonly experience restrictions in orientation, such as resulting from wobbling-in-cone-type motions (Kinosita et al., Citation1977). In this situation, a simple method and computer program for estimation of κ2 distributions and averages is given by Loura (Citation2012), which requires as inputs estimates of donor and acceptor transverse locations and dipole orientation (average tilt relative to bilayer normal, width of angular distribution).
A popular quantification of the extent of FRET is given by the FRET efficiency, E, which is defined by:
In this equation, iD(t) and iDA(t) are the D decays in absence and presence of A (respectively). The effect of FRET on D fluorescence is the reduction of lifetime and quantum yield (note that, in this simple case, the D decay law remains exponential, albeit faster than in the absence of A). The relationship between the D lifetime in absence and presence of A (τ0 and τ, respectively) is given by
where R is the D-A separation. An expression identical to Equation (4) can be written for the fluorescence quantum yield. By rearranging this equation one obtains R
This relationship emphasizes the sensitivity of FRET to donor-acceptor distances R ≅ R0 (or equivalently, for E ≅ 0.5). In this range, a change of 5% in R (≅2 Å for a typical R0 value of 40 Å) corresponds to a 7% variation of the experimental FRET efficiency, which is measurable experimentally. This steep dependence of E on D-A distance warrants the traditional use of FRET as a “spectroscopic ruler” (Stryer, Citation1978).
However, in membranes, each D molecule is usually surrounded by a distribution of A molecules. Therefore, measurement of single D/A distances is neither meaningful nor feasible. The decay of D’s emission becomes complex and dependent on the topology of the system under study, as well as on the concentration of A. Analytical solutions can still be derived for uniform distribution of chromophores, such as planar distributions of D and A, for which the decay of D in presence of A becomes (Fung & Stryer, Citation1978; Wolber & Hudson, Citation1979):
In this equation, γ is the incomplete gamma function, defined as:
Re is the minimal D/A distance (exclusion distance) and n2 is the numerical concentration of A (molecules/unit area). Equation (6) is valid both for a plane of A molecules containing D (cis transfer) and in case the D and A molecules lie in parallel planes with closest D/A distance Re (trans transfer), a situation common on membranes, as D and A are often located at different depths in the bilayer.
Upon preparation of lipid vesicles, D and A molecules are frequently inserted in both of the bilayer leaflets, with equal probability. In this case, one must consider two planes of acceptors for a given donor, one corresponding to the acceptors lying in the same bilayer leaflet as the donor, and another for those located in the opposite leaflet. The decay law in this case is obtained by simply multiplying the intrinsic donor decay by the FRET terms corresponding to each plane of acceptors. Another common occurrence in membrane systems is a complex decay of donor even in the absence of acceptors, with a sum of two or three exponentials being required for a proper description. In this case, the above equations can be still used, provided that the exponential donor intrinsic decay term is replaced by this function, and τ0 is replaced by the intensity-average (Lakowicz, Citation2006) decay lifetime, such as described elsewhere (Loura et al., Citation1996, Citation2000). It should be stressed that in the determination of FRET efficiency (Equation (3)), the so-called lifetime-weighted quantum yield (Lakowicz, Citation2006) should be used, since the latter is proportional to all the photons emitted by the sample.
Although there are important advantages associated with model fitting from time-resolved data (see Loura et al., Citation2010), steady-state data obtained in a conventional spectrofluorimeter can be used. In this case, Equation (6) can be integrated numerically (in a program or spreadsheet) to produce curves of FRET efficiency E (calculated using Equation (3)) as function of acceptor concentration n2, with Re as a parameter. Alternatively, Re is fixed and experimental FRET decays/efficiencies are compared with theoretical expectations. In the scenario explored in this article, FRET is used to study differential distribution of lipid acceptors between the vicinity of a protein (annular region) and the bulk bilayer, which, as shown below, may be, in simple terms, characterized by a single parameter (μ or Ks below). In this case, fixing the value of Re (e.g. taking into account the size of the protein) allows retrieval of that parameter.
Specifically, we now consider FRET from a cylindrical membrane protein, labeled with a D group in an axially symmetric position, to A-labeled lipid probes (otherwise, the ensuing loss of symmetry prevents an analytical derivation of the FRET efficiency. Note that the approximate validity of this assumption should always be verified). The donor fluorescence decay curve is affected by FRET contributions to both annular and bulk (uniformly distributed outside the annular layer) acceptor populations (ρannular and ρrandom, respectively).
Calculation of ρrandom proceeds by using Equation (6). In the more general case, two exclusion distances Re1 and Re2 should be considered, one for each membrane leaflet (). In each case, Re is estimated as Re = (Re,lateral² + Re,z²)1/2, where Re,lateral is the distance along the bilayer plane between the cylindrical protein axis and second phospholipid layer around the protein (estimated as equal to the protein radius plus the diameter of the phospholipid cross-section) and Re,z is the transverse distance between the planes of the donor and acceptor chromophores.
Figure 1. Schematic depiction of the geometrical model considered for the FRET formalism. A cylindrical membrane protein (light grey) bears a FRET donor group in an axially symmetric position (D in the right panel, which represents a side view), and is surrounded by N annular lipids (dark grey; in the left panel, which represents a top view, n = 9) in each membrane leaflet. Bulk lipids are shown with white headgroup. The donor-acceptor (A) distances to annular layer-located probes (d1 and d2, for annular lipids located at the top or bottom leaflet, respectively) are shown in the right panel, together with the exclusions distances to bulk-located probes (Re1 and Re2).
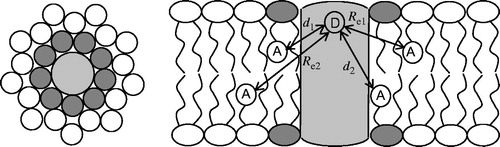
All annular acceptors within each leaflet are assumed to be at the same distance (di, i = 1 or 2, for top- or bottom-leaflet acceptors, respectively) to the protein fluorophore, and the FRET rate to each of these acceptors, kTi. is calculated by Equation (1), replacing R with di. The probability μ of each of the N annular sites in each leaflet to be occupied by an acceptor depends on the acceptor molar fraction and on a selectivity constant (KS) which quantifies the degree of acceptor enrichment in the annular layer, relative to its bulk concentration,
where NAcceptor is the concentration of labeled lipid, and NLipid is the concentration of unlabeled lipid. As shown in the Supplementary material, in the very dilute acceptor probe limit (which is assumed here), Ks is a thermodynamically consistent equilibrium constant. A binomial distribution describing the probability of each occupation number assuming a given μ (0–N sites occupied simultaneously by labeled lipid), is then calculated:
Finally, the FRET contribution arising from energy transfer to non-annular lipids is dictated by Equation (6), where Re = Re1 or Re2, for the top or bottom leaflets, respectively (see ), is calculated from the expected exclusion distance between the protein and lipids outside the annular shell. The donor decay in presence of acceptor can then be calculated using Equation (8), and in turn the FRET efficiency is obtained by integration of the donor decays in presence and absence of acceptor (Equation (3)). In practice, all parameters are fixed except μ (or, equivalently, Ks), so that the latter can be optimized by varying its value until the calculated FRET efficiency matches the experimentally measured value. This can be conveniently carried out using the spreadsheet in the Supplementary Material file (available online).
Application of FRET to LacY lipid selectivity
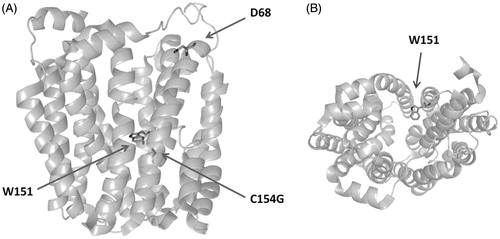
LacY (), one of the best studied cytoplasmic membrane proteins, is often considered as a paradigm for secondary transport proteins that couple the energy stored in an electrochemical ion gradient to a concentration gradient (β-galactoside/H+ symport). LacY was the first gene-encoding transmembrane protein (TMP) to be cloned into a recombinant plasmid and sequenced. This led to the overexpression, solubilization and purification of LacY, and its reconstitution into proteoliposomes in fully functional state. Hence, LacY has been one of the most, if not the most, investigated TMPs (Guan & Kaback, Citation2006).
Following years of effort, the first three-dimensional structure of LacY was resolved from X-ray diffraction studies using the conformationally restricted mutant (C154G; Cys 154 → Gly) (Abramson et al., Citation2003) and, shortly after, the wild-type structure was also completed (Guan et al., Citation2007). In side view, the monomer of LacY is heart-shaped, and accounts for a diameter of 6 nm displaying a large internal hydrophobic cavity open to the cytoplasmic side. A large number of studies have demonstrated the dependence of LacY on the lipid environment (Bogdanov & Dowhan, 1995; Bogdanov et al., Citation2010; Chen & Wilson, Citation1984; Vitrac et al., Citation2013). Earlier works based on the use of pyrene-labelled phospholipids and low protein-to-lipid ratios, already pointed to the existence of an annular phospholipid region around LacY (Lehtonen & Kinnunen, Citation1997), which was also demonstrated by atomic force microscopy of supported lipid bilayers where the protein has been reconstituted (Picas et al., 2010c).
PE/PG/CL selectivity of W151/C154G LacY
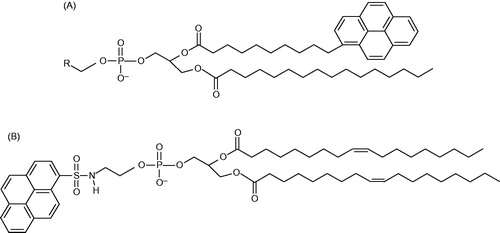
In an earlier study, the influence of composition of PE/PG mixtures upon the annular lipid surrounding LacY was investigated (Picas et al., Citation2010a). The actual objective of these experiments was to investigate if there were a particular affinity between the protein and any of the phospholipid species that predominate in the natural phospholipid environment. In the inner membrane of E. coli, were LacY resides, the most abundant phospholipid species found are 70% PE %, 20% PG and 5% cardiolipin (CL). The experimental approach consisted in the preparation of large unilamellar vesicles of varying proportions of POPE and POPG (1:3, 1:1 and 3:1), with partial replacement of host lipid PE or PG with pyrene-labeled derivatives 1-hexadecanoyl-2-(1-pyrenedecanoyl)-sn-glycero-3-phosphoethanolamine (Pyr-PE) or 1-hexadecanoyl-2-(1-pyrenedecanoyl)-sn-glycero-3-phosphoglycerol (Pyr-PG), respectively (see general structure of pyrene tail-labelled lipids in ). Then, single tryptophan W151/C154G LacY mutant was reconstituted in these lipid systems at 1:40 protein:lipid (w/w) ratio.
Figure 3. Structures of the pyrene-labelled lipids used as acceptors. (A) Tail-labeled lipids. R = ,
, and CH(OH)CH2OH for Pyr-PE, Pyr-PC, and Pyr-PG, respectively. (B) Head-labeled lipid, HPyr-PE.
On the one hand, the FRET efficiency data revealed a remarkable dependence on the composition of the system. For 1:3 PE/PG, low values (0.1–0.2) were measured for Pyr-PG acceptor, whereas no significant FRET was observed for Pyr-PE acceptor at either temperature. For the equimolar mixture, FRET to Pyr-PE (∼0.15–0.20) could now be measured, and FRET to Pyr-PG was particularly efficient at 10 °C (∼0.35–0.40). For the biomimetic 3:1 PE/PG system, efficient FRET (0.6–0.7) to both probes could be measured. At variance with the other compositions, FRET to Pyr-PE was more efficient than that for Pyr-PG. From these results one can extract two main conclusions: (i) FRET from LacY Trp to pyrene-labeled lipid probes is sensitive to the bilayer composition. In particular, the protein is able to recruit PE or PG, in some way being able to adapt to the changes in the composition of its environment; and (ii) since the FRET values are higher for PE in the biomimetic 3:1 composition, it appears that PE is more abundant in the annular region than PE for this system, and PE may thus provide a more adequate matching than PG to the size of the protein.
Ensuing research focused on this PE/PG mole ratio, and quantitative analysis of the FRET data was attempted on the framework of the above described quantitative formalism. Different PE lipids were used, with varying degrees of acyl chain saturation. Besides monounsaturated POPE, saturated 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE) and diunsaturated 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) were also employed. Additionally, the effect of cardiolipin (CL) on the annular lipid composition was also investigated (Picas et al., Citation2010b).
FRET efficiencies were analyzed essentially as outlined in the modeling section above, with an estimated N = 23 annular sites in each leaflet. The D LacY W151 was assumed to be located in the axis of a cylindrically symmetrical protein (though the location of W151 is not strictly axial, it is not very far, as seen in ), near the membrane interface. All A fluorophores were assumed to be located near the center of the bilayer. In this way, the distance d between D and annular A molecules was taken as identical for all annular A, independently of their membrane leaflet. Relevant to this approximation, a molecular dynamics study of pyrene-labeled 1-hexadecanoyl-2-(1-pyrenedecanoyl)-sn-glycero-3-phosphocholine (Pyr-PC) in fluid 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC; Repáková et al., Citation2006) revealed that the pyrene group has a deep location within the bilayer, with the most probable location being ∼0.6 nm from the midplane in probe dilute (0.8 mol%) systems. Because this displacement is five times smaller than the Förster radius for the Trp/Pyr pair, it is reasonable to consider all acceptors as equivalently located in the centre of the bilayers, also given that the faster FRET to the same-leaflet acceptors and the slower FRET to those in the opposite monolayer partially cancel out in the computation of the FRET efficiency. Using l = 1.2 nm as the transverse distance between W151 and the A plane (typical of interphase-anchored Trp residues; see, e.g. de Planque et al., Citation2003), and 3.0 nm (the approximate protein radius; Guan & Kaback, Citation2006) as the distance along the bilayer plane between the protein axis and the annular lipid molecules, the D-annular A distance is given by d = ((1.2)2 + (3.0)2)1/2 =3.2 nm. For the Förster radius, the value R0 = 3 nm, reported for the Trp/pyrene pair (Tahara et al., Citation1992), was used, whereas for the calculation of n2, area/lipid values of 0.56 nm2, 0.56 nm2, and 1.26 nm2 were assumed for POPE, POPG, and CL, respectively (Gutberlet et al., Citation2000; Rand & Parsegian, Citation1989).
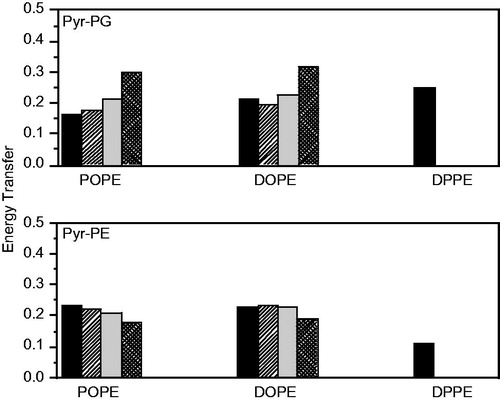
shows the experimental FRET efficiencies between the single Trp151 of LacY and either Pyr-PG or Pyr-PE as acceptors in the different lipid systems at 37 °C, together with the theoretical values, calculated using different values of the fitting parameter μ. This parameter represents the probability of finding a given phospholipid in the annular region of LacY. The fact that higher FRET efficiencies are obtained for transfer to Pyr-PE (0.232 ± 0.028) compared to Pyr-PG (0.165 ± 0.018) in the POPE/POPG system, and, in a similar way for Pyr-PE (0.231 ± 0.023) compared to Pyr-PG (0.211 ± 0.038) in the DOPE/POPG system, is a first indicator of the selectivity of LacY for PE relative to PG in these systems. This is confirmed by the quantitative model calculations. Best agreement with experimental values requires an annular region composed of approximately ∼90 mol% PE in these systems, whereas 75 mol% would be expected for random distribution of both phospholipids. In the POPE/POPG mixture, the FRET data are compatible with a complete PG exclusion from the annular layer, which is therefore composed solely of PE (μ(PE) = 1.00, μ(PG) = 0.00). Notably, when LacY is reconstituted in DOPE/POPG, the experimental FRET efficiencies indicate an enrichment of PG in the annular region (μ(PE) = 0.86, μ(PG) = 0.14) compared to the POPE:POPG system, but still a lower concentration than the one expected for uniform lipid distribution. Regarding the DPPE/POPG mixture, gel/fluid phase coexistence is expected, with POPG-enriched fluid domains coexisting with DPPE-enriched gel-phase bilayer regions. In this system, an increase in the efficiency of FRET to Pyr-PG and a decrease in that to Pyr-PE are verified (), to an extent that the efficiency of FRET to Pyr-PG now clearly surpasses that to Pyr-PE. This is a clear indication that LacY is preferably located in the fluid domains, where the PG A probe is more abundant.
Figure 4. Comparison of experimental and theoretical values of FRET efficiency between W151 and Pyr-PG (top) and Pyr-PE (bottom) at 37 °C in POPE:POPG (3:1, mol/mol) (left), DOPE:POPG (3:1, mol/mol) (center) and DPPE:POPG (3:1, mol/mol) (right) proteoliposomes (1.5 μM LacY). Reprinted with permission from Picas et al. (Citation2010b). © 2010 Elsevier.
In addition to PE and PG, the lipid composition of E. coli’s membrane contains 5–7% of CL. CL or diphosphatidylglycerol is a double negative-charged phospholipid that is believed to have strong influence in TMPs activity (Lee, Citation2003). To test the presence and effects of this phospholipid on the annular region two CL species differing in the length and composition of the acyl chains, myristoyl-CL and oleoyl-CL were incorporated in the POPE/POPG matrix. Incorporation of CL decreases the efficiencies of FRET when comparing to the same phospholipid mixtures containing no CL, especially when A is Pyr-PG. This suggests that CL displaces PE and, more extensively, PG from the annular region of LacY (see ). The fact that the effect is more pronounced for PG than for PE is probably related to the preference of the protein for PE species as described above for the binary systems. Upon applying the FRET quantitative model, when the acceptor is Pyr-PG, even by imposing segregation of this probe from the first annular layer (μ(PG) = 0), it is still not possible to conciliate the theoretical (0.162) and the experimental values (0.143 for oleoyl-CL, 0.142 for myristoyl-CL). This indicates that, besides being totally excluded from the first layer, PG is also somewhat rarefied beyond it. On the other hand, when A is Pyr-PE, a model matching to the experimental efficiencies (0.183 for oleo CL, 0.196 for myristoyl-CL) requires only partial replacement of PE by CL. When the CL lipid is oleoyl-CL, the retrieved composition of the annular layer is 40 mol% PE and 60 mol% CL. However, when the CL lipid is myristoyl-CL, the composition of the annular layer is 68 mol% PE and 32 mol% CL, indicating that in this case PE is kept in close proximity of the protein, in the same proportion as in the bulk. In the latter case, CL enrichment in the annular layer is solely produced by replacing PG. The fact that myristoyl-CL is not able to displace PE in the same way that oleoyl-CL does is probably due to the hydrophobic mismatch between the short myristoyl acyl chains and the protein. On the whole, this study indicated that, for LacY, PE is the most relevant component of the annular region and that, because it is not displaced by PG or (completely) by CL, it appears to be tightly bounded to the protein. Selectivity of LacY for PE and predominance of this phospholipid at the annular region, verified and characterized by FRET measurements and modelling, provide support for a hypothetical coupling between this lipid and LacY during the transport cycle.
Table 1. Comparison of experimental and theoretical FRET efficiencies for ternary mixtures PE:PG:CL 67:23:10 at 37 °C. Reprinted with permission from Picas et al. (Citation2010b). © 2010 Elsevier.
Enhanced FRET sensitivity and the role of acyl chain composition
One inherent problem with these experiments is the great degree of dilution of the labeled within the unlabeled lipids, which results in a little sensitivity for FRET efficiency values. To overcome this problem, two essential modifications were made (Suárez-Germà et al., Citation2012a). First, acceptor concentration was increased from 0.25 to 1.5 mol%, in order to produce efficiencies of E ≈ 0.5, for which FRET sensitivity to distance is maximal. Second, the use of binary 3:1 PE/PG mixtures to mimic E. coli’s membrane precluded the recovery of selectivity constants Ks, which are ill-defined for host lipid mixtures (as they depend on particular proportion of the mixture components). For this purpose, FRET was measured in pure POPE, POPG, DOPC and POPC vesicles. Acceptor probes were either Pyr-PE, Pyr-PG or a PC-derivative (1-hexadecanoyl-2-(1-pyrenedecanoyl)-sn-glycero-3-phosphocholine or Pyr-PC).
and show the experimental FRET efficiency E, average probe mole fraction in the annular region μ, and selectivity constant Ks values at both room (25 °C) and physiological (37 °C) temperatures, for the non-PC and PC systems, respectively.
Table 2. Experimental efficiencies, probabilities of each site in the annular ring of POPE or POPG vesicles being occupied by a pyrene labeled phopholipid and relative association constant toward LacY. Reprinted with permission from Suárez-Germà et al. (Citation2012a). © 2012 American Chemical Society.
Table 3. Experimental efficiencies, probabilities of each site in the annular ring of POPC or DOPC vesicles being occupied by a pyrene labeled phospholipid and relative association constant toward LacY. Reprinted with permission from Suárez-Germà et al. (Citation2012a). © 2012 American Chemical Society.
These results show that, for all host lipids and at both temperatures, FRET to Pyr-PE is consistently more efficient than for the other acceptor probes. Pyr-PE is preferred over Pyr-PG, and the latter over Pyr-PC, in the POPE and POPG matrixes at 25 °C (), and in the DOPC matrix at both temperatures (). This preference is reversed in the POPE and POPG matrixes at 37 °C () and in the POPC matrix at both temperatures (). Interestingly, Pyr-PC shows a higher efficiency in POPC than in DOPC, indicating a possible influence of the degree of unsaturation of the acyl chains. An opposite behavior is observed for Pyr-PG, for which the efficiency in DOPC is higher than in the POPC matrix.
In the POPE matrix, μ values indicate that Pyr-PG is excluded at both temperatures and that Pyr-PC is excluded at 25 °C and shows a small enrichment at 37 °C. Since Ks = 0 means no acceptor in the annular region, it becomes clear that at 25 °C, Pyr-PG and Pyr-PC are completely excluded. Although Pyr-PG behaves in the same way at 37 °C, LacY shows an increased preference for Pyr-PC at this temperature. The overall results in the POPE matrix, in which LacY is folded closely to the in vivo conditions (Suárez-Germà et al., Citation2012a), point to the fact that Pyr-PE should be in closer proximity than the other probes. On the other hand, Ks for Pyr-PE in the POPG matrix are compatible with a moderate enrichment of this probe in the annular region. Pyr-PG is depleted from the annular region at both temperatures when the host phospholipid is POPG. Similarly, we can observe that Pyr-PC is also depleted when hosted by POPG at 25 °C, and that a very slight enrichment is observed at 37 °C.
These observations may be likely related to the inverted topology of domains C6 and P7 of LacY when reconstituted in POPG proteoliposomes (Wang et al., Citation2002). Our FRET data confirm the preference of LacY for PE and its probable predominance in the annular layer. However, the most interesting result is possibly that, according to the Ks values, Pyr-PC is enriched in the annular region in a POPC matrix (Ks > 1) and excluded from it in a DOPC matrix (Ks ∼ 0). Given that DOPC and POPC share the same headgroup and have very similar hydrophobic lengths in the bilayer (2.48 nm for DOPC vs. 2.54 nm for POPC; Soubias et al., Citation2010), this difference is probably related to the different specific curvature of the two lipid species. It has been reported that whereas proper topology of LacY depends on a dilution of high negative surface charge density (and hence, probably the decreased affinity of the protein for PG), rather than on spontaneous curvature (C0; Bogdanov et al., Citation2008), the latter appears to be crucial regarding uphill transport of lactose by LacY in vivo (Wikström et al., Citation2009) with negative curvature lipids such as PE being required. C0(POPC) is essentially zero, and DOPC, due to its additional unsaturated acyl chain, has a negative specific curvature [C0(DOPC) = −0.11 nm−1; Soubias et al., Citation2010], which, though still far from the nonbilayer lipid DOPE [C0(DOPE) = −0.35 nm−1; Soubias et al., Citation2010], may justify the preference of properly reconstituted LacY for DOPC rather than POPC, and hence, the differential behavior in DOPC and POPC matrixes regarding selectivity for labeled probes. The fact that an opposite trend is observed for Pyr-PG () is suspicious and probably related to the above-mentioned improper organization of LacY in PG.
Head-labeled acceptor probes and the role of spontaneous curvature
For a potentially better evaluation of this effect of lipid acyl chain composition, we resorted to a head-labeled pyrene probe as acceptor, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(1-pyrenesulfonyl) (ammonium salt) (HPyr-PE; see for structure). We expected that, by using a head-labeled probe, we would avoid perturbation induced by the bulky pyrene group on the acyl chain region of the bilayer. FRET between W151/C154G LacY and HPyr-PE was measured in DOPE/POPG, POPE/POPG and DPPC/POPG 3:1, both at 25 °C and 37 °C ().
Figure 5. FRET efficiency between W151 and Pyr-PE head-labeled in different lipid matrices at 25 °C (black columns) and at 37 °C (grey columns). Proteoliposomes (1.5 μM LacY) of DOPE:POPG 3:1 (mol:mol), POPE:POPG 3:1 (mol:mol) and DPPE:POPG 3:1 (mol:mol) were doped with x = 0.0025 of label. The error bars stand for σ/√n, σ being the standard deviation and n the number of measurements performed. Reprinted with permission from Suárez-Germà et al. (Citation2013). © 2013 American Chemical Society.
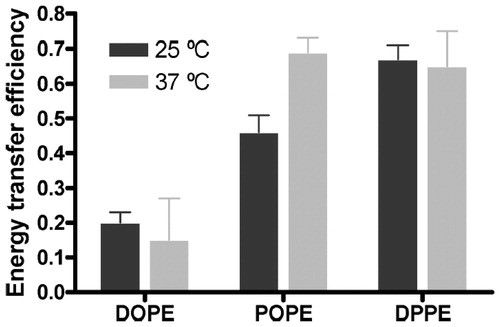
From these data it is apparent that the system in which FRET efficiency at both temperatures is consistently the highest is DPPE:POPG. Taking into account the preference of LacY for the Lα phase (Picas et al., Citation2010b; see also above), this result implies the seemingly unphysical result of preference of HPyr-PE for the Lα PG-rich phase, rather than for the Lβ PE-rich phase. A possible reason behind this observation is that the unsaturated acyl chains of HPyr-PE are better accommodated in the fluid phase rather than in the Lβ gel, and this would supersede the headgroup effect difference. Since the main transition temperature of DOPE (which HPyr-PE most resembles in terms of acyl chain composition) is around ∼−9 °C (Feng et al., Citation2002), LacY and HPyrPE will both be preferentially located inside the fluid domains and the obtained FRET values will be higher. On the other hand, within the fluid phase domains, LacY probably interacts preferentially with PE probe rather than POPG. Thus, the increased FRET efficiency in the DPPE:POPG system stems from probe and protein co-localization in the fluid phase of this phase-separated system and indicates a preference of LacY for: (i) Fluid over gel phase; and (ii) PE over PG headgroups.
Regarding the POPE:POPG system, the high FRET efficiency obtained at 37 °C indicates, primarily, that LacY much prefers to be surrounded by PE lipid (including HPyr-PE probe) than by POPG, which is in agreement with the above-described results obtained using Pyr-PE (Picas et al., Citation2010b; Suárez-Germà et al., Citation2012a). The difference in FRET efficiency between 25 °C and 37 °C is probably related to the thermotropic behavior of this mixture. Indeed, if the system is in a gel/fluid mixture under room temperature, because of its preference for fluid phases, LacY will show some preference for the fluid, PE-impoverished, domains. Hence, some annular sites may be occupied by POPG at 25 °C, decreasing FRET efficiency to HPyr-PE when compared to that in a single fluid phase at 37 °C.
The DOPE:POPG system shows lower FRET efficiencies (and consequently lower μ values, not shown) than expected at both temperatures. This indicates that the local concentration of HPyr-PE is significantly lower in the case of DOPE:POPG in comparison to the other mixtures, which was not observed with tail-labeled Pyr-PE (Picas et al., Citation2010b; see above).
Since it has been suggested that lipid spontaneous curvature C0 (Gruner, Citation1985) appears to be crucial regarding uphill transport of lactose by LacY in vivo (Wikström et al., Citation2009) it becomes tempting to relate this parameter with our FRET data. Thus, whilst DOPE is known for its highly negative spontaneous curvature, resulting from its inverted conical shape, with small headgroup and disordered acyl chains [C0(DOPE) = −0.35 nm−1; Soubias et al., Citation2010], to the best of our knowledge, the spontaneous curvature of POPE has not been determined. However, replacement of an oleoyl chain with a palmitoyl one in POPE will reduce the cross-sectional area, resulting in an increase of the lateral pressure at the hydrophobic region of the lipid (Suárez-Germà et al., Citation2011), and therefore −C0(POPE) < −C0(DOPE) is also expected, similarly to what is observed with POPC (C0 = 0) and DOPC (C0 = −0.11 nm−1; Soubias et al., Citation2010).
Because HPyr-PE bears two oleoyl acyl chains, just as in DOPE, it would be reasonable to assume that the probe reports the behavior of this latter phospholipid. However, in terms of specific curvature, pyrene-labeling at the headgroup will most probably have a severe effect. It has been shown that the replacement of headgroup ethanolamine H atoms with methyl groups decreases the absolute C0 value (C0(DOPE(Me1) = −0.27 nm−1, C0(DOPE(Me2) = −0.19 nm−1; Soubias et al., Citation2010), which is understandable since these replacements change the shape of DOPE from an truncated cone to something closer to a cylinder. A similar effect could be expected for pyrene-labelling at the headgroup, hence we may hypothesize that −C0(HPyr-PE) < −C0(DOPE). Thus, DOPE is clearly expected to be the PE lipid component with the most negative spontaneous curvature, compared to POPE and HPyr-PE. The higher FRET efficiency shown by POPE:POPG in seemingly indicates that HPyr-PE and POPE compete for the annular sites of LacY, whereas that the low efficiency and corresponding μ values for DOPE:POPG at both temperatures reflect, in comparative terms, more extensive occupation by DOPE of the annular sites, with concomitant probe exclusion. Thus, our data seem to confirm that spontaneous curvature is a major determinant of LacY-lipid interaction, with PE lipids bearing more negative C0 values being preferred relative to others with a more cylindrical shape. This seems to be at odds with a recent study indicating that POPE provides to LacY significantly higher uphill transport activity than DOPE (Vitrac et al., Citation2013). It is worth mentioning here that our mutant (LacY/C154G/single-W151) is different from the ones used in that study (C-less LacY/H205C or C-less LacY/F250C; Vitrac et al., Citation2013). Whereas these are fully functional, LacY (C154G) is severely restricted conformationally and does not transport H+ (Garcia-Celma et al., Citation2009; Smirnova & Kaback, Citation2003). A rationalization of our results may involve one of the following scenarios: (i) The design of our experiment presents limitations, possibly stemming from less adequate physicochemical behavior of HPyr-PE as a PE-lipid reporter (also note that, unlike zwitterionic unlabeled PE, HPyr-PE bears a negative charge); (ii) annular lipid composition is not the only determinant – and perhaps not the main determinant – of uphill transport activity. Additionally, the use of the C154G mutant provides information about a specific condition of the protein, without taking into account its need for adaptability when facing structural transitions (Shaikh et al., Citation2013). These latter possibilities reinforce the fact that there are still many aspects of LacY-lipid interactions which need to be clarified. In particular, a more definite clarification of the differential interaction with pyrene head-labeled lipid HPyr-PE will probably involve FRET experiments in pure POPE and DOPE, similarly to our above described study with tail-labeled Pyr-PE (Suárez-Germà et al., Citation2012a), though in these kind of experiments, the biomimetics of the matrix would be inevitably lost. Additionally, the use of different pyrene-labeled PE lipids as acceptor probes calls to the necessity of complementary studies to clarify these probes’ behavior, using, e.g. molecular dynamics simulations, which were recently employed to study free pyrene in bilayers (Loura et al., Citation2013).
W151/C154G/D68C LacY mutant and the role of the D68 amino acid
Whereas all the previously described results used single-W151/C154G LacY as donor, we next switched to a mutant in which the aspartic acid in position 68 was replaced by cysteine (single-W151/C154G/D68C LacY; Suárez-Germà et al., Citation2012b). It has been demonstrated that D68 is important for the protein to be sensitive to the proton gradient, but also that it plays a role in facilitating conformational changes associated with substrate translocation (Liu et al., Citation2010). In fact, D68 position is very sensitive to replacement (even the most conservative replacement inactivates transport). However, as long as several second-site activity revertants have been described (Jessen-Marshall & Brooker, Citation1996), it has been postulated that D68 is not absolutely irreplaceable for the transport mechanism. D68 mutants are still able to bind substrate but its translocation is locked. Our aim was to verify it replacement of D68 resulted in changes in relative protein-lipid affinity in biomimetic 3:1 PE/POPG vesicles (PE = DOPE, POPE, DPPE), which could be related to LacY function.
The results, shown in , indicate that, also for W151/C154G/D68C LacY, Pyr-PE is preferred over Pyr-PG, at both temperatures and all phospholipid matrixes (μ in are always the highest for Pyr-PE). These results coincide qualitatively with those observed in POPE:POPG and DOPE:POPG systems, using the W151/C154G mutant of LacY. Because our results with the latter () were obtained at 37 °C using a different probe concentration (0.25 mol%), for the sake of comparison, the FRET efficiencies to Pyr-PE obtained at this temperature with both mutants were normalized, and are thus represented in . These normalized values of E were similar for both mutants in DOPE:POPG, and lower and higher normalized E values were observed in POPE:POPG and DPPE:POPG, respectively, for the systems where the 68 residue has been mutated into cysteine.
Figure 6. Comparison of normalised FRET efficiency at 37 °C between W151 of the mutant single-W C154G D68C LacY and Pyr-PE (black columns) and W151 of the single-W C154G LacY and Pyr-PE (white columns). Reprinted with permission from Suárez-Germà et al. (Citation2012b). © 2012 American Chemical Society.
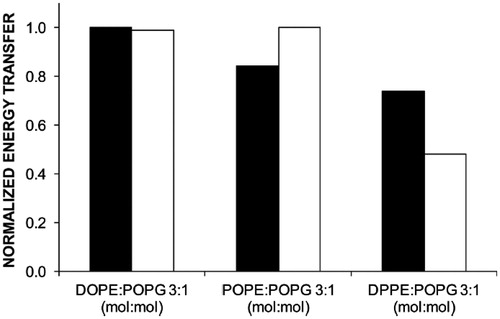
Table 4. Experimental efficiencies and probabilities µ for each site in the LacY D68C annular ring being occupied by a pyrene tail-labeled phospholipid. Reprinted with permission from Suárez-Germà et al. (Citation2012b). © 2012 American Chemical Society.
These results indicate that whilst the lipid selectivity for D68 is DOPE ∼ POPE > DPPE, the mutation increases the protein selectivity for DOPE over POPE and DPPE. Although POPE is still present in the boundary of LacY D68C, the mutation decreases the preference of the protein for this phospholipid. This might support the thesis that this amino acid is related to the interaction between the protein and POPE (Hakizimana et al., Citation2008), as well as the findings related to conformational changes aroused in the protein because of the mutated amino acid (Liu et al., Citation2010).
Concluding remarks
This review addressed the problem of studying protein/lipid selectivity by FRET, using LacY as a case study. The basic FRET formalism, which can be easily adapted to other systems, was described. This work illustrates some of the limitations of the methodology, namely those stemming from the reliance on acceptor membrane probes, which may not be proper analogs of the host lipids. Its assumption of an axially-located protein donor excludes its applicability to systems where this is clearly not the case (however, even in this situation, less general FRET models can be derived; see, e.g. Fernandes et al., Citation2007). The complexity of the formalisms may constitute a drawback, and, for this purpose, a spreadsheet for numerical calculation of the FRET efficiency for a given annular acceptor concentration (or for fitting this concentration to the experimental FRET efficiency) is provided as online Supplementary material with this article. This allows convenient application of the FRET model to steady-state experimental data, without need for an expensive and complex time-resolved fluorescence set-up. The model uses as inputs independent information on the size of the annular region (number of lipids), which are estimated from geometrical considerations for membrane proteins, depending on the number of transmembranar helices.
Nevertheless, it is clear that a wealth of relevant information may be obtained. For example, in the case of LacY, the preference for PE (and CL) lipids could be firmly established, as well as the importance of spontaneous curvature of the lipids, and sensitivity to crucial mutations in the protein. Advantages over the usual ESR approach to lipid/protein selectivity include the intrinsic sensitivity of fluorescence, and the possibility of exploring not only the nature of the first layer of lipids, but also the other adjacent ones (depending on the relative values of the Förster and protein radii). Conjugation of these types of FRET studies with FRET-independent measurements, including molecular dynamics simulations for investigating probe adequacy (Loura & Prates Ramalho, Citation2011), will provide a way forward to better understand the molecular details behind protein/lipid selectivity and its role on membrane protein function.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
C. S. G. is the recipient of a FPI fellowship from the Ministerio de Economía y Competitividad of Spain. This study was supported by grant CTQ-2008-03922/BQU from the Ministerio de Ciencia e Innovación of Spain, and Fundação para a Ciência e Tecnologia, Portugal (projects PTDC/QUI-BIQ/112067/2009, RECI/CTM-POL/0342/2012, PEst-OE/QUI/UI0313/2014, and FEDER, through the COMPETE program, project reference FCOMP-01-0124-FEDER-010787 [FCT PTDC/QUI-QUI/098198/2008]). C. S. G. and J. H. B. thank the Universitat de Barcelona for financial support.
Supplementary material available online
Supplementary Material
Download PDF (50.6 KB)Supplementary Material
Download MS Excel (15.1 MB)References
- Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. 2003. Structure and mechanism of the lactose permease of Escherichia coli. Science 301:610–615
- Bogdanov M, Dowhan W. 1995. Phosphatidylethanolamine is required for in-vivo function of the membrane-associated lactose permease of Escherichia coli. J Biol Chem 270:732–739
- Bogdanov M, Xie J, Heacock P, Dowhan W. 2008. To flip or not to flip: lipid-protein charge interactions are a determinant of final membrane protein topology. J Cell Biol 182:925–935
- Bogdanov M, Heacock P, Guan A, Dowhan W. 2010. Plasticity of lipid-protein interactions in the function and topogenesis of the membrane protein lactose permease from Escherichia coli. Proc Natl Acad Sci USA 107:15057–15062
- Cabré EJ, Loura LMS, Fedorov A, Perez-Gil J, Prieto M. 2012. Topology and lipid selectivity of pulmonary surfactant protein SP-B in membranes: answers from fluorescence. Biochim Biophys Acta 1818:1717–1725
- Chen CC, Wilson TH. 1984. The phospholipid requirement for activity of the lactose carrier of Escherichia coli. J Biol Chem 259:732–739
- de Planque MRR, Bonev BB, Demmers JAA, Greathouse DV, Koeppe RE, Separovic F, et al. 2003. Interfacial anchor properties of tryptophan residues in transmembrane peptides can dominate over hydrophobic matching effects in peptide-lipid interactions. Biochemistry 42:5341–5348
- Everett J, Zlotnick A, Tennyson J, Holloway PW. 1986. Fluorescence quenching of cytochrome b5 in vesicles with an asymmetric transbilayer distribution of brominated phosphatidylcholine. J Biol Chem 261:6725–6729
- Feng Y, Yu ZW, Quinn PJ. 2002. Effect of urea, dimethylurea, and tetramethylurea on the phase behavior of dioleoylphosphatidylethanolamine. Chem Phys Lipids 114:149–157
- Fernandes F, Loura LMS, Koehorst R, Spruijt RB, Hemminga MA, Fedorov A, Prieto M. 2004. Quantification of protein-lipid selectivity using FRET: application to the M13 major coat protein. Biophys J 87:344–352
- Fernandes F, Neves P, Gameiro P, Loura LMS, Prieto M. 2007. Ciprofloxacin interactions with bacterial protein OmpF: modelling of FRET from a multi-tryptophan protein trimer. Biochim Biophys Acta 1768:2822–2830
- Förster T. 1949. Experimentelle und theoretische Untersuchung des Zwischenmolekularen übergangs von Elektrinenanregungsenergie [Experimental and theoretical investigation of the intermolecular transfer of electronic excitation energy]. Z Naturforsch 4a:321–327
- Fung BK, Stryer L. 1978. Surface density determination in membranes by fluorescence energy transfer. Biochemistry 17:5241–5248
- Garcia-Celma JJ, Smirnova IN, Kaback HR, Fendler K. 2009. Electrophysiological characterization of LacY. Proc Natl Acad Sci USA 106:7373–7378
- Gruner SM. 1985. Intrinsic curvature hypothesis for biomembrane lipid composition: a role for nonbilayer lipids. Proc Natl Acad Sci USA 82:3665–3669
- Guan L, Kaback HR. 2006. Lessons from lactose permease. Annu Rev Biophys Biomol Struct 35:67–91
- Guan L, Mirza O, Verner G, Iwata S, Kaback HR. 2007. Structural determination of wild-type lactose permease. Proc Natl Acad Sci USA 104:15294–15298
- Gutberlet T, Dietrich U, Bradaczek H, Pohlentz G, Leopold K, Fischer W. 2000. Cardiolipin, alpha-D-glucopyranosyl, and L-lysylcardiolipin from gram-positive bacteria: FAB MS, monofilm and X-ray powder diffraction studies. Biochim Biophys Acta 1463:307–322
- Hakizimana P, Masureel M, Gbaguidi B, Ruysschaert JM. 2008. Interactions between phosphatidylethanolamine headgroupand LmrP, a multidrug transporter a conserved mechanism for proton gradient. J Biol Chem 283:9369–9376
- Jessen-Marshall A, Brooker RJ. 1996. Evidence that transmembrane segment 2 of the lactose permease is part of a conformationally sensitive interface between the two halves of the protein. J Biol Chem 271:1400–1404
- Kinosita K, Kawato S, Ikegami A. 1977. A theory of fluorescence polarization decay in membranes. Biophys J 20:289–305
- Lakowicz JR. 2006. Principles of Fluorescence Spectroscopy. 3rd ed. New York: Kluwer Academic/Plenum
- Lee AG. 2003. Lipid-protein interactions in biological membranes: a structural perspective. Biochimic Biophys Acta 1612:1–40
- Lehtonen JY, Kinnunen PKJ. 1997. Evidence of phospholipid microdomain formation in liquid crystalline liposomes reconstituted with Escherichia coli lactose permease. Biophys J 72:1247–1257
- Liu Z, Maej MJ, Kaback HR. 2010. Helix dynamics in LacY: Helices II and IV. J Mol Biol 396:617–626
- Loura LMS, Fedorov A, Prieto M. 1996. Resonance energy transfer in a model system of membranes. Application to gel and liquid crystalline phases. Biophys J 71:1823–1836
- Loura LMS, Fedorov A, Prieto M. 2000. Membrane probe distribution heterogeneity: a resonance energy transfer study. J Phys Chem B 104:6920–6931
- Loura LMS, Fernandes F, Prieto M. 2010. Membrane microheterogeneity: Förster resonance energy transfer characterization of lateral membrane domains. Eur Biophys J 39:589–607
- Loura LMS, Prates Ramalho, JP. 2011. Recent developments in molecular dynamics simulations of fluorescent membrane probes. Molecules 16:5437–5452
- Loura LMS. 2012. Simple estimation of Förster resonance energy transfer (FRET) orientation factor distribution in membranes. Int J Mol Sci 13:15252–15270
- Loura LMS, do Canto, AMTM, Martins J. 2013. Sensing hydration and behavior of pyrene in POPC and POPC/cholesterol bilayers: A molecular dynamics study. Biochim Biophys Acta 1828:1094–1101
- Marsh D, Horváth LI. 1998. Structure, dynamics and composition of the lipid-protein interface. Perspectives from spin-labelling. Biochim Biophys Acta 1376:267–296
- O’Keefe AH, East JM, Lee AG. 2000. Selectivity in lipid binding to the bacterial outer membrane protein OmpF. Biophys J 79:2066–2074
- Picas L, Montero MT, Morros A, Vázquez-Ibar JL, Hernández-Borrell J. 2010a. Evidence of phosphatidylethanolamine and phosphatidylglycerol presence at the annular region of lactose permease of Escherichia coli. Biochim Biophys Acta 1798:291–296
- Picas L, Suárez-Germà C, Montero MT, Vázquez-Ibar JL, Hernández-Borrell J, Prieto M, Loura LMS. 2010b. Lactose permease lipid selectivity using Förster resonance energy transfer. Biochim Biophys Acta 1798:1707–1713
- Picas L, Carretero-Genevrier A, Montero MT, Vázquez-Ibar JL, Seantier B, Milhiet PE, Hernández-Borrell J. 2010c. Preferential insertion of lactose permease in phospholipid domains: AFM observations. Biochim Biophys Acta 1798:1014–1019
- Rand RP, Parsegian VA. 1989. Hydration forces between phospholipid–bilayers. Biochim Biophys Acta 988:351–376
- Repáková J, Holopainen JM, Karttunen M, Vattulainen I. 2006. Influence of pyrene-labeling on fluid lipid membranes. J Phys Chem B 110:15403–15410
- Shaikh SA, Li J, Enkavi G, Wen PC, Huang Z, Tajkhorshid E. 2013. Visualizing functional motions of membrane transporters with molecular dynamics simulations. Biochemistry 52:569–587
- Smirnova IN, Kaback HR. 2003. A mutation in the lactose permease of Escherichia coli that decreases conformational flexibility and increases protein stability. Biochemistry 42:3025–3031
- Soubias O, Teague WE Jr, Hines KG, Mitchell DC, Gawrisch K. 2010. Contribution of membrane elastic energy to rhodopsin function. Biophys J 99:817–824
- Stryer L. 1978. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem 47:819–846
- Suárez-Germà C, Montero MT, Ignés-Mullol J, Hernández-Borrell J, Domènech O. 2011. Acyl chain differences in phosphatidylethanolamine determine domain formation and LacY distribution in biomimetic model membranes. J Phys Chem B 115:12778–12784
- Suárez-Germà C, Loura LMS, Prieto M, Domènech Ò, Montero MT, Rodríguez-Banqueri A, et al. 2012a. Membrane protein-lipid selectivity: enhancing sensitivity for modeling FRET data. J Phys Chem B 116:2438–2445
- Suárez-Germà C, Loura LMS, Domènech O, Montero MT, Vázquez-Ibar JL, Hernández-Borrell J. 2012b. Phosphatidylethanolamine-lactose permease interaction: a comparative study based on FRET. J Phys Chem B 116:14023–14028
- Suárez-Germà C, Loura LMS, Prieto M, Domènech Ò, Campanera JM, Montero MT, Hernández-Borrell J. 2013. Phospholipid-lactose permease interaction as reported by a head-labeled pyrene phosphatidylethanolamine: a FRET study. J Phys Chem B 117:6741–6748
- Tahara Y, Murata M, Ohnishi S, Fujiyoshi Y, Kikuchi M, Yamamoto Y. 1992. Functional signal peptide reduces bilayer thickness of phosphatidylcholine liposomes. Biochemistry 31:8747–8754
- van der Meer BW, van der Meer DM, Vogel SS. 2013. Optimizing the orientation factor kappa-squared for more accurate FRET measurements. In: Medintz I, Hildebrandt N, eds. FRET – Förster Resonance Energy Transfer: From Theory to Applications. Weinheim, Germany: Wiley-VCH, 63–104
- Visser AJWG, Vysotski ES, Lee J. 2011. Critical transfer distance determination between FRET pairs. Available at: http://www.photobiology.info/Experiments/Biolum-Expt.html [Accessed 12 February 2014]
- Vitrac H, Bogdanov M, Dowhan, W. 2013. Proper fatty acid composition rather than an ionizable lipid amine is required for full transport function of lactose permease from Escherichia coli. J Biol Chem 288:1–24
- Wang X, Bogdanov M, Dowhan W. 2002. Topology of polytopic membrane protein subdomains is dictated by membrane phospholipid composition. EMBO J 21:5673–5681
- Wikström M, Kelly AA, Georgiev A, Eriksson HM, Klement MR, Bogdanov M, et al. 2009. Lipid-engineered Escherichia coli membranes reveal critical lipid headgroup size for protein function. J Biol Chem 284:954–965
- Williamson IM, Alvis SJ, East JM, Lee AG. 2002. Interactions of phospholipids with the potassium channel KcsA. Biophys J 83:2026–2038
- Wolber PK, Hudson BS. 1979. An analytical solution to the Förster energy transfer problem in two dimensions. Biophys J 28:197–210