Abstract
This mini-review addresses advances in understanding the transmembrane topologies of two unrelated, single-subunit bicarbonate transporters from cyanobacteria, namely BicA and SbtA. BicA is a Na+-dependent bicarbonate transporter that belongs to the SulP/SLC26 family that is widespread in both eukaryotes and prokaryotes. Topology mapping of BicA via the phoA/lacZ fusion reporter method identified 12 transmembrane helices with an unresolved hydrophobic region just beyond helix 8. Re-interpreting this data in the light of a recent topology study on rat prestin leads to a consensus topology of 14 transmembrane domains with a 7+7 inverted repeat structure. SbtA is also a Na+-dependent bicarbonate transporter, but of considerably higher affinity (Km 2–5 μM versus >100 μM for BicA). Whilst SbtA is widespread in cyanobacteria and a few bacteria, it appears to be absent from eukaryotes. Topology mapping of SbtA via the phoA/lacZ fusion reporter method identified 10 transmembrane helices. The topology consists of a 5+5 inverted repeat, with the two repeats separated by a large intracellular loop. The unusual location of the N and C-termini outside the cell raises the possibility that SbtA forms a novel fold, not so far identified by structural and topological studies on transport proteins.
Introduction
This mini-review considers the use of topology mapping to uncover the basic topologies of two unrelated, single-subunit bicarbonate transporters from cyanobacteria, namely BicA (SulP/SLC26A family) and SbtA. Recent data on the likely topology of SLC26 family members are also considered with reference to BicA structure.
BicA and SbtA are two key Na+-dependent bicarbonate transporters that contribute to the functionality of the cyanobacterial CO2 concentrating mechanism (CCM). transporters, in combination with active CO2 uptake systems, allow
to be actively accumulated inside the cell, and then dehydrated to CO2 within carboxysome micro-compartments to support optimal rates of photosynthetic CO2 fixation via the primary carboxylase, ribulose bisphosphate carboxylase-oxygenase or Rubisco (Price et al., Citation2008; Price, Citation2011). A functional CCM enhances growth performance, and a basal form of the cyanobacterial CCM is obligatory for survival in the natural environment. Largely due to the contribution of oceanic strains, cyanobacteria are estimated to contribute as much as 25% to annual global net primary productivity (Field et al., Citation1998; Liu et al., Citation1997). Uptake of CO2 and
represents the largest macro-nutrient flux in support of photosynthesis and biosynthesis in cyanobacteria, with a large fraction of this flux expected to be handled by BicA and SbtA. In the case of the prevalent Prochlorococcus species, BicA and SbtA homologs are the only identifiable transporters capable of handling photosynthetic CO2/
uptake (Price, Citation2011; Rae et al., Citation2011).
BicA is a Na+-dependent bicarbonate transporter of relatively low substrate-affinity, but with high maximum flux rate relative to photosynthesis; it belongs to the widespread SulP/SLC26 family (Price et al., Citation2004) and is a probable Na+/ symporter with potential 1:1 stoichiometry. Three different BicA transporters have been shown to display transport affinities (Km) of 74–353 μM (Price, Citation2011).
SbtA, is a high-affinity Na+-dependent bicarbonate transporter (Shibata et al., 2002) acting as a probable Na+/ symporter (potentially 2Na+:
stoichiometry). In Synechococcus PCC7002, the SbtA transporter has been estimated to have a high photosynthetic affinity for
(approx. equivalent to Km) of around 2 μM and of relatively low flux rate (Price et al., Citation2004).
The BicA and SbtA transporters are believed to be inactive in the dark, a process that potentially involves protein phosphorylation events (Price et al., Citation2008; Price, Citation2011). In addition to determining the structure of two interesting transporters, topology mapping of BicA and SbtA was undertaken as prelude to understanding the post-translational or allosteric activation of BicA and SbtA. The other purpose was to allow rational cloning/fusion approaches to introducing these transporters into the chloroplast of higher plants to improve the efficiency of CO2 fixation and final crop yield (Price et al., Citation2011a, Citation2013).
Methods of topology determination
Transmembrane topology can be determined by a number of methods; while it does not have the resolution of a crystal structure, knowledge of the topology does provide a useful basis for further studies. All methods rely on tagging the protein in some way at multiple sites and then assessing accessibility of the tag from the intracellular or extracellular side of the membrane or from within the membrane to determine its location relative to the membrane. We have used the phoA-lacZ dual reporter system to determine the topologies of BicA and SbtA but, in the case of BicA, have drawn on data from other approaches to generate the topology shown here. We will begin by briefly reviewing these approaches.
Enzyme reporter systems rely on using enzymes that function only in the cytoplasm or periplasm and therefore enzyme activity functions as a reporter of topology. In the phoA-lacZ system a cassette including the genes for alkaline phosphatase (phoA) and β-galactosidase (lacZ) is fused to successive C-terminal truncations of the transporter gene (Alexeyev & Winkler, Citation1999; van Geest & Lolkema, Citation2000), or can be inserted into the gene as a sandwich fusion. The activity of both enzymes is then determined. Since alkaline phosphatase is active only in the periplasm while β-galactosidase is active only in the cytoplasm, the relative activity indicates the topological orientation of the construct. An advantage of the dual reporter system is that the ratio of the two activities can be calculated and this corrects for any differences in the expression level of different fusion constructs (Alexeyev & Winkler, Citation1999). Dual reporter systems have been shown to provide accurate topological information by comparing results with subsequent structures as they become available (Cassel et al., Citation2008).
The reliability of reporter fusion topology mapping was more thoroughly tested by determining the topology of the Escherichia coli ClcA H+/Cl− exchange transporter using a phoA/GFP reporter (Cassel et al., Citation2008) after the structure had been determined by X-ray crystal structure (Dutzler et al., Citation2002). This allowed the construction of fusions at different points along the known structure to test for potential difficulties of the dual reporter system. It was concluded that the dual reporter accurately predicted “normal” TMH although it did not detect the two shorter, and less hydrophobic, helical hairpins seen in the ClcA structure because the size of the fusion protein disrupted normal folding. Another disadvantage of this approach is that it is restricted to use in bacteria; however, as many eukaryotic membrane proteins have bacterial homologues, topologies can be deduced from studies on these homologues in the same way that crystal structures of bacterial proteins are being used to develop homology models of their eukaryotic counterparts. Bacterial systems for the analysis of topology provide advantages in the ease with which the multiple constructs required can be generated and expressed and because well-developed methodologies exist.
Scanning cysteine accessibility mutagenesis (SCAM) is another commonly used approach. This approach relies on the reactivity of the sulfhydryl group of cysteine. Cysteine residues are introduced at key points along the length of the protein, which is then expressed in an appropriate system and treated with various reagents that covalently bind to cysteine residues. These reagents can be introduced from either side of the membrane and can be chosen with a range of properties to scan particular aspects of structure or function (Bogdanov et al., Citation2005; Zhu & Casey, Citation2007). By mapping accessibility of introduced cysteines from the intra- and extracellular side of the membrane, topology can be determined. This method overcomes several of the disadvantages of reporter fusion methods. First, since only a single cysteine residue is needed in each construct the method does not rely on substantial modification of the protein and as the modified protein often retains function, normal folding is assured. Second, this approach can also be used in many different expression systems. Finally, the choice of sulfhydryl reagents available can lead to fine scale mapping of regions of the transporter of interest. However, it requires a functional cysteine-less protein as the starting point so is unsuitable for any protein where a cysteine residue has an essential functional role. Methods for the analysis of binding to cysteine residues can also be laborious, depending on the system used.
Glycosylation and epitope scanning are similar approaches, in that both require introduction of a small protein sequence to analyse accessibility; examples (Kast & Gros, Citation1998; Popov et al., Citation1997). They are therefore intermediate in their potential to disrupt protein folding when compared with reporter-fusion methods and SCAM. Glycosylation scanning is restricted to eukaryotic systems and depends on the introduction of a three amino acid N-glycosylation consensus sequence (Cheung & Reithmeier, Citation2007). Since glycosylation occurs in the lumen of the endoplasmic reticulum, it is found only on extracellular loops and therefore acts as a topology marker, although only for extracellular locations. As it occurs co-translationally, it can also provide information on the folding pathway of a membrane protein as any part of the protein exposed to the lumen endoplasmic reticulum during folding can potentially be glycosylated, regardless of its final location. Epitope scanning requires the introduction of an epitope at various points in the protein sequence. The protein can then be assessed for reactivity to the relevant antibody when supplied from either side of the membrane (see for example, Czachorowski et al., Citation2009). In both techniques, it is difficult to interpret a negative result as local folding and proximity to the membrane surface could inhibit either glycosylation or antibody binding, leading to false negatives.
BicA topology
Members of the SulP/SLC26 family are found in plants, animals and bacteria and function in the transport of anions (Alper & Sharma, Citation2013; Hawkesford, Citation2003; Mount & Romero, Citation2004; Price & Howitt, Citation2011). Many have been well characterized physiologically, with roles including nutrient uptake in plants, bicarbonate uptake in photosynthetic bacteria and anion transport and regulation in mammalian tissues, with mutations in some members of the family causing genetic disease. Prestin is a molecular motor protein that responds to voltage, rather than having a transport function but is clearly homologous to the rest of the family (Zheng et al., Citation2000).
Bioinformatic approaches initially predicted that SulP transporters included a hydrophobic domain consistent with the presence of multiple transmembrane helices and an intracellular domain, the Sulphate Transporter Anti-Sigma Antagonist or STAS domain, named because of a low level of homology to a bacterial anti-sigma antagonist, SpoIIAA (Aravind & Koonin, Citation2000). This basic structure has been confirmed by a low resolution structure from a bacterial SulP protein (Compton et al., Citation2011; Hallworth et al., Citation2013), which was found to be dimeric. Interactions within the transmembrane region stabilized the dimer and the STAS domains were not in contact with each other (Compton et al., Citation2011). In contrast, it has recently been suggested that tetramers are the preferred structure, at least in vertebrates (Hallworth et al., Citation2013). So far there is no high resolution structure for any member of the family; however, the structure of the STAS domain from several sources has been determined (Babu et al., Citation2010; Pasqualetto et al., Citation2008; Sharma et al., Citation2011b). Furthermore, removal or mutation of the STAS domain affects transport function (reviewed by Sharma et al., Citation2011a).
The topology of SulP transporters has been controversial, but has recently been comprehensively analyzed by two studies on different members of the family, leading to the topology shown in which uses BicA as example. The results of both studies are consistent with a 14-transmembrane structure, comprising a 7+7 inverted repeat based on the structure of the bacterial uracil transporter, UraA (Lu et al., Citation2011). The topology of BicA was analysed through the generation of random phoA-lacZ fusions followed by targeted constructs to distinguish between different models consistent with the initial data (Shelden et al., Citation2010). This clearly showed 12 full transmembrane regions with intracellular N- and C-termini. While the topology was largely consistent with hydropathy predictions, there was one exception. A hydrophobic region between transmembrane helices 8 and 9, corresponding to one of the two conserved motifs within the family (Saier et al., Citation1999), was found to be located cytoplasmically instead of in the membrane. At the time, we suggested this region may form a re-entrant loop or interact with another part of the protein, consistent with its hydrophobicity, sequence conservation and implication in transport function (Price & Howitt, Citation2011; Shelden et al., Citation2010). Interaction between this region and another part of the transporter was likely because mutations within the loop result in little or no transport activity, due to an effect on trafficking of the mutant proteins to the plasma membrane (Coyle et al., Citation1998; Khurana et al., Citation2000; Karniski, Citation2001; Pera et al., Citation2008). This suggested that the mutations prevent correct folding of the protein, implying that this region is important for the final folded form of the protein.
Figure 1. Topology of the BicA-7002 bicarbonate transporter showing 14 transmembrane (TM) segments with a 7+7 inverted repeat structure. The transmembrane region shown in orange was not positively identified as crossing the membrane in the BicA mapping study (Shelden et al., Citation2010) but has been included based on the topology of prestin (Gorbunov et al., Citation2014) to provide a consensus topology for the SulP/SLC26 family. By analogy to UraA and rPRES, TM3 of BicA (shaded green) could also be partially unwound. The Saier motif refers to a 13 amino acid hydrophobic region after transmembrane helix 8 that is the second of two conserved motifs within the SulP (TC #2.53) family (Saier et al., Citation1999). STAS refers to the Sulphate Transporter Anti-Sigma Antagonist domain, named because of a low level of homology to a bacterial anti-sigma antagonist, SpoIIAA (Aravind & Koonin, Citation2000).
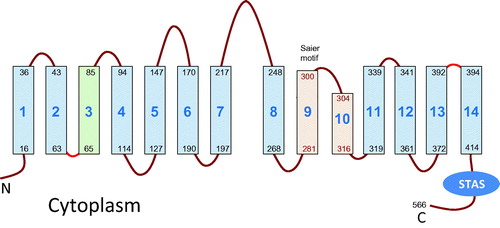
The location of this region was recently resolved by the second topology study which used homology modelling, cysteine accessibility scanning (SCAM) and molecular dynamics to show that rat prestin (rPres) contains 14 transmembrane segments (Gorbunov et al., Citation2014). An important advance was to recognize UraA as a structural homolog for SulP/SLC26, which was determined both by bioinformatic approaches (Vastermark & Saier, Citation2014) and homology modelling (Gorbunov et al., Citation2014). This allowed the solved crystal structure of UraA to be used as a modelling template for prestin, followed by confirmation of the topology of the modelled structure by labelling strategically placed cysteine residues from either the intracellular or extracellular medium (Gorbunov et al., Citation2014). Twelve of the transmembrane segments in UraA and rPres are most likely helical and equivalent to those identified in BicA. The remaining two are formed from the putative re-entrant loop region. The 14 transmembrane segments comprise a 7+7 inverted repeat structure with helices 3 and 10 being partially unfolded and containing a short region of β-strand (see ).
Taken together, the two topology studies on SulP family members provide a convincing argument for the 14 transmembrane segments, and to therefore refute or resolve other previously proposed topologies, such as (Alper & Sharma, Citation2013; Leves et al., Citation2008; Lohi et al., Citation2003; Navaratnam et al., Citation2005; Rajagopalan et al., Citation2006; Zheng et al., Citation2001). Our study (Shelden et al., Citation2010) likely missed segments 9 and 10 because the presence of the fusion protein can interfere with normal membrane targeting. This is particularly likely where the structures are less hydrophobic (Cassel et al., Citation2008), which may be the case here because of the β-strand segment within the corresponding sequence of helix 10 of UraA. It is also possible that the BicA loop between helices 9 and 10 does not extend from the outer face of the membrane. The prestin study was unable to confirm a cytoplasmic location between helices 2 and 3 (which contains another β-strand region) even though cysteine labelling is usually less disruptive. However, the BicA study did locate this tight loop to the cytoplasmic face. Again, the loop may normally remain inside the membrane but the fusion protein might have resulted in part of the normally hidden loop extending into the cytoplasm whereas a single cysteine substitution is unlikely to disrupt structure, and so remain inaccessible. For human SLC26 members the extracellular location of loops between helices 3/4 and 7/8 has been verified by analyzing the functionality of putative glycosylation sites (Li et al., Citation2014; Matsuda et al., Citation2004).
The 14-transmembrane-segment-structure provides an explanation for much of the existing structure/function work on members of the SulP family. Helices 9 and 10 contain one of the two most highly conserved regions of the SulP family; the other is found in the first two helices. Structure/function studies using analysis of mutants have identified both regions as functionally important (Khurana et al., Citation2000; Leves et al., Citation2008; Rajagopalan et al., Citation2006). Disease-causing mutations in human homologues are also found in helices 9 and 10 (Coyle et al., Citation1998; Karniski, Citation2001; Khurana et al., Citation2000; Pera et al., Citation2008). Studies with chimeras of prestin from mammals, which show electromotility, and non-mammals, where prestin has a transport function, have led to the proposal that these two regions interact and contribute to both functions (Schaechinger et al., Citation2011). Exchange of both regions was required to convert the non-electromotile prestin from zebrafish to a transporter displaying electromotility. The proposed 14-transmembrane segment topology explains these data by showing interaction between these two conserved regions to form the ion binding site. This is consistent with the positioning of the uracil binding site in UraA and was supported by molecular dynamics simulation to confirm this site as solute accessible (Gorbunov et al., Citation2014). It is also noteworthy that the red cell bicarbonate/chloride exchanger, SLC4A1, has also been modelled on UraA as a 14-transmembrane (Barneaud-Rocca et al., Citation2013; Cordat & Reithmeier, Citation2014).
SbtA topology
SbtA, the high affinity Na+-dependent bicarbonate transporter (typical Km of 2–5 μM bicarbonate), is important in cyanobacterial photosynthetic CO2 fixation (Price, Citation2011), but it belongs to a family of transporters much more restricted in distribution. Bioinformatic analysis of the SbtA family shows that is largely restricted to cyanobacteria and a few bacteria, although a low level similarity to the arabinose transporter AraH of the ABC superfamily (TC 3.A.1.2.2) has been detected (von Rozycki et al., Citation2004). The family is apparently absent from eukaryotes. Because of this, the family has been much less studied than the SulP family, resulting in an almost complete absence of structural information or structure/function studies. There is, however, evidence that SbtA, a predicted monomer of 40 kDa, exists in the membrane of cyanobacteria as a 160 kDa tetramer (Zhang et al., Citation2004), suggesting a tetrameric structure. Topology mapping of SbtA from Synechocystis PCC6803 was attained with the same dual reporter system (see above) as that used for BicA (Price et al., Citation2011b). SbtA was found to have 10 transmembrane helices (). Further sequence analysis revealed an internal duplication suggesting a 5+5 inverted repeat structure with a large intracellular loop separating the two repeat domains, confirming previous analysis (von Rozycki et al., Citation2004). Unusually, the N- and C-termini are located outside the cell.
Figure 2. Topology of SbtA-6803 bicarbonate transporter showing 10 transmembrane segments with 5+5 inverted repeat structure based on the phoA-lacZ reporter topology study (Price et al., Citation2011b).
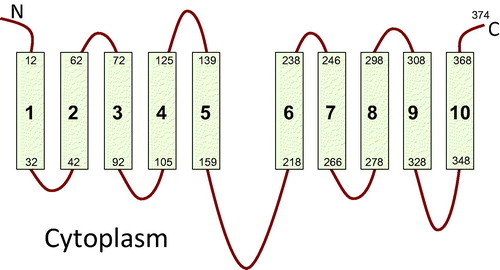
It is unclear whether SbtA, like BicA, belongs to a larger family making it difficult to identify an appropriate structural model. It is increasingly clear that secondary transporters adopt a relatively small number of common folds, with proteins that show no sequence similarity adopting the same fold (Shi, Citation2013). While other transporters with a 5+5 inverted repeat structure exist, such as those with the LeuT or NhaA folds (Abramson & Wright, Citation2009; Shi, Citation2013), these have their N- and C-termini on the cytoplasmic side of the membrane. Whether complete inversion of a transporter structure is possible remains unknown. The large intracellular loop in SbtA is reminiscent of structures from the major facilitator superfamily, except that these transporters have 12 or more transmembrane helices and form 6+6 repeats so would require that SbtA had lost the first and 12th helices (Yan, Citation2013). Whether SbtA represents a distinct fold or is a variant on a known structure is only likely to be resolved when a crystal structure becomes available.
Summation
In the absence of a solved crystal structure for a particular membrane protein, the application of topology mapping techniques has demonstrated capacity to yield valuable structural information about the number and location of transmembrane helices and placement of cytoplasmic and periplasmic loops. Topology determination is most reliable when data is confirmed by more than one approach as each has distinct advantages and disadvantages. Topology studies can also benefit from combined approaches incorporating phylogenetic studies, topology prediction programs and/or homology modelling. As we have shown with the SulP family, the convergence of a number of studies using different methodologies and different members of the family has produced a well-supported consensus topology. For both BicA and SbtA, knowledge of their topologies is proving to be an invaluable aid to interpreting and designing structure/function studies which may eventually lead to optimization of these transporters for expression in plants.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abramson J, Wright EM. 2009. Structure and function of Na+-symporters with inverted repeats [Review]. Curr Opin Struct Biol 19:425–432
- Alexeyev MF, Winkler HH. 1999. Membrane topology of the Rickettsia prowazekii ATP/ADP translocase revealed by novel dual pho-lac reporters. J Mol Biol 285:1503–1513
- Alper SL, Sharma AK. 2013. The SLC26 gene family of anion transporters and channels. Molec Asp Med 34:494–515
- Aravind L, Koonin EV. 2000. The STAS domain – a link between anion transporters and antisigma-factor antagonists. Curr Biol 10:R53–55
- Babu M, Greenblatt JF, Emili A, Strynadka NCJ, Reithmeier RAF, Moraes TF. 2010. Structure of a SLC26 anion transporter STAS domain in complex with acyl carrier protein: implications for E. coli YchM in fatty acid metabolism. Structure 18:1450–1462
- Barneaud-Rocca D, Etchebest C, Guizouarn H. 2013. Structural model of the anion exchanger 1 (SLC4A1) and identification of transmembrane segments forming the transport site. J Biologic Chem 288:26372–26384
- Bogdanov M, Zhang W, Xie J, Dowhan W. 2005. Transmembrane protein topology mapping by the substituted cysteine accessibility method (SCAMTM): application to lipid-specific membrane protein topogenesis. Methods 36:148–171
- Cassel M, Seppala S, von Heijne G. 2008. Confronting fusion protein-based membrane protein topology mapping with reality: The Escherichia coli ClcA H+/Cl- exchange transporter. J Mol Biol 381:860–866
- Cheung JC, Reithmeier RA. 2007. Scanning N-glycosylation mutagenesis of membrane proteins. Methods 41:451–459
- Compton ELR, Karinou E, Naismith JH, Gabel F, Javelle A. 2011. Low resolution structure of a bacterial SLC26 transporter reveals dimeric stoichiometry and mobile intracellular domains. J Biol Chem 286:27058–27067
- Cordat E, Reithmeier RAF. 2014. Structure, function, and trafficking of SLC4 and SLC26 anion transporters. In Bevensee MO, ed. Exchangers (Current Topics in Membranes). Burlington (MA): Elsevier Inc. Academic Press, 1–67
- Coyle B, Reardon W, Herbrick JA, Tsui LC, Gausden E, Lee J, et al. 1998. Molecular analysis of the Pds gene in pendred-syndrome (sensorineural hearing loss and goitre). Human Mol Genet 7:1105–1112
- Czachorowski M, Lam-Yuk-Tseung S, Cellier M, Gros P. 2009. Transmembrane topology of the mammalian Slc11a2 iron transporter. Biochemistry 48:8422–8434
- Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. 2002. X-ray structure of a ClC chloride channel at 3.0A reveals the molecular basis of anion selectivity. Nature 415:287–294
- Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. 1998. Primary production of the biosphere – integrating terrestrial and oceanic components. Science 281:237–240
- Gorbunov D, Mattia Sturlese M, Florian Nies F, Murielle Kluge M, Massimo Bellanda M, Roberto Battistutta R, Dominik Oliver D. 2014. Molecular architecture and the structural basis for anion interaction in prestin and SLC26 transporters. Nat Commun 5:3622–3635
- Hallworth R, Stark K, Zholudeva L, Currall BB, Nichols MG. 2013. The conserved tetrameric subunit stoichiometry of Slc26 proteins. Microsc Microanal 19:799–807
- Hawkesford MJ. 2003. Transporter gene families in plants: The sulphate transporter gene family – redundancy or specialization? Physiologia Plantarum 117:155–163
- Karniski LP. 2001. Mutations in the diastrophic dysplasia sulfate transporter (DTDST) gene: Correlation between sulfate transport activity and chondrodysplasia phenotype. Human Mol Genet 10:1485–1490
- Kast C, Gros P. 1998. Epitope insertion favors a six transmembrane domain model for the carboxy-terminal portion of the multidrug resistance-associated protein. Biochemistry 37:2305–2313
- Khurana OK, Coupland LA, Shelden MC, Howitt SM. 2000. Homologous mutations in two diverse sulphate transporters have similar effects. FEBS Lett 477:118–122
- Leves FP, Tierney ML, Howitt SM. 2008. Polar residues in a conserved motif spanning helices 1 and 2 are functionally important in the SulP transporter family. Int J Biochem Cell Biol 40:2596–2605
- Li J, Xia F, Reithmeier R. 2014. N-glycosylation and topology of the human SLC26 family of anion transport membrane proteins. Am J Physiol Cell Physiol 306:C943–C960
- Liu HB, Nolla HA, Campbell L. 1997. Prochlorococcus growth rate and contribution to primary production in the equatorial and subtropical North Pacific Ocean. Aquatic Microb Ecol 12:39–47
- Lohi H, Lamprecht G, Markovich D, Heil A, Kujala M, Seidler U, Kere J. 2003. Isoforms of SLC26A6 mediate anion transport and have functional PDZ interaction domains. Am J Physiol Cell Physiol 284:C769–C779
- Lu FR, Li S, Jiang Y, Jiang J, Fan H, Lu GF, et al. 2011. Structure and mechanism of the uracil transporter UraA. Nature 472:243–246
- Matsuda K, Zheng J, Du GG, Klocker N, Madison LD, Dallos P. 2004. N-linked glycosylation sites of the motor protein prestin: effects on membrane targeting and electrophysiological function. J Neurochem 89:928–938
- Mount DB, Romero MF. 2004. The SLC26 gene family of multifunctional anion exchangers [Review]. Eur J Physiol 447:710–721
- Navaratnam D, Bai JP, Samaranayake H, Santos-Sacchi J. 2005. N-terminal-mediated homomultimerization of prestin, the outer hair cell motor protein. Biophys J 89:3345–3352
- Pasqualetto E, Seydel A, Pellini A, Battistutta R. 2008. Expression, purification and characterisation of the C-terminal STAS domain of the SLC26 anion transporter prestin. Protein Expr Purif 58:249–256
- Pera A, Dossena S, Rodighiero S, Gandia M, Botta G, Meyer G, et al. 2008. Functional assessment of allelic variants in the SLC26A4 gene involved in Pendred syndrome and nonsyndromic EVA. Proc Natl Acad Sci USA 105:18608–18613
- Popov M, Tam LY, Li J, Reithmeier RA. 1997. Mapping the ends of transmembrane segments in a polytopic membrane protein. Scanning N-glycosylation mutagenesis of extracytosolic loops in the anion exchanger, band 3. J Biol Chem 272:18325–18332
- Price GD. 2011. Inorganic carbon transporters of the cyanobacterial CO2 concentrating mechanism. Photosynthesis Res 109:47–57
- Price GD, Badger MR, von Caemmerer S. 2011a. The prospect of using cyanobacterial bicarbonate transporters to improve leaf photosynthesis in C3 crop plants. Plant Physiol 155:20–26
- Price GD, Badger MR, Woodger FJ, Long BM. 2008. Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): Functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J Exp Bot 59:1441–1461
- Price GD, Howitt SM. 2011. The cyanobacterial bicarbonate transporter BicA: Its physiological role and the implications of structural similarities with human SLC26 transporters. Biochem Cell Biol 89:178–188
- Price GD, Pengelly JJL, Forster B, Du JH, Whitney SM, von Caemmerer S, et al. 2013. The cyanobacterial CCM as a source of genes for improving photosynthetic CO2 fixation in crop species. J Exp Botany 64:753–768
- Price GD, Shelden MC, Howitt SM. 2011b. Membrane topology of the cyanobacterial bicarbonate transporter, SbtA, and identification of potential regulatory loops. Mol Membr Biol 28:265–275
- Price GD, Woodger FJ, Badger MR, Howitt SM, Tucker L. 2004. Identification of a SulP-type bicarbonate transporter in marine cyanobacteria. Proc Natl Acad Sci USA 101:18228–18233
- Rae BD, Forster B, Badger MR, Price GD. 2011. The CO2-concentrating mechanism of Synechococcus WH5701 is composed of native and horizontally-acquired components. Photosynth Res 109:59–72
- Rajagopalan L, Patel N, Madabushi S, Goddard JA, Anjan V, Lin F, et al. 2006. Essential helix interactions in the anion transporter domain of prestin revealed by evolutionary trace analysis. J Neurosci 26:12727–12734
- Saier MH, Eng BH, Fard S, Garg J, Haggerty DA, Hutchinson WJ, et al. 1999. Phylogenetic characterization of novel transport protein families revealed by genome analyses [Review]. Biochim Biophys Acta Rev Biomemb 1422:1–56
- Schaechinger TJ, Gorbunov D, Halaszovich CR, Moser T, Kugler S, Fakler B, Oliver D. 2011. A synthetic prestin reveals protein domains and molecular operation of outer hair cell piezoelectricity. EMBO J 30:2793–2804
- Sharma AK, Rigby AC, Alper SL. 2011a. STAS domain structure and function. Cellular Physiol Biochem 28:407–422
- Sharma AK, Ye LW, Baer CE, Shanmugasundaram K, Alber T, Alper SL, Rigby AC. 2011b. Solution structure of the guanine nucleotide-binding STAS domain of SLC26-related SulP protein Rv1739c from Mycobacterium tuberculosis. J Biol Chem 286:8534–8544
- Shelden MC, Howitt SM, Price GD. 2010. Membrane topology of the cyanobacterial bicarbonate transporter, BicA, a member of the SulP (SLC26A) family. Mol Membr Biol 27:12–23
- Shi Y. 2013. Common folds and transport mechanisms of secondary active transporters. Annu Rev Biophys 42:51–72
- Shibata M, Katoh H, Sonoda M, Ohkawa H, Shimoyama M, Fukuzawa H, et al. 2002. Genes essential to sodium-dependent bicarbonate transport in cyanobacteria – function and phylogenetic analysis. J Biol Chem 277:18658–18664
- van Geest M, Lolkema JS. 2000. Membrane topology and insertion of membrane proteins: Search for topogenic signals [Review]. Micro Molec Biol Rev 64:13–33
- Vastermark A, Saier MH Jr. 2014. Evolutionary relationship between 5+5 and 7+7 inverted repeat folds within the amino acid-polyamine-organocation superfamily. Proteins 82:336–346
- von Rozycki T, Schultzel MA, Saier MH Jr. 2004. Sequence analyses of cyanobacterial bicarbonate transporters and their homologues. J Mol Microbiol Biotechnol 7:102–108
- Yan N. 2013. Structural advances for the major facilitator superfamily (MFS) transporters. Trends Biochem Sci 38:151–159
- Zhang PP, Battchikova N, Jansen T, Appel J, Ogawa T, Aro EM. 2004. Expression and functional roles of the two distinct NDH-1 complexes and the carbon acquisition complex NdhD3/NdhF3/CupA/Sll1735 in Synechocystis sp PCC 6803. Plant Cell 16:3326–3340
- Zheng J, Long KB, Shen WX, Madison LD, Dallos P. 2001. Prestin topology: Localization of protein epitopes in relation to the plasma membrane. Neuroreport 12:1929–1935
- Zheng J, Shen WX, He DZZ, Kevin BL, Madison LD, Dallos P. 2000. Prestin is the motor protein of cochlear outer hair cells. Nature 405:149–155
- Zhu Q, Casey JR. 2007. Topology of transmembrane proteins by scanning cysteine accessibility mutagenesis methodology. Methods 41:439–450
