Abstract
Membrane microdomains enriched in cholesterol, sphingolipids (rafts), and specific proteins are involved in important physiological functions. However their structure, size and stability are still controversial. Given that detergent-resistant membranes (DRMs) are in the liquid-ordered state and are rich in raft-like components, they might correspond to rafts at least to some extent. Here we monitor the lateral order of biological membranes by characterizing DRMs from erythrocytes obtained with Brij-98, Brij-58, and TX-100 at 4 °C and 37 °C. All DRMs were enriched in cholesterol and contained the raft markers flotillin-2 and stomatin. However, sphingomyelin (SM) was only found to be enriched in TX-100-DRMs – a detergent that preferentially solubilizes the membrane inner leaflet – while Band 3 was present solely in Brij-DRMs. Electron paramagnetic resonance spectra showed that the acyl chain packing of Brij-DRMs was lower than TX-100-DRMs, providing evidence of their diverse lipid composition. Fatty acid analysis revealed that the SM fraction of the DRMs was enriched in lignoceric acid, which should specifically contribute to the resistance of SM to detergents. These results indicate that lipids from the outer leaflet, particularly SM, are essential for the formation of the liquid-ordered phase of DRMs. At last, the differential solubilization process induced by Brij-98 and TX-100 was monitored using giant unilamellar vesicles. This study suggests that Brij and TX-100-DRMs reflect different degrees of lateral order of the membrane microdomains. Additionally, Brij DRMs are composed by both inner and outer leaflet components, making them more physiologically relevant than TX-100-DRMs to the studies of membrane rafts.
Introduction
In recent years, evidence has been presented to show that the Singer-Nicolson membrane model (Singer & Nicolson, Citation1972) is not entirely true. Not only do lipid half-layer differences exist, as proposed by the bilayer-couple hypothesis (Sheetz & Singer, Citation1974), but it is also well known that membrane molecules are not homogeneously distributed throughout the plasma membrane, resulting in segregation of these molecules into domains. However, the mechanisms of formation and stability of such segregated microdomains are not well understood.
The lipid rafts model (Lingwood et al., Citation2009) considers that the driving force for formation of domains is the preferential interaction between cholesterol and sphingolipids (Quinn, Citation2010). Cholesterol and sphingomyelin are believed to be the major lipid components of rafts due to their capacity to form a liquid-ordered phase. Given that rafts are important functional membrane components, they have attracted considerable research attention. Considering the operational definition of rafts, they are assumed to be resistant to detergent extraction (Pike, Citation2004). Thus, the properties of rafts have commonly been described based on the isolation of detergent-resistant membranes (DRMs) at low temperature. Although the result of detergent insolubility of membranes depends on the experimental procedure, DRM fractions reflect to some extent the properties of rafts in living cells (Sonnino & Prinetti, Citation2008). In particular, the resistance of the red blood cell membrane to nonionic detergents has been reported since the 1970s (Sheetz, Citation1979; Steck, Citation1974; Yu et al., Citation1973), but only recently has evidence for the association of lipid rafts with the membrane skeleton in human erythrocytes been presented (Ciana et al., Citation2005, Citation2011, Citation2014; Crepaldi Domingues et al., Citation2009; Domingues et al., Citation2010). The standard detergent used to isolate DRMs from erythrocytes and other cells is TX-100. However, Brij detergents have been described for the disruption of cell membranes, with a reduced tendency to solubilize components from the inner leaflet when compared with TX-100 (Schuck et al., Citation2003).
In order to further investigate the composition and organization of DRMs from human erythrocytes, and to obtain new insights into the biological membrane architecture, we used Brij 98 and Brij 58 detergents to partially disrupt those cell membranes, at low (4 °C) and physiological (37 °C) temperatures. To our knowledge, this is the first time that these polyoxyethylene ether detergents have been used to prepare DRMs from human erythrocytes. Through a detailed phospholipid and fatty acid composition determination we provide evidence that Brij DRMs are composed by both inner and outer leaflet components of the erythrocyte membrane. In addition, this study suggests that the anion transporter, band 3, may play an important role in erythrocyte raft microdomains.
Materials and methods
Materials
Methyl-β-cyclodextrin (MβCD), detergents (TX-100, Brij 98, and Brij 58), and 5- and 16-doxyl-stearic acid spin labels (5- and 16-SASL) were obtained from Sigma-Aldrich (St Louis, MO). Mouse monoclonal anti-flotillin-2 and goat polyclonal anti-human stomatin primary antibodies were purchased from BD Biosciences (San Jose, CA) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. HRP-conjugated rabbit anti-mouse and anti-goat polyclonal secondary antibodies were obtained from Rheabiotech (Campinas, SP, Brazil). All other chemicals were of analytical grade.
Blood processing and preparation of DRMs
Fresh human blood from healthy donors was obtained from a blood bank (approved by the Ethics in Research Committee, FCM/Unicamp, protocol number 227/2009). Erythrocytes were isolated by centrifugation at 1000 g for 5 min followed by three washes in phosphate-buffered saline (PBS, 5 mM Na-phosphate, 154.5 mM NaCl, 4.5 mM KCl, pH 7.4). After dilution of the erythrocytes in PBS (1:1, v:v), the suspension was then filtered through α-cellulose/microcrystalline cellulose to isolate erythrocytes from platelets and leukocytes (Beutler et al., Citation1976). The purified erythrocyte suspension was washed three times in TN buffer (25 mM Tris/HCl, 150 mM NaCl, pH 7.4), and the packed cells were used to prepare DRMs. For some experiments, purified erythrocyte suspensions (Ht = 20%) were treated with 5 mM MβCD in PBS for 30 min at 37 °C and washed three times in TN buffer, prior to detergent solubilization (see below). To quantify the amount of cholesterol removed from the cell membrane, MβCD-treated and untreated (control) erythrocytes were lysed and subjected to lipid extraction (Rose & Oklander, Citation1965). Ghost membranes were also used in some experiments, prepared as previously described by Dodge et al. (Citation1963), with minor modifications (Crepaldi Domingues et al., Citation2009).
Purified erythrocytes (approximately 2.5 × 109 cells) were incubated with 16 mM TX-100, Brij 98, or Brij 58 in TN buffer for 30 min at either 4 °C or 37 °C. Sucrose was then added to the cell extracts to a final concentration of 40%, in the presence of 0.15 M Na2CO3, and overlaid with 5 ml of a 30% sucrose solution in TN, followed by 2.5 ml of a 5% sucrose solution in TN. The DRMs were isolated by ultracentrifugation (Optima L series, Beckman Coulter, Brea, CA) at 225,000 gmax, for 16 h at 4 °C, using a SW41 rotor. After ultracentrifugation, 10 fractions (from top to bottom) of 1 ml each were collected and saved for subsequent characterization. Fraction 3 (low density fraction) contained all the DRMs.
Cholesterol, phosphate and protein assays
Total cholesterol was quantified using an enzymatic/colorimetric assay kit (#743051, Laborclin, Pinhais, Brazil). Total phospholipid concentration was performed by phosphomolybdate determination, according to (Chen et al., Citation1956). Protein content was determined with bicinchoninic acid (Bio Agency Biotecnologia, São Paulo, Brazil), using bovine serum albumin as a standard.
Protein immunoblot
Samples were separated using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), as described by Laemmli (Citation1970). Proteins from the gels were electrophoretically transferred to polyvinylidene difluoride membranes (PVDF). The membranes were then blocked for 1 h in TN containing 5% non-fat milk and 0.05% Tween-20, followed by incubation with primary antibody overnight at 4 °C. After washing, the PVDF membranes were then incubated with the appropriate peroxidase conjugated secondary antibody. Antibodies were detected using the enhanced chemiluminescent West-Pico kit (Pierce Biotechnology, Rockford, IL).
Preparation of lipid extracts and phospholipid analysis
Lipid extraction from both ghost membranes and DRMs was performed with chloroform, methanol, and water (2:1:1, v:v:v), according to Folch’s method (Folch et al., Citation1957). Lipid samples were subjected to analysis by high performance thin-layer chromatography (HPTLC) on silica gel plates (J. T. Baker, Phillipsburg, NJ) using a solvent system consisting of chloroform, ethanol, water, and triethylamine (35:35:7:35, v:v:v:v). Phospholipid bands were visualized by spraying a solution of molybdenum blue reagent (Sigma-Aldrich, St Louis, MO) onto the silica gel plates (Toledo et al., Citation1995), and image analyses were performed using Scion Image Beta 4.02 (Scion Corporation) software.
Fatty acids analysis
Phospholipid bands containing all relevant lipids were scraped off the HPTLC plate and extracted with chloroform/methanol (2:1, v:v) prior to gas chromatography (GC) analysis.
The fatty acids of the original phospholipids were methylated using the boron trifluoride-methanol (methanol-BF3) complex, as described by the American Oil Chemists’ Society (AOCS, Citation1998), and analyzed by gas chromatography (Chrompack GC equipped with a flame ionization detector). The separations were carried out using a 50 mm fused silica capillary column (WCOT CP-Sil 88, Chrompack, Chromtech, MN). The oven temperature was programmed from 180–210 °C at 10 °C/min, and hydrogen was used as the carrier gas. The injector temperature was set at 250 °C, and the detector temperature at 280 °C. The fatty acids composition was determined by comparing the retention times with authentic standards (Sigma-Aldrich, St Louis, MO) and calculating the relative percentages.
EPR measurements
The lipid acyl chain packing of the DRMs and their original membranes was monitored by electron paramagnetic resonance (EPR) with the use of doxyl-stearate (5- and 16-SASL) probes. Spectra were recorded at 25 °C using a Bruker EMX spectrometer (Bruker GmbH, Germany), operated at 9.4 GHz (X-band) with field-modulation amplitude of 1 G.
A film of 5- or 16-SASL was formed from a chloroform stock solution, dried under a stream of N2, and left under reduced pressure for a minimum of 2 h to remove traces of the organic solvent. Samples (DRMs or intact erythrocyte cells) were incubated with these films for 30 min at 37 °C in order to incorporate the spin label molecules (up to 1 mol% of the total lipid content of the sample). The order parameter (S) of the membranes was calculated from the hyperfine splitting of the SASL probes spectra (Hubbell & McConnell, 1971), as described previously (Crepaldi Domingues et al., 2009; Domingues et al., 2010). The order parameter is directly related to the tilt angle of the acyl chains, and inversely related to the trans-gauche distribution of chain dihedrals, so that larger S values (near unity) correspond to small amplitudes of motion and more ordered lipid chains (Schreier et al., Citation1978), as observed in liquid-ordered lipid phases (Crepaldi Domingues et al., Citation2009; Domingues et al., Citation2010).
Preparation and solubilization of giant unilamellar vesicles (GUVs)
GUVs made from erythrocyte membrane (ghosts) lipid extracts were prepared by the electroformation method developed by Angelova and Dimitrov (Citation1986). Briefly, 15 μl of a lipid chloroform solution were spread on the surfaces of two glasses coated with FTO (fluor tin oxide) and sealed with a Teflon spacer to form a chamber, which was filled with 0.2 M sucrose solution. This electroformation chamber was connected to a function generator (1 V, 10 Hz) and placed in the incubator at 50 °C for 1 h. Observation of GUVs was performed on a Zeiss Axiovert 200 inverted microscope (Jena, Germany) equipped with a 63 × Ph2 objective. A 5 μl aliquot of the GUV suspension was added to a chamber containing 0.2 M glucose and a fixed detergent concentration. Time sequences of GUVs were recorded with a Zeiss AxioCam HSm digital camera (Jena, Germany).
Results
In order to obtain and characterize DRMs from human erythrocytes prepared with Brij 98 and Brij 58, cells were lysed using the same detergent molar concentration (16 mM – equivalent of 1% TX-100), corresponding to a detergent:phospholipid molar ratio of 16:1. After ultracentrifugation in a nonlinear sucrose density gradient, a floating material was clearly visible at the 5–30% sucrose interface (fraction 3), where the viscosity was determined to be approximately 1.54 mPa.s (data not shown).
Because this is the first time that Brij DRMs have been obtained from erythrocytes, we have also tested a reduction in Brij concentration to half (8 mM) of that described above and no differences were observed related to the amount of cholesterol and total proteins confined into DRMs (data not shown). Moreover, these resistant membranes were obtained not only at 4 °C but also at physiological temperature (37 °C) (see below), as we have previously reported for TX-100 and C12E8 DRMs (Domingues et al., Citation2010). Visually, the floating material (DRMs) in the sucrose gradient tubes obtained at 37 °C was very similar to those obtained at 4 °C, except for Brij 98 which presented a more diffuse band collected mostly as fraction 4 (See Supplementary material, Figure S1, available online). Therefore, to obtain more accurate results all the analysis performed for Brij 98 DRMs obtained at 37 °C correspond to fractions 3–5 which were pooled and concentrated before use. For all other samples the DRMs were collected as fraction 3.
Characterization of cholesterol and protein content in Brij DRMs
The cholesterol content determination () revealed that at least 30% of the total cholesterol of the original membranes (intact erythrocytes) was retained in the Brij DRM fraction. This finding agrees well with TX-100 and previous DRM reports in the literature (Ciana et al., Citation2005; Crepaldi Domingues et al., Citation2009; Murphy et al., Citation2004), and that is also consistent with membrane rafts, which are cholesterol-enriched. Based on the cholesterol and protein contents, erythrocyte membranes were found to be more resistant to Brij 98 than to treatment with either Brij 58 or TX-100, at 4 °C. In fact, at low temperature, approximately 1.5-fold more cholesterol () and 3-fold more protein () were detected in the Brij 98 DRMs, than in Brij 58 or TX-100 DRMs. When TX-100 and Brij DRMs were prepared at 37 °C, no significant differences were observed in their cholesterol contents (). In contrast, the amount of protein in the Brij 98 DRMs obtained at 37 °C was 2- to 3-fold higher than in the Brij 58 and TX-100 DRMs prepared under the same condition. shows the increased cholesterol-to-protein mass ratio in the DRMs, compared to the original erythrocyte membrane. This distinguishing feature of DRMs was observed in all cases, irrespective of the detergent and temperature used, even for Brij 98 DRMs, which presented the highest amounts of cholesterol and total proteins. Additionally, we have also performed ultracentrifugation at room temperature (25 °C) when the detergent (TX-100) solubilization was carried out at 37 °C. The amount of cholesterol measured in those DRM samples (data not shown) were very similar to the ones obtained when DRMs were isolated by centrifugation at 4 °C (). These results indicate that the solubilized material does not segregate from detergent micelles when the samples are cooled.
Figure 1. Cholesterol (A) and protein (B) content, and cholesterol/protein mass ratio (C) in DRMs obtained by treatment of erythrocytes (RBC) with TX-100, Brij 98, and Brij 58 under different conditions: 4 °C or 37 °C for intact RBC, and 4 °C for cholesterol-depleted RBC (DRMs-MβCD). Cholesterol and protein were quantified in erythrocyte membranes corresponding to 2.5 × 109 RBCs and in their DRMs obtained after centrifugation of the same amount of starting material. **p < 0.001, *p < 0.05 (unpaired Student’s t-test, n = 3–6).
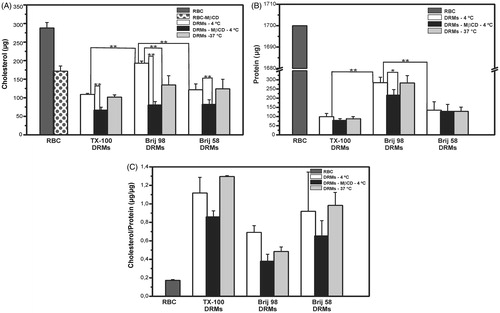
Interesting results were also obtained when experiments were conducted with cholesterol-depleted erythrocyte membranes. As we have previously reported (Crepaldi Domingues et al., Citation2009), when erythrocytes (Ht = 20%) were incubated with a sublytic concentration of MβCD (5 mM), approximately 40% of the cholesterol was removed from the cell membrane (). As for DRMs prepared from MβCD-treated cells, the decrease in the amount of cholesterol was greater for the Brij 98 DRMs (approximately 60%), compared to the cholesterol present in DRMs from intact erythrocytes at 4 °C (). For the Brij 58 and TX-100 DRMs, the decrease in cholesterol amount occurred in the same proportion for both MβCD-treated and intact cells (approximately 40%).
Influence of detergent, cholesterol, and temperature on raft and non-raft proteins
Flotillin-2 and stomatin are proteins known as lipid raft markers of different cell types, and their association with DRMs from intact erythrocytes obtained using TX-100 at 4 °C has been previously described in the literature (Ciana et al., Citation2011; Crepaldi Domingues et al., Citation2009; Domingues et al., Citation2010; Salzer & Prohaska, Citation2001). These proteins were also present in the Brij 98 and Brij 58 DRMs prepared at 4 °C (). Moreover, cholesterol depletion (MβCD-treated cells) partially disrupted the association of flotillin-2 with these DRMs (), as formerly reported for TX-100 DRMs (Domingues et al., Citation2010). Interestingly, no reduction was observed in the flotillin-2 and stomatin contents when the Brij 98 and Brij 58 DRMs were obtained at 37 °C. In contrast, the action of TX-100 over these proteins was found to be temperature-sensitive, and they were partially solubilized from TX-100 DRMs obtained at 37 °C (Domingues et al., Citation2010).
Figure 2. (A) Western blot of the raft markers stomatin and flotillin-2 present in DRM fractions obtained from intact (at 4 °C and 37 °C) and cholesterol-depleted (MβCD, at 4 °C) erythrocytes treated with Brij 98 and Brij 58. (B) Western blot of band 3 present in 10 fractions (from top to bottom, 1–10) obtained at 4 °C and 37 °C. Results are representative of three independent experiments.
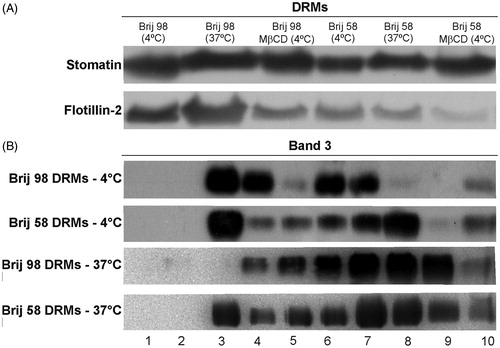
Regarding band 3, a non-lipid raft protein marker which has barely been detected in TX-100 DRMs (Ciana et al., Citation2011; Crepaldi Domingues et al., Citation2009; Domingues et al., Citation2010), we found that this integral protein is present in Brij 98 and Brij 58 DRMs (). However, band 3 was partially solubilized from DRMs when cells were treated at 37 °C. These results indicate that the presence of band 3 into DRMs is not only detergent-dependent but also temperature-sensitive. The presence of band 3 and membrane skeletal proteins, such as spectrin, was also detected by SDS-PAGE (see Supplementary material, Figure S2, available online).
Phospholipid analysis of DRMs
The phospholipids detected by HPTLC and analyzed in this work were phosphatidylcholine (PC), phosphatidylethanolamine (PE), and sphingomyelin (SM) (see Supplementary material, Figure S3, available online). Although a slight band of phosphatidylserine (PS) could be visualized in some DRM samples, its detection was not reproducible. Hence, neither PS nor minor membrane lipids (PI, PA, PG) quantification were analyzed here. The relative composition of phospholipids (mass % of PC, PE, and SM) () in the Brij DRMs, but not in the TX-100 DRMs, resembled that in the original ghost membranes. Since PE and SM are mainly present in the inner and outer monolayers, respectively, these results indicate that lipids from both leaflets were equally preserved in the Brij 98 and Brij 58-resistant membranes.
Figure 3. Distribution (mass %) of phospholipid classes (SM – sphingomyelin, PC – phosphatidylcholine, and PE – phosphatidylethanolamine) present in the samples of ghosts, TX-100 DRMs, Brij 98 DRMs, and Brij 58 DRMs analyzed by HPTLC and quantified by densitometry. DRMs were obtained from erythrocytes treated with detergents at 4 °C.
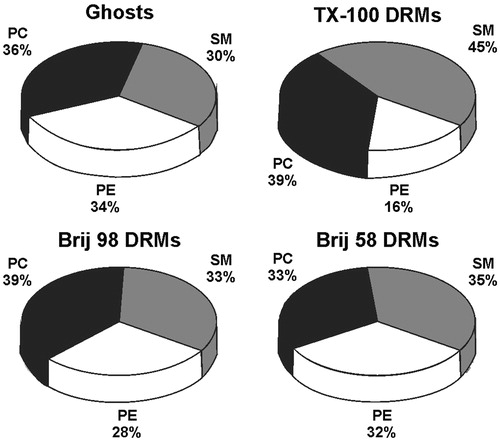
Among the three DRM samples, the greatest differences were observed for SM and PE. The TX-100 DRMs were enriched in SM (45%) and depleted in PE (16%), indicating a differential solubilization promoted by that detergent relatively to Brij 98 and Brij 58. The SM/glycerophospholipid (PC+PE) relative mass ratios (0.4 for ghost membranes) also reflected the differences in phospholipid composition, with mass ratios of 0.8, 0.5 and 0.5 obtained for the TX-100, Brij 98, and Brij 58 DRMs, respectively.
To further investigate the lipid composition of the DRMs, their phospholipid (PC, PE, and SM) fatty acid contents () were also analyzed using GC (see Supplementary material, Figure S4, available online). In ghost membranes (control), palmitic (16:0) and stearic (18:0) acids were the most abundant saturated fatty acids in the PC and PE fractions (), while the predominant saturated fatty acids in SM were palmitic and lignoceric (24:0) acids, providing further evidence of the increased content of long-chain fatty acids (>20C) in SM (). Moreover, the content of unsaturated fatty acids (18:1; 18:2 and 20:4) was significantly higher for PE () than for SM and PC.
Figure 4. Distributions of fatty acids in the phosphatidylcholine (A), phosphatidylethanolamine (B), and sphingomyelin (C) fractions extracted by HPTLC from ghost membranes or DRMs, as shown in . The saturated/unsaturated fatty acids ratios in the PC, PE, and SM fractions present in ghost membranes and DRMs is given in (D). Palmitic acid (16:0), stearic acid (18:0), oleic acid (18:1), linoleic acid (18:2), arachidonic acid (20:4), behenic acid (22:0), lignoceric acid (24:0), nervonic acid (24:1) (only detected in SM fractions). *p < 0.001 and **p < 0.05: a ghosts vs. all DRMs, b ghosts vs. TX-100 and Brij 58 DRMs, c ghosts vs. Brij 98 DRMs, d Brij 98 DRMs vs. TX-100 DRMs, e Brij 58 DRMs vs. TX-100 DRMs (unpaired Student’s t-test, n = 3). DRMs were obtained from erythrocytes treated with detergents at 4 °C.
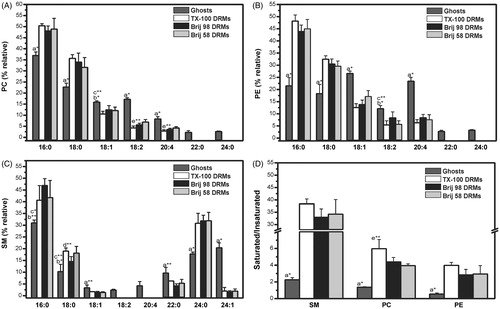
shows that for all phospholipids analyzed, saturated fatty acids were more abundant in the DRM fractions than in ghost membranes, as expected for lipid rafts and DRMs (Koumanov et al., Citation2005; Pike et al., Citation2005). This effect was even more evident in the case of SM, for which the saturated/unsaturated ratio increased more than 10-fold in all the DRMs, relative to ghost membranes. Looking carefully at , it can be seen that the amount of long chain (>20C) fatty acids (saturated and unsaturated) always decreased in the DRMs; however, this was not the case for lignoceric acid in SM. In fact, the most important changes in fatty acid composition detected in all DRM preparations were the increase in lignoceric acid (24:0) of SM and the decrease in arachidonic acid (20:4) of PE.
Lipid packing in DRMs
The spin-labeling method was used to monitor the order of the acyl chains moiety in the Brij DRMs, and also to give further information on the effects of cholesterol depletion and temperature on the organization of such membrane fractions. shows EPR spectra of 5-SASL in intact erythrocytes, and in TX-100, Brij 98, and Brij 58 DRMs prepared at 4 °C. The order parameters (S) calculated from the spectra obtained with the 5- and 16-SASL probes at 25 °C are given in , respectively.
Figure 5. EPR spectra of 5-SASL in intact erythrocytes (RBC), TX-100, Brij 98, and Brij 58 DRMs (A). Order parameter (S) values obtained from EPR spectra of 5- and 16-SASL (B and C, respectively) in intact (RBC) and cholesterol-depleted (MβCD) erythrocyte cells and their respective DRMs prepared at 4 °C or 37 °C with TX-100, Brij 98, and Brij 58. All EPR spectra were recorded at 25 °C. **p < 0.001, *p < 0.05 (unpaired Student’s t-test, n = 3–6).
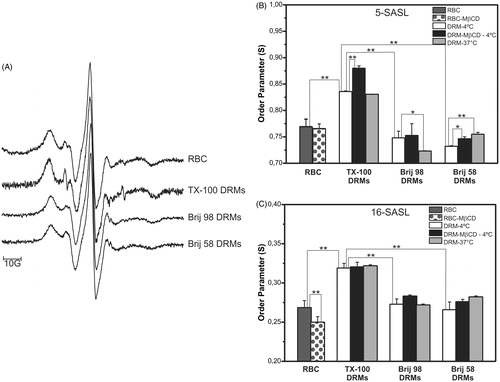
The 5-SASL probe is able to monitor the first carbon atoms of the phospholipid acyl chain moiety (Mainali et al., Citation2012), and for erythrocyte membranes the S value obtained for that portion of the bilayer was higher (S = 0.77) than that determined at the hydrophobic core of the bilayers (sensed by the 16-SASL probe, S = 0.27) (), as expected from the membrane organization profile and in agreement with previous reports in the literature (Cassera et al., Citation2002; Crepaldi Domingues et al., Citation2009; Domingues et al., Citation2010). The TX-100 DRMs presented significantly higher S values than the Brij DRMs, at both membrane depths (). These results indicated that acyl chain packing in the TX-100 DRMs was consistent with the liquid-ordered state, as we have previously described for TX-100 and C12E8 DRMs (Crepaldi Domingues et al., Citation2009). However, no significant decrease in the membrane fluidity of the Brij 98 and Brij 58 DRMs was observed, compared to intact erythrocytes.
The effect of cholesterol depletion (40%) on intact erythrocyte membrane (control) organization was registered as a decrease in the S value, sensed only by 16-SASL (), which monitors deeper levels within the lipid bilayer. Regarding DRMs prepared from such membranes, cholesterol depletion affected the order of their lipids in a different way: The S values monitored by 5-SASL increased, while 16-SASL was not sensitive to any differences in acyl chain packing for the TX-100 and Brij 58 DRMs. For the DRMs prepared at 37 °C, the 5-SASL spectra revealed small and opposite changes for Brij 98 (lower S ∼increased fluidity) and Brij 58 (higher S ∼decreased fluidity) DRMs, in comparison to those obtained at low temperature (). However, it is important to highlight that the S values determined for the Brij DRMs () were never significantly higher than those for intact erythrocytes at the same condition, in a different way than observed for TX-100 DRMs.
Solubilization of erythro-GUVs
The effects of the detergents Brij 98 and TX-100 on GUVs made from lipid extracts of erythrocyte ghosts (these ghost-like GUVs were named here as erythro-GUVs) were followed with optical microscopy. A small aliquot of the GUV suspension was added to solutions of glucose containing either Brij98 or TX-100. The effects caused by each detergent on erythro-GUVs were quite different (). shows one erythro-GUV in the presence of 0.1 mM Brij 98. The second snapshot shows the moment when the erythro-GUV is destabilized and restructured into several small vesicles of 1–2 μm diameter, which can be better viewed in the last snapshot. It is not clear whether Brij 98 caused a partial solubilization of the membrane or whether it is an all-or-none phenomenon, since the erythro-GUV remains intact until a sudden restructuring into small vesicles occurs. All erythro-GUVs in contact with Brij 98 behave in a similar way.
Figure 6. Sequence of images of erythro-GUVs in the presence of (A) 0.1 mM Brij 98 and (B) 0.5 mM TX-100. The time shown in each snapshot is relative to the moment when the erythro-GUVs were added to the detergent suspension. At least 20 GUVs were followed in the presence of Brij 98 and 100 GUVs in the presence of TX-100. The scale bars represent 10 μm.
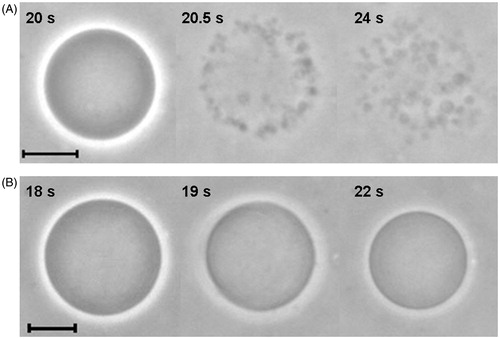
The effects caused on erythro-GUVs by TX-100 were very different (). Clearly, TX-100 is able to partially solubilize the erythro-GUV, as can be seen from the reduction in vesicle size in the second snapshot. The last snapshot shows the insoluble fraction of the erythro-GUV, without the original optical contrast conferred by the initial sucrose/glucose asymmetry inside and outside the vesicle. This partial solubilization and contrast loss was observed for all erythro-GUVs treated with TX-100 above its cmc and in our previous work (Casadei et al., 2014).
Discussion
In this study we provide evidence that Brij 98 and Brij 58 solubilize the erythrocyte membrane in a different way when compared with TX-100. As a result of this differential solubilization process, DRMs presented different lipid and protein composition unraveling new faces of membrane domains. Because most of the studies involving phase separation are available only for simple mixtures of lipids (Bezlyepkina et al., Citation2013; Goni et al., Citation2008), here we have used erythrocytes to prepare DRMs as an approach to explore the lateral order of biological membranes. Although membrane models can be very useful to study the behavior of membrane lipids, one should be aware that they do not provide the complexity of a biological membrane that is given by a wide variety of lipids and a large number of proteins.
The use of detergents and low temperature to isolate DRMs from different cells has been intensely debated in the literature. Despite several criticisms (Lichtenberg et al., Citation2005), DRMs are still very useful as a model to explore the structure and function of membranes. To some extent, DRMs are also a good tool to study lipid rafts (Sonnino & Prinetti, Citation2013), since these structures are enriched in cholesterol and sphingolipids and are in a liquid ordered-like phase. However, we are aware that DRMs are not identical to pre-existing rafts and to better understand the similarity/differences between DRMs and the microdomains of living cells, the composition of these detergent-insoluble membrane fractions must be more carefully analyzed. In addition, a recent study showed that TX-100 did not induce the formation of liquid-ordered domains in a model membrane system (sphingomyelin/POPC/cholesterol), but increased domain size by coalescing pre-existing nanodomains (Pathak & London, Citation2011). Thus, although the use of detergent can disturb membrane organization, DRMs are still a reliable approach to study membrane microdomains.
The composition and structure of erythrocyte membrane are very well known, but there are still many questions regarding lipid rafts in such membranes (Ciana et al., Citation2013, Citation2014; Domingues et al., Citation2010; Mikhalyov & Samsonov, Citation2011; Nagao et al., Citation2002; Salzer & Prohaska, Citation2001; Samuel et al., Citation2001). We have previously characterized DRMs from intact erythrocytes using TX-100 and C12E8 in the presence of sodium carbonate (Crepaldi Domingues et al., Citation2009; Domingues et al., Citation2010), and the results suggested the association of lipid rafts with the membrane skeleton. Indeed, Ciana et al. (Citation2011) have clearly demonstrated the necessity of using a high pH and ionic strength (as provided by sodium carbonate) to release DRMs from the erythrocyte membrane skeleton during the sucrose gradient preparation procedure. In this study, we used for the first time the nonionic polyoxythylene ethers Brij 98 and Brij 58 to isolate and characterize DRMs from erythrocytes. The fact that Brij DRMs were obtained only in the presence of sodium carbonate is consistent with the hypothesis that DRMs are anchored to the membrane skeleton through electrostatic interactions (Ciana et al., Citation2005, Citation2011; Crepaldi Domingues et al., Citation2009; Domingues et al., Citation2010). For detailed information about the effects of carbonate on DRMs isolation, see a recent review published by Ciana et al. (Citation2014). Temperature does not seem to be a critical factor in obtaining DRMs from erythrocytes, since Brij DRMs were obtained not only at low temperature (4 °C), but also at 37 °C, as we have previously described for TX-100 and C12E8 DRMs (Domingues et al., Citation2010). Mikhalyov & Samsonov (Citation2011) have also shown the existence of raft microdomains in live erythrocytes using fluorescent markers, observed at both 4 °C and 37 °C, which stability was highly cholesterol-dependent.
Brij DRMs prepared at 4 °C were found to contain stomatin and flotillin-2 (protein markers of lipid rafts). The partial solubilization of flotillin-2 after cholesterol depletion confirms our previous data regarding its dependence on the cholesterol content (Domingues et al., Citation2010). Additionally, cholesterol depletion did not affect stomatin domains when cell membranes were solubilized by Brij, as previously observed for TX-100 (Domingues et al., Citation2010). On the other hand, there were differences in the distribution of these proteins in Brij and TX-100 DRMs prepared at 37 °C. Since TX-100 has a tendency to selectively solubilize phospholipids present on the cytoplasmic leaflet of the cell membrane (Ingelmo-Torres et al., Citation2009; Koumanov et al., Citation2005; Lichtenberg et al., Citation2005; Waugh & Hsuan, Citation2009), it can easily favor the solubilization of proteins located in the inner monolayer, such as flotillin-2 and stomatin (Domingues et al., Citation2010). However, the stability of the inner monolayer of DRMs seems to be preserved when Brij detergents are used at physiological temperature, since flotillin-2 and stomatin remain associated with the DRMs ().
Lipid analysis showed that only the TX-100 DRMs were clearly enriched in SM, and contained a small percentage of PE (). On the other hand, the Brij DRMs were enriched in cholesterol () but not in SM, confirming that TX-100 but not the Brij detergents preferentially solubilize lipids from the cytoplasmic leaflet of the cell membrane (). These results indicate that Brij 98 and Brij 58 are more suitable than TX-100 in terms of preserving the lipid bilayer of DRMs, and therefore the characterization of these structures could reveal more details about the association between rafts and the membrane skeleton (Ciana et al., Citation2005, Citation2011; Crepaldi Domingues et al., Citation2009; Domingues et al., Citation2010). In addition, fatty acyl chain analysis revealed that not only cholesterol and SM, but also other phospholipids such as PC and PE, play an important role in the formation and properties of membrane domain, contributing to the typical high saturated/unsaturated lipid ratio of DRMs. The dissimilarities between the TX-100 and Brij DRMs can also be explained by the different structures and physicochemical properties of the detergents. Since the acyl chains of Brij 98 and Brij 58 present different numbers of carbons, and only Brij 98 is unsaturated, these detergents can disrupt the membrane components differently. For instance, cholesterol was more resistant to Brij 98 than to Brij 58 (). Moreover, the results shown here with erythro-GUVs in contact with Brij 98 and TX-100 can also be interpreted in terms of different detergent incorporation in the bilayer and flip-flop rates. In a previous work, it was shown that TX-100 initially induced a large increase in the surface area of GUVs composed of POPC, indicating a fast transfer of TX-100 to the inner leaflet, followed by complete vesicle solubilization. On the other hand, the anionic detergent SDS initially caused an increase in membrane spontaneous curvature and ultimately a vesicle burst, suggesting that SDS was not able to flip-flop because of its charged headgroup (Sudbrack et al., Citation2011). Here, considering that Brij 98 bears a quite large hydrophilic portion, we propose that it is mainly incorporated in the outer leaflet, which ultimately causes destabilization of the vesicle structure, allowing equilibrium of the detergent population to take place between the two bilayer halves, and rearrangement into several small vesicles. Therefore, the effects caused by Brij 98 are similar to those previously reported for SDS (Sudbrack et al., Citation2011), although the increase in spontaneous curvature caused by Brij 98 seems to be smaller than by SDS, which could be explained either by a faster flip-flop rate or a lower partition coefficient of Brij 98 into the lipid bilayer. The addition of TX-100 to erythro-GUVs did not cause a clear increase in either surface area or spontaneous curvature. Most likely, TX-100 is able to incorporate and flip-flop but to a lesser extent in erythro-GUVs as compared with pure fluid POPC GUVs (Sudbrack et al., Citation2011). However, TX-100 is clearly able to partially solubilize erythro-GUVs (). It can be speculated that TX-100 preferentially solubilizes the fluid phase and leaves an insoluble fraction, probably rich in sphyngolipids and cholesterol, to which the detergent cannot be incorporated to a great extent.
Band 3 protein, the most abundant integral protein of the erythrocytes, was almost absent in TX-100 DRMs (corresponding to approximately 2% of the content in the intact membrane) and it was considered unlikely to be associated with lipids rafts (Crepaldi Domingues et al., Citation2009). Here we clearly show that band 3 was detected in Brij DRMs (). Considering these results, band 3 still remains a potential candidate for DRMs linkage to the membrane-skeleton. Although there is evidence indicating the association of cholesterol-enriched domains with the membrane-skeleton, the identification of an anchor for such association is still unknown. However, Ciana et al. (Citation2014) have recently proposed the existence of a direct interaction between rafts and spectrin which phosphatidylserine plays a key role. On the other hand, our results revealed that band 3 could also be involved in the linkage of DRMs (and lipid rafts) to the membrane-skeleton through an electrostatic interaction (a carbonate-sensitive linkage). Moreover, the stability of band 3 in Brij DRMs can be explained by the fact that Brij detergents do not preferentially solubilize lipids from the inner leaflet, as TX-100 does. Actually, using atomic force microscopy, Cai et al. (Citation2012) showed direct evidence of lipid rafts in erythrocytes and band 3 was found to be localized in those microdomains when the inner monolayer was preserved.
The presence of cytoskeletal proteins in DRMs from different cells has been very controversial in the literature. In erythrocytes, particularly, PIP2 is known to promote changes in the membrane skeleton (An et al., Citation2005, Citation2006) and for other cells it has been shown that cholesterol modulates PIP2 activity and, consequently, the organization and stability of actin cytoskeleton (Kwik et al., Citation2003). Hence, alterations in such DRM proteins could be expected, as a result of cholesterol depletion. If this is the case here, it is evidenced for spectrin in Brij 98 DRMs (Supplementary Figure S1, online). Thus, considering that Brij does not preferentially solubilize the inner leaflet of the plasma membrane, the association of membrane skeletal proteins with membrane domains could be revealed by Brij DRMs. On the other hand, it has been shown that the presence of membrane skeletal proteins (i.e. spectrin) in TX-100 DRMs may reflect the contamination of the erythrocyte suspension by granulocytes and consequent proteolysis; as a result of the proteolysis the formed fragments can migrate to lower density regions in the sucrose gradient during the centrifugation (Ciana et al., Citation2011). However, even using carefully purified erythrocytes in the presence of protease inhibitors and keeping some maneuvers under control, TX-100 DRMs occasionally presented membrane skeletal fragments (Ciana et al., Citation2011, Citation2014). This association of membrane skeletal proteins with DRMs described for either Brij or TX-100 remains still unclear and requires further investigation.
In a thorough review, Quinn (Citation2010) proposed a lipid matrix model for the membrane raft structure, and described the association between cholesterol and SM in model membranes. Such interaction preferentially occurs when SM is symmetric (16–18 carbons in the acyl chain), while asymmetric (22–26 C) SM preferentially associates with glycerophospholipids instead of cholesterol. We have shown here that both symmetric and asymmetric SM are present in TX-100 and Brij DRMs from erythrocytes, so that specific associations between SM and cholesterol might occur. Interestingly, high amounts of lignoceric acid (24:0) were found only in SM and not in the other phospholipid fractions (PC and PE) of these DRMs. This could also be a relevant feature of SM, explaining its resistance to detergent solubilization. Koumanov et al. (Citation2005) have previously reported that the SM fraction of DRMs from human erythrocytes is enriched in lignoceric acid (62%), but gave no information about the fatty acid composition of the other phospholipid species. In our samples lignoceric acid was approximately 35% of the SM fractions extracted from the DRMs (). Such differences in the lignoceric acid content could be explained by the distinct protocols (for DRM preparation) and different original samples (ghosts or intact erythrocytes, respectively) used.
EPR is a very useful tool for membrane studies, providing information on the existence of membrane domains or the coexisting membrane phases (Subczynski et al., Citation2010). We have previously employed EPR to characterize DRMs from erythrocyte membranes prepared with TX-100 and C12E8, which were found to be in the liquid-ordered phase (Crepaldi Domingues et al., Citation2009; Domingues et al., Citation2010). Surprisingly, Brij 98 and Brij 58 DRMs prepared under different conditions (4 °C and 37 °C, normal and reduced cholesterol content – ) presented order parameters similar to those of the intact erythrocyte membranes. The higher S values () of TX-100 DRMs relative to Brij (98 and 58) DRMs indicate their different lipid compositions, which is a result of the selective disruption of the inner monolayer promoted by TX-100 (as discussed before). Since DRMs are known to be well-organized structures, enriched in SM and cholesterol, our results show that the high acyl chain packing of TX-100 DRMs can be directly related to lipids of the outer leaflet (cholesterol and SM). However, SM seems to contribute much more than cholesterol to the formation of a liquid-ordered phase. The increased S value sensed by 5-SASL () for cholesterol-depleted TX-100 DRMs (which are enriched in SM) reflects these highly ordered membrane fractions. These results indicate that cholesterol depletion per se is not enough to disrupt the interactions that maintain the liquid-ordered phase of DRMs. Additionally, the increased SM/cholesterol ratio in such DRMs might impose new interactions amongst the insoluble membrane lipids and proteins, resulting in an even more organized structure, with increased lipid packing.
The differential solubilization process promoted by Brij and TX-100 detergents was specifically confirmed using GUVs made from erythrocyte membrane lipid extracts and this finding reflects the remarkable differences found in the insoluble membranes obtained in the sucrose gradient. Thus, whether DRMs can be extrapolated to rafts, all the analysis conducted in this study could reflect the existence of different subpopulation of lipid rafts. These results can be understood on the basis of the heterogeneity of rafts, as proposed before (Pike, Citation2004). In that model, rafts containing different lipid composition coexist in cells and thus domains enriched in cholesterol and sphingolipid are resistant to TX-100 extraction, while domains containing higher level of glycerophospholipids are easily solubilized. Because Brij detergents are less selective in removing glycerophospholipids than TX-100, the isolated insoluble material contains more subtypes of rafts. In this work we showed that Brij DRMs present remarkable features of DRMs/rafts such as a low density in the sucrose gradient, a high cholesterol/protein mass ratio, and the presence of raft-marker proteins (flotillin-2 and stomatin). Hence, the increased level of PE and Band 3 in Brij DRMs might reflect their association with rafts. On the other hand, the different lipid and protein composition found in Brij DRMs could also be a consequence of an incomplete solubilization of non-raft domains. The fact that the solubilization of the lipid bilayer is a detergent-dependent process suggests that DRMs are only one way to approach the structure of lipid rafts and additional techniques are required. Other experiments are being conducted by our group to better understand the interaction of these detergents with membranes and the solubilization process.
Conclusion
This work provides results that differentiate the lipid and protein compositions of Brij and TX-100 DRMs obtained from erythrocytes. Although detergents can affect the lipid distribution on membranes, the use of Triton X-100 and Brij revealed different aspects of the lateral organization on erythrocyte membrane giving new insights into the membrane microdomain composition. Since components of both inner and outer leaflets were found to be present in Brij 98 and Brij 58 DRMs (differently from TX-100 DRMs), our data suggest that Brij DRMs are an alternative strategy to explore the membrane structural biology. We also provide evidence of the involvement of band 3 in lipid rafts which could play an important role in the structure and function of those microdomains. Altogether these results may impact future studies on cell membrane in either physiological or pathophysiological conditions.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by grants #2009/00904-1 from São Paulo Research Foundation (FAPESP) and #479993/2011-4 from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). BRC was the recipient of a fellowship from CNPq (#132836/2009-2) and CCD was a recipient of a fellowship from São Paulo Research Foundation (FAPESP #2010/18516-5).
Supplementary Material available online
Supplementary Figures S1–S4.
Supplementary Material
Download PDF (1.4 MB)Acknowledgements
The authors would like to thank Dr M. T. Lamy (University of São Paulo) for the use of the EPR facilities.
References
- American Oil Chemists’ Society (AOCS). 1998. Official Methods and Recommended Practices of the American Oil Chemists’ Society; 5th ed. Champaign (IL): AOCS
- An X, Guo X, Liu S, Lux SE, Baines A, Gratzer W, Mohandas N. 2005. Identification and functional characterization of protein 4.1R and actin-binding sites in erythrocyte beta spectrin: regulation of the interactions by phosphatidylinositol-4,5-bisphosphate. Biochemistry 44:10681–10688
- An X, Zhang X, Debnath G, Baines AJ, Mohandas N. 2006. Phosphatidylinositol-4,5-biphosphate (PIP2) differentially regulates the interaction of human erythrocyte protein 4.1 (4.1R) with membrane proteins. Biochemistry 45:5725–5732
- Angelova MI, Dimitrov DS. 1986. Liposome electroformation. Faraday Discuss Chem Soc 81:303–311
- Beutler E, West C, Blume KG. 1976. The removal of leukocytes and platelets from whole blood. J Lab Clin Med 88:328–333
- Bezlyepkina N, Gracià RS, Shchelokovskyy P, Lipowsky R, Dimova R. 2013. Phase diagram and tie-line determination for the ternary mixture DOPC/eSM/cholesterol. Biophys J 104:1456–1464
- Cai M, Zhao W, Shang X, Jiang J, Ji H, Tang Z, Wang H. 2012. Direct evidence of lipid rafts by in situ atomic force microscopy. Small 8:1243–12450
- Casadei BR, Domingues CC, de Paula E, Riske KA. 2014. Direct visualization of the action of Triton X-100 on giant vesicles of erythrocyte membrane lipids. Biophys J 106:2417–2425
- Cassera MB, Silber AM, Gennaro AM. 2002. Differential effects of cholesterol on acyl chain order in erythrocyte membranes as a function of depth from the surface. An electron paramagnetic resonance (EPR) spin label study. Biophys Chem 99:117–127
- Chen PS, Toribara JTY, Warner H. 1956. Microdetermination of phosphorus. Anal Chem 28:1756–1758
- Ciana A, Achilli C, Hannoush RN, Risso A, Balduini C, Minetti G. 2013. Freely turning over palmitate in erythrocyte membrane proteins is not responsible for the anchoring of lipid rafts to the spectrin skeleton: A study with bio-orthogonal chemical probes. Biochim Biophys Acta 1828:924–931
- Ciana A, Achilli C, Balduini C, Minetti G. 2011. On the association of lipid rafts to the spectrin skeleton in human erythrocytes. Biochim Biophys Acta 1808:183–190
- Ciana A, Balduini C, Minetti G. 2005. Detergent-resistant membranes in human erythrocytes and their connection to the membrane-skeleton. J Biosci 30:317–328
- Ciana A, Achilli C, Minetti G. 2014. Membrane rafts of the human red blood cell. Mol Membr Biol 31:47–57
- Crepaldi Domingues C, Ciana A, Buttafava A, Balduini C, De Paula E, Minetti G. 2009. Resistance of human erythrocyte membranes to Triton X-100 and C12E8. J Membr Biol 227:39–48
- Dodge JT, Mitchell C, Hanahan DJ. 1963. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys 100:119–130
- Domingues CC, Ciana A, Buttafava A, Casadei BR, Balduini C, De Paula E, Minetti G. 2010. Effect of cholesterol depletion and temperature on the isolation of detergent-resistant membranes from human erythrocytes. J Membr Biol 234:195–205
- Folch J, Lees M, Sloane Stanley GH. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
- Goni F, Alonso A, Bagatolli L, Brown R, Marsh D, Prieto M, Thewalt J. 2008. Phase diagrams of lipid mixtures relevant to the study of membrane rafts. Biochim Biophys Acta 1781:665–684
- Hubbell WL, McConnell HM. 1971. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc 93:314–326
- Ingelmo-Torres M, Gaus K, Herms A, Gonzalez-Moreno E, Kassan A, Bosch M, et al. 2009. Triton X-100 promotes a cholesterol-dependent condensation of the plasma membrane. Biochem J 420:373–381
- Koumanov K, Tessier C, Momchilova A, Rainteau D, Wolf C, Quinn P. 2005. Comparative lipid analysis and structure of detergent-resistant membrane raft fractions isolated from human and ruminant erythrocytes. Arch Biochem Biophys 434:150–158
- Kwik J, Boyle S, Fooksman D, Margolis L, Sheetz MP, Edidin M. 2003. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc Natl Acad Sci USA 100:13964–13969
- Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
- Lichtenberg D, Goni F, Heerklotz H. 2005. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem Sci 30:430–436
- Lingwood D, Kaiser H, Levental I, Simons K. 2009. Lipid rafts as functional heterogeneity in cell membranes. Biochem Soc Trans 37:955–960
- Mainali L, Raguz M, O'brien WJ, Subczynski WK. 2012. Properties of fiber cell plasma membranes isolated from the cortex and nucleus of the porcine eye lens. Exp Eye Res 97:117–129
- Mikhalyov I, Samsonov A. 2011. Lipid raft detecting in membranes of live erythrocytes. Biochim Biophys Acta 1808:1930–1939
- Murphy SC, Samuel BU, Harrison T, Speicher KD, Speicher DW, Reid ME, et al. 2004. Erythrocyte detergent-resistant membrane proteins: Their characterization and selective uptake during malarial infection. Blood 103:1920–1928
- Nagao E, Seydel K B, Dvorak JA. 2002. Detergent-resistant erythrocyte membrane rafts are modified by a Plasmodium falciparum infection. Exp Parasitol 102:57–59
- Pathak P, London E. 2011. Measurement of lipid nanodomain (raft) formation and size in sphingomyelin/POPC/cholesterol vesicles shows TX-100 and transmembrane helices increase domain size by coalescing preexisting nanodomains but do not induce domain formation. Biophys J 101:2417–2425
- Pike L. 2004. Lipid rafts: heterogeneity on the high seas. Biochem J 378:281–292
- Pike L, Han X, Gross RW. 2005. Epidermal growth factor receptors are localized to lipid rafts that contain a balance of inner and outer leaflet lipids: a shotgun lipidomics study. J Biol Chem 280:26796–26804
- Quinn P. 2010. A lipid matrix model of membrane raft structure. Prog Lipid Res 49:390–406
- Rose HG, Oklander M. 1965. Improved procedure for the extraction of lipids from human erythrocytes. J Lipid Res 6:428–431
- Salzer U, Prohaska R. 2001. Stomatin, flotillin-1, and flotillin-2 are major integral proteins of erythrocyte lipid rafts. Blood 97:1141–1143
- Samuel BU, Mohandas N, Harrison T, Mcmanus H, Rosse W, Reid M, Haldar K. 2001. The role of cholesterol and glycosylphosphatidylinositol-anchored proteins of erythrocyte rafts in regulating raft protein content and malarial infection. J Biol Chem 276:29319–29329
- Schreier S, Polnaszek CF, Smith IC. 1978. Spin labels in membranes. Problems in practice. Biochim Biophys Acta 515:395–436
- Schuck S, Honsho M, Ekroos K, Shevchenko A, Simons K. 2003. Resistance of cell membranes to different detergents. Proc Natl Acad Sci USA 100:5795–5800
- Sheetz MP. 1979. Integral membrane protein interaction with Triton cytoskeletons of erythrocytes. Biochim Biophys Acta 557:122–134
- Sheetz MP, Singer SJ. 1974. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci USA 71:4457–4461
- Singer SJ, Nicolson G. 1972. The fluid mosaic model of the structure of cell membranes. Science 175:720–731
- Sonnino S, Prinetti A. 2008. Membrane lipid domains and membrane lipid domain preparations: are they the same thing? Trends Glycosci Glycotechnol 20:315–340
- Sonnino S, Prinetti A. 2013. Membrane domains and the ‘lipid raft’ concept. Curr Med Chem 20:4–21
- Steck TL. 1974. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol 62:1–19
- Subczynski W, Raguz M, Widomska J. 2010. Studying lipid organization in biological membranes using liposomes and EPR spin labeling. Methods Mol Biol 606:247–269
- Sudbrack TP, Archilha NL, Itri R, Riske KA. 2011. Observing the solubilization of lipid bilayers by detergents with optical microscopy of GUVs. J Phys Chem B 115:269–277
- Toledo MS, Suzuki E, Straus AH, Takahashi HK. 1995. Glycolipids from Paracoccidioides brasiliensis. Isolation of a galactofuranose-containing glycolipid reactive with sera of patients with paracoccidioidomycosis. J Med Vet Mycol 33:247–251
- Waugh M, Hsuan J. 2009. Preparation of membrane rafts. Methods Mol Biol 462:403–414
- Yu J, Fischman DA, Steck TL. 1973. Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents. J Supramol Struct 1:233–248
