Abstract
Aquaporins (AQP) are transmembrane channels for small, predominantly uncharged solutes. Their selectivity is partly determined by the aromatic/arginine constriction. Ammonia is similar in size and polarity to water, yet a subset of aquaporins distinguishes between the two. We mutated the constriction of water-selective rat AQP1 to mimic that of the ammonia-permeable human AQP8 by replacing Phenylalanine 56 with histidine, Histidine 180 with isoleucine, and Cysteine 189 with glycine, alone and in combination. Only AQP1 mutants including the H180I exchange increased the ammonia and methylamine tolerance of yeast. In a second set of mutations, we replaced Histidine 180 with alanine, leucine, methionine, phenylalanine, asparagine or glutamine. AQP1 H180A was equivalent to AQP1 H180I. AQP1 H180L increased ammonia but not methylamine tolerance of yeast. AQP1 mutants with methionine, phenylalanine, asparagine or glutamine in place of Histidine 180, increased neither ammonia nor methylamine tolerance of yeast. All mutants conducted water, as judged by osmotic assays with yeast sphaeroplasts. We propose that the arginine-facing amino acid residue is the most versatile selector of aquaporin constrictions, excluding Escherichia coli glycerol facilitator-type aquaporins.
Introduction
Major intrinsic proteins (MIP), thus named because they are a major protein of eye lens fibre cell membranes, are homotetrameric channels with four individual pores that allow passage of small solutes across biological membranes (Bok et al., Citation1982; Mulders et al., Citation1995). They are found in bacteria, archaea and in eukaryotes (Calamita et al., Citation1995; Lee et al., Citation2005; Preston et al., Citation1992). MIPs are now more commonly named aquaporins or aquaglyceroporins, since most function as water and/or glycerol channels (Wu & Beitz, Citation2007). In the following, we will use the short form AQP for all members of this protein family.
Solute selectivity of AQPs is determined by two channel sections: The conserved asparagine proline alanine (NPA) region, and the aromatic/arginine (ar/R) constriction.
The NPA region is located at the centre of the AQP monomer (). Two half-helices are positioned end to end, their cumulative backbone amide dipoles repelling cations (de Groot et al., Citation2003; Hol et al., Citation1978; Wree et al., Citation2011). The NPA motifs are helix-capping (Richardson & Richardson, Citation1988).
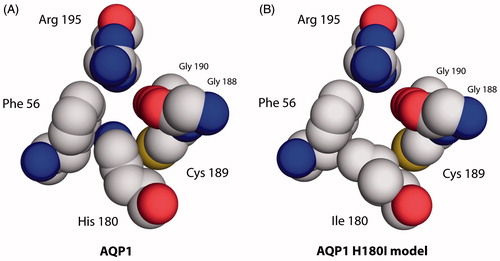
Figure 1. Aromatic/arginine (ar/R) constriction of primarily water-conducting aquaporins. (A) Ribbon representation of an AQP monomer based on the X-ray crystal structure of Pichia pastoris AQY1 (PDB: 3ZOJ). Oxygen atoms of water molecules are shown as spheres. (B) Close-up of the selectivity filter of the same AQP monomer. Its two half-helices are outlined, the NPA motifs and the ar/R constriction’s arginine and histidine are depicted in stick representation, the latter including hydrogen atoms. Two interlocking pairs of water molecules in the constriction are shown simultaneously. (C) Extracellular view of the ar/R constriction of the water-selective bovine AQP1 (PDB: 1J4N, Sui et al. Citation2001). The numbering is that of the rat AQP1 used in our experiments. (D) Model of the ar/R constriction of the water- and ammonia-permeable human AQP8, based on the crystal structure of bovine AQP1.
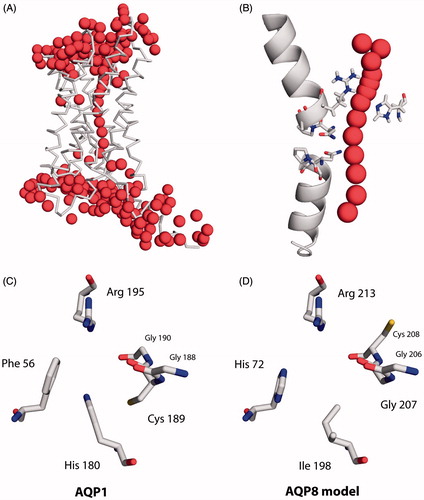
The ar/R constriction, more generally named selectivity filter, is located at the extracellular, or intraorganelle, channel opening of AQPs (). It is usually the narrowest part of the channel, with an average cross section of less than 3 Å in water-selective AQPs (Savage et al., Citation2010; Sui et al., Citation2001). Its positively charged arginine residue completes proton exclusion and provides two hydrogen bond donor sites (de Groot & Grubmüller, Citation2001; Li et al., Citation2011; Wu et al., Citation2009). In some AQPs an aliphatic amino acid is predicted in place of arginine (Gupta et al., Citation2012). Protein backbone carbonyl groups accept hydrogen bonds, with the corresponding amino acid residues contributing to the channel lining in some aquaglyceroporins (de Groot & Grubmüller, Citation2001; Fu et al., Citation2000; Murata et al., Citation2000). The remaining two amino acids of the ar/R constriction vary, and their identity often allows one to predict whether an aquaporin predominantly conducts water, glycerol, or other molecules (Wu & Beitz, Citation2007).
A histidine residue faces the arginine in water-selective AQPs (). According to the most highly resolved AQP X-ray crystal structure to date, the histidine’s ε-nitrogen atom is probably unprotonated (Kosinska Eriksson et al., Citation2013). It can accept hydrogen bonds (). In most AQPs, an aromatic side chain faces the string of backbone carbonyl groups (“aromatic/arginine” constriction). It interacts weakly with solutes.
Unlike other molecules, water can be fully hydrogen-bonded in the ar/R constriction (Hub & de Groot, Citation2008). Yet, water molecules do not get stuck here. Instead, they appear to slip through the ar/R constriction more readily than through other channel portions, and in a coordinated manner involving pairs of water molecules, as suggested by highly resolved electron density distribution (Kosinska Eriksson et al., Citation2013).
Among biologically relevant molecules, ammonia (NH3) resembles water most closely in size and polarity (Weast, Citation1977). Unlike water, it carries three hydrogen atoms. It is a good hydrogen bond acceptor but a poor donor (Nelson et al., Citation1987; Zhu & Yang, Citation1994). Due to its basicity (pKb ≈4.75 at 25 °C, Khoo et al., Citation1977), its conjugated acid ammonium (NH4+) is the predominant species at physiological pH. Nevertheless, AQP-mediated permeation of unprotonated ammonia through biological membranes is important, e.g. in Malaria parasites, cyanobacteria, barley roots and rat hepatocytes (Coskun et al., Citation2013; Ritchie, Citation2013; Soria et al., Citation2013; Zeuthen et al., Citation2006).
Water-selective AQPs tend to be poor ammonia channels (Geyer et al., Citation2013; Nakhoul et al., Citation2001; Zeidel et al., Citation1994). A group of AQPs occasionally referred to as aquaammonioporins, conducts both species with similar efficiency (Holm et al., Citation2005; Saparov et al., Citation2007). It includes plant tonoplast intrinsic proteins (TIP) and mammalian AQP8, the crystal structures of which remain to be published (Agemark et al., Citation2012; Iacovache et al., Citation2010). They are predicted to have very similar ar/R constrictions (): Facing the arginine is a lipophilic isoleucine, and the aromatic amino acid is histidine. Mutating either of these to, respectively, histidine or phenylalanine (as in AQP1, ) reduces ammonia permeability in Triticum aestivum TIP2;1, in the former case (Ile → His) barely affecting water permeability (Holm et al., Citation2005; Jahn et al., Citation2004). Bertl and Kaldenhoff propose that ammonia traverses the central pore of the T. aestivum TIP2;2 tetramer, but their conclusion rests on HgCl2-inhibition studies, and they do not mention the high affinity of ammonia for mercuric ions (Bertl & Kaldenhoff, Citation2007; Pugh & Hirsch Quastel, Citation1937). In water-selective rat AQP1, the mutations R195V and H180A increase ammonia permeability while leaving water permeability unaffected (Beitz et al., Citation2006). Both findings point to the necessity of a wider and more hydrophobic ar/R constriction for ammonia to permeate as efficiently as water.
Here, we present the results of more detailed studies of rat AQP1 (rAQP1) mutants mimicking the ar/R constriction of human AQP8 (hAQP8). Similar experiments, in which the selectivity filter of the water-selective AQP Spinacia oleracea plasma membrane intrinsic protein 2;1 (SoPIP2;1) was made to resemble that of four TIPs and five NIPs (Nodulin26-like intrinsic proteins), yielded three ammonia-conducting mutants, all of them with multiple amino acid exchanges. Its major finding was the significance of an amino acid not directly contacting the pore (Dynowski et al., Citation2008). By contrast, our experiments included single mutants of rAQP1. We studied their ammonia and methylamine permeability using yeast growth assays, verifying channel integrity by osmotic assays with the same yeast transformants. As will be seen, the arginine-facing amino acid residue appears to be the most versatile selectivity-determining barrier of the rAQP1 constriction.
Methods
Yeast strains and plasmids
Saccharomyces cerevisiae strain 31019b fps1Δ (MATa ura3Δ mep1Δ mep2::LEU2 mep3::kanMX2 yll043wΔ0) lacking its three ammonium transporters, as well as its aquaglyceroporin Fps1 (Tamás et al., Citation1999), was used for the ammonia assay. It was generated from strain 31019b (Marini et al., Citation1997). Saccharomyces cerevisiae strain BY4742 fps1Δ (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 yll043w::kanMX), lacking only Fps1, was used for the methylamine assay. It was generated from strain BY4742 (Brachmann et al., Citation1998, obtained from Euroscarf). Cells were transformed using the lithium acetate/single stranded carrier DNA/PEG procedure described by Gietz et al. (Citation1995). Most aquaporins were expressed from the plasmid pRS426MET25 (Mumberg et al., Citation1994). Fps1 was expressed from pRS416MET25. Point mutations were generated according to the QuikChange Site-Directed Mutagenesis Kit (Stratagene) and verified by DNA-sequencing.
Growth media
SD KHL medium contained 0.17% yeast nitrogen base (YNB) without amino acids and ammonium (Beckton Dickinson), 38 mM ammonium sulfate, 100 mM D-glucose, 0.11 mM L-lysine HCl, 0.095 mM L-histidine HCl, and 0.76 mM L-leucine. Its pH was set to 5.6 with NaOH before autoclaving. Ammonia assay medium was made of 20 mM ammonium sulfate or 40 mM L-glutamine, 0.17% YNB, 150 mM D-glucose, 40 mM MOPS/NaOH pH 6.9, unless indicated otherwise. Methylamine assay medium was made of 10 mM methylammonium chloride, 0.17% YNB, 150 mM D-glucose, 8.7 mM L-proline, 0.11 mM L-lysine HCl, 0.095 mM L-histidine HCl, 0.76 mM L-leucine, 20 mM MES/NaOH pH 5.5, unless indicated otherwise. Liquid media were filtered sterile with polyether sulfone or polyvinylidene difluoride filters of 0.2 μm mean pore diameter (Filtropur S, Sarstedt). To prepare solid media, 2% agar (Oxoid) was used, with filter-sterilized YNB, glucose and amino acid solutions added after autoclaving. Chemicals were obtained from Roth or Sigma-Aldrich, with purities of at least 98%.
Yeast growth assays
A fresh yeast transformant colony or a scraping from a glycerol stock (aqueous yeast suspension, 40% V/V glycerol, −70 °C) was grown in 1.6 ml SD KHL medium inside a glass tube at 29 °C and 180 rpm orbital shaking overnight or until turbid. Cells were harvested by centrifugation, washed twice with 1 ml, and resuspended in 0.5 ml, doubly deionized water. The apparent absorption at 600 nm (OD600) was determined after appropriate dilution, and the suspension was diluted to the desired OD600. Yeast cell suspensions were either pipetted onto agar plates (5 μl) or into microplate wells (Honeycomb 2, Oy Growth Curves Ab Ltd) containing growth medium (10 μl yeast suspension into 290 μl medium). Agar plates were incubated at 29 °C, and yeast growth was documented by scanning. Liquid cultures were incubated in the Bioscreen C MBR (Oy Growth Curves Ab Ltd), with the following settings: 29 °C, 10 s medium intensity shaking before measurements, 30 min measurement interval, 420–580 nm wideband filter. The net starting OD420–580 was approximately 0.05–0.08 (7.5 mm pathlength due to the fill level). Areas under growth curves (AUC, Skyttä & Mattila-Sandholm, Citation1991) were calculated by adding rectangle areas obtained with the formula 0.5 (ODi + ODi+1) (ti+1 − ti), i denoting the ith measurement, and t the time. The starting OD (t = 0 h) was subtracted from each value.
Yeast sphaeroplast buffers
Four buffers were used for yeast sphaeroplast preparation and osmotic assays (Bertl & Kaldenhoff, Citation2007; Song et al., Citation2012): Buffer I (50 mM KH2PO4/KOH pH 7.2, 0.2% V/V 2-mercaptoethanol), Buffer II (1.8 M sorbitol, 50 mM KH2PO4/KOH pH 7.2, 0.2% V/V 2-mercaptoethanol), Buffer III (1.5 M sorbitol, 50 mM NaCl, 5 mM CaCl2, 10 mM MOPS/NaOH pH 7.2), and Buffer IV (1.2 M sorbitol, 50 mM NaCl, 5 mM CaCl2, 10 mM MOPS/NaOH pH 7.2). Buffers were filtered sterile with polyether sulfone filters of 0.45 μm mean pore diameter (Filtropur S, Sarstedt), the 2-mercaptoethanol being added afterwards under laminar flow. For experiments with BY4742 fps1Δ sphaeroplasts, buffers were not filtered but frozen in aliquots (−20 °C), and Buffer III contained 1.8 M instead of 1.5 M sorbitol.
Yeast sphaeroplast preparation
Yeast cultures were grown as described above (yeast growth assays), except for resuspending them in 0.5 ml SD KHL, and without washing. They were then grown in 50–100 ml SD KHL (29 °C, 180–200 rpm) to an OD600 of 1–1.7 (1–3 for BY4742 fps1Δ), and harvested in 50 ml tubes (centrifuged 2500 g, 5 min). The pellet was resuspended in Buffer I (3 ml/40 mg wet pellet) and incubated (29 °C, 100 rpm in a tilted position, 15 min). This was followed by the addition of bovine serum albumin (BSA, Albumin fraction V, Genaxxon, 100 mg/40 mg wet pellet), Buffer II (6 ml/40 mg wet pellet), and zymolyase-20 T (MP Biomedicals, 0.5 mg/40 mg wet pellet), and by gentle mixing before further incubation (29 °C, 100 rpm in a tilted position, 60 min). Digested yeast were washed twice with 1–2 ml Buffer IV (4 °C, centrifuged 2000 g, 5 min), and resuspended in 2–4 ml Buffer IV. The OD600 was measured, and the suspension stored at 4 °C until use. The ratio of wet pellet weight to OD600 units (OD600 multiplied by the culture volume in ml) was consistently found to be 1–2 mg/ml.
Osmotic assays with yeast sphaeroplasts
Osmotic assays with yeast sphaeroplast suspensions were performed using a stopped-flow device (SFM-300, BioLogic). The sphaeroplast suspension was kept on ice, and an appropriate amount of it was diluted with cold (4 °C) Buffer IV inside a 10 ml glass beaker, to a total volume of 2.0 ml at OD600 = 2. When needed, 2.0 μl of aqueous HgCl2 stock solution (25 mM) was added and diluted before adding the yeast, to obtain a final concentration of 25 μM HgCl2. The diluted yeast suspension was taken up in a 6 ml plastic syringe which was then mounted on the stopped-flow module. The same was done with Buffer III. Each sample was driven into and out of its reservoir several times, until no bubbles appeared. At least 10 min were allowed for temperature equilibration (20 °C unless mentioned otherwise). Measurements were performed by mixing the sphaeroplast suspension with an equal volume of Buffer III (150 μl total volume per injection, 6.5 ml/s) and recording the intensity of scattered light (546 nm, 90°). At least 9 curves were recorded for each sample (at least 3 curves for BY4742 fps1Δ sphaeroplasts). Each curve was individually normalized and its nominal relaxation time (τ) determined using the Bio-Kine 32 V4.46 software (BioLogic). Light scattering measurements with yeast-free Buffer IV allowed for the calculation of net light scattering signal changes from detector voltages (ΔU/U0). The average ratio of tonicity change to light scattering change was 1.00 ± 0.18 (n = 24) for yeast strain 31019b fps1Δ, and 0.28 ± 0.06 (n = 62) for yeast strain BY4742 fps1Δ, i.e., the former seemed to yield a larger proportion of intact sphaeroplasts (Soveral et al., Citation2008).
Yeast membrane protein detection
Yeast were grown in 100 ml SD KHL medium inside 500 ml Erlenmeyer flasks at 29 °C and 180 rpm orbital shaking to an OD600 of ∼1. They were collected in 50 ml tubes (1268 g, 10 min), and washed with 25 ml water and 10 ml extraction buffer (5 mM Na2EDTA, 25 mM Tris/HCl pH 7.5). The unified pellets were frozen at −20 °C. On the day of microsome isolation they were thawed on ice, and resuspended in 0.5 ml extraction buffer containing protease inhibitor (Complete EDTA-free protease inhibitor cocktail tablets, Roche) and 0.5 g acid-washed glass beads (Sigma-Aldrich, Ø ≈0.5 mm). Cells were disrupted by 10 vortexing cycles of 30 s each at maximum setting, with cooling periods of at least 30 s, and centrifuged (2500 g, 10 min). Following a second extraction, the combined supernatants were transferred to 25 ml ultracentrifuge tubes (polycarbonate, Beckman Coulter). These were filled up with extraction buffer and centrifuged at ∼100,000 g for 45 min including acceleration. The pellets were resuspended in 0.2 ml Buffer M (50 mM NaCl, 100 mM NaH2PO4/Na2HPO4 pH 8.0). Following protein concentration measurement (Bio-Rad Protein Assay, Bradford, Citation1976), DDM was added for solubilisation (2.5% w/V).
Proteins were separated by SDS PAGE (2–6 μg BSA equivalent per sample), blotted on PVDF membranes (Hybond-P, Amersham Biosciences), AQP1 marked with mouse monoclonal anti-AQP1 IgG antibody (Santa Cruz Biotechnology, 0.2 mg/ml diluted 1:1000) and HRP-conjugated goat anti-mouse IgG (Jackson Immuno Research Europe, 0.4 mg/ml diluted 1:2000–10000), and detected by chemiluminescence (ECL Plus Western blotting detection system and Hyperfilm ECL, GE Healthcare).
Osmotic assays with Xenopus laevis oocytes
cRNA synthesis, Xenopus laevis oocyte preparation, cRNA injection, and swelling assays were performed as described before (von Bülow et al., Citation2012).
Graphs, images and statistical analysis
Data was evaluated on spreadsheets (Microsoft Excel), one way analysis of variance was carried out with SigmaPlot 11.0, graphs and images were generated using the Swiss-PDB Viewer, PyMOL, SigmaPlot, Adobe Illustrator and Adobe Photoshop.
Results
Changing Histidine 180 to isoleucine increases the apparent ammonia and methylamine permeability of rAQP1
summarizes the effect of rAQP1 and its hAQP8-mimicking mutants on yeast growth in the presence of toxic amounts of ammonia or methylammonium.
Figure 2. Apparent ammonia and methylammonia permeability of rAQP1 mutants. (A) Yeast transformants lacking all three ammonium transporters Mep1-3, as well as the endogenous aquaglyceroporin Fps1 (31019b fps1Δ), were grown at 29 °C in liquid medium containing ammonium as the only nitrogen source (NH4+ 40 mM, pH 6.9). Growth was detected turbidimetrically (OD420-580 nm) and evaluated by calculating areas under curves (AUC48 h, dashed lines indicate the limits used). Four sample growth curves are shown. (B) Average AUC48h (n = 4) for the first set of rAQP1 mutants. The grey line denotes the value for yeast transformants producing no AQP. (C) As in B, with ammonium replaced by glutamine. (D) Presence of rAQP1 mutants in 31019b fps1Δ transformants was verified by SDS-PAGE and Western blotting. (E) Yeast transformants lacking the endogenous aquaglyceroporin Fps1 (BY4742 fps1Δ) were grown at 29 °C in liquid medium containing a toxic amount of methylammonium (MA, 10 mM, pH 5.5). The major nitrogen source was 8.7 mM proline. (F) Average AUC48 h (n = 4) for the first set of rAQP1 mutants. (G) As in F, with no methylammonium. Error bars denote SD, referring to the number of yeast cultures. Please note the varying ordinate scales.
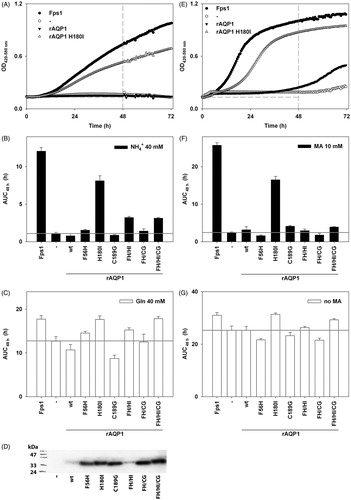
Wild-type rAQP1 failed to improve yeast growth on ammonia as the only nitrogen source (AUC48h = 0.8 h as compared to 1.1 h for AQP-deficient yeast, ). Among single mutants, only rAQP1 H180I led to a substantial increase in yeast growth (AUC48h = 8.1 h), comparable to that enabled by the aquaglyceroporin Fps1 (AUC48h = 12.1 h). Among the multiple mutants, rAQP1 F56H H180I and rAQP1 F56H H180I C189G increased yeast growth (AUC48h = 3.2 h and 3.1 h, respectively). These results were repeated in four independent experiments, an example of which is shown in Supplementary Figure S1A (available online).
Corresponding results were obtained with yeast grown on methylammonium-containing medium, the major difference being that the multiple rAQP1 mutants failed to improve growth (). One independent experiment had previously shown the double mutant rAQP1 F56H H180I to also increase yeast growth (Supplementary Figure S1B).
Differences in yeast transformant growth were largely abolished by exchanging ammonium ions for glutamine, or by leaving out methylammonium, depending on the assay ( and ). The presence of rAQP1 and its mutants in the ammonia assay yeast strain 31019b fps1Δ was verified by SDS PAGE and Western blotting (). Total production of wild-type rAQP1, and that of the mutant F56H H180I, appeared lower than that of the other mutants. The extraction procedure did not discriminate between the plasma membrane and other, more abundant ones such as the endoplasmic reticulum membrane. To find out whether all rAQP1 mutants were functional within the plasma membrane, their effect on yeast sphaeroplast water permeability was tested.
Water permeability of rAQP1 seems least affected by mutating Cysteine 189 to glycine, and most affected by the double mutation F56H H180I
The water permeability of the rAQP1 mutants was tested with sphaeroplasts made from both yeast strains (). All rAQP1 mutants conducted water (). Since their concentration in the yeast plasma membrane was not known, the data does not represent single channel water permeability. Nevertheless, it allows for the following observations: (i) In the absence of AQPs, the shrinkage rate (τ−1, 20 °C) was constant at 0.11 s−1 for 31019b fps1Δ sphaeroplasts and 0.10 s−1 for BY4742 fps1Δ sphaeroplasts; (ii) rAQP1-producing yeast were the most water-permeable (τ−1 = 1.30 s−1 and 0.86 s−1); and (iii) The mutation C189G appeared to be the least disruptive (τ−1 = 0.85 s−1 and 0.63 s−1), while F56H appeared to be the most disruptive one (τ−1 = 0.28 s−1 and 0.23 s−1), in particular when combined with H180I in rAQP1 F56H H180I (τ−1 = 0.18 s−1 and 0.16 s−1).
Figure 3. Water permeability of rAQP1 mutants as determined with yeast sphaeroplasts. (A) Sphaeroplasts were subjected to a hyperosmotic sorbitol gradient (150 mM, tonicity increase of ∼11%), and their shrinkage was followed by measuring light scattering at 20 °C. Three normalized average curves are shown (n = 9–11). The yeast strain in this example was 31019b fps1Δ. The horizontal line marks the ordinate value at which the nominal relaxation time (τ) was read. (B) Inverse nominal relaxation times (τ−1) of sphaeroplast shrinkage (n = 3–11). Data obtained with both yeast strains (31019b fps1Δ and BY4742 fps1Δ). The grey line marks the smallest value. (C) Mercuric chloride inhibition of rAQP1 mutants. BY4742 fps1Δ sphaeroplasts were incubated with 25 μM HgCl2 for at least 10 min. τ−1 ratios are shown, with values below 1 indicating inhibition (n = 3–7). Error bars denote SD, referring to the number of individually evaluated curves. No error is shown for rAQP1 (n = 2). Yeast transformants were from the same frozen stocks as those used in the ammonia and methylamine assays shown in .
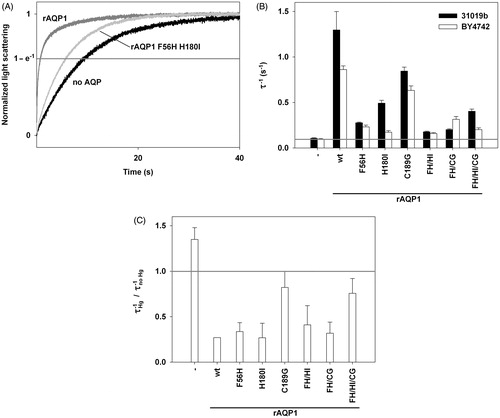
Mercuric chloride (25 μM HgCl2) reduced the shrinkage rate of most yeast transformants to about a third (). As expected, this was not the case for AQP-deficient yeast and for those producing the rAQP1 mutants C189G and F56H H180I C189G, although rAQP1 F56H C189G was inhibited (Preston et al., Citation1993).
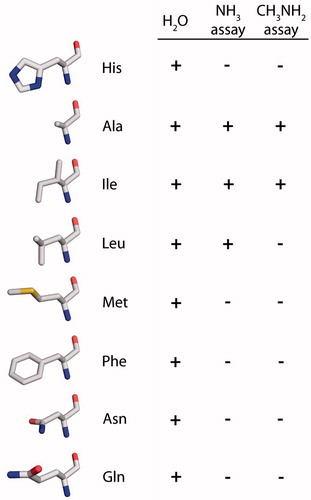
In summary, the apparent ammonia and methylamine permeability of rAQP1 could only be increased by mutating the arginine-facing Histidine 180 to isoleucine. At the same time, this mutation decreased the water permeability. Since the altered permeability seemed to be due to changing the histidine residue to a shorter and more hydrophobic one, it was decided to vary the length and the hydrophobicity by mutating histidine to a range of suitable amino acids ().
Leucine in place of Histidine 180 increases apparent ammonia, but not methylamine, permeability of rAQP1
The amino acids selected to replace Histidine 180 in rAQP1 were alanine (Beitz et al., Citation2006), leucine, methionine and phenylalanine, with hydrophobic and increasingly large side chains. Asparagine was chosen as a hydrogen-bonding and similarly sized alternative to leucine, while the glutamine residue is comparable in length to that of histidine (incidentally, the genetic codes for histidine and glutamine vary in the third position only).
summarizes the effect of these mutations on the apparent ammonia and methylamine permeability. rAQP1 H180A closely resembled rAQP1 H180I in its ability to increase yeast growth in the presence of toxic amounts of ammonia or methylamine. rAQP1 H180L increased yeast growth significantly in case of the ammonia assay, at an intermediate level when compared to rAQP1 and rAQP1 H180I (AUC48h = 3.8 h versus 0.8 h and 8.1 h, respectively). This intermediate result was reproduced four out of four times. rAQP1 H180L did not increase methylamine permeability in two independent experiments ( and Supplementary Figure S2B). No other rAQP1 Histidine 180 mutant had a significant effect on yeast growth. rAQP1 H180Q increased the ammonia tolerance in the experiment shown in , but not in three other experiments (Supplementary Figure S2A).
Figure 5. Apparent ammonia and methylammonia permeability of rAQP1 mutants. (A) Yeast transformants lacking all three ammonium transporters Mep1-3, and the endogenous aquaglyceroporin Fps1 (31019b fps1Δ), were grown at 29 °C in liquid medium containing ammonium as the only nitrogen source (NH4+ 40 mM, pH 6.9). Growth was detected turbidimetrically (OD420–580 nm) and evaluated by calculating areas under curves (AUC 48 h, dashed lines indicate the limits used). Four sample growth curves are shown. (B) Average AUC48 h (n = 4) for the second set of rAQP1 mutants. The grey line denotes the value for yeast transformants producing no AQP. (C) As in B, with ammonium replaced by glutamine. (D) Presence of rAQP1 mutants in 31019b fps1Δ transformants was verified by SDS-PAGE and Western blotting. Please note that preperations of AQP-deficient, rAQP1 wild-type and H180I-producing yeast were different from those shown in . (E) Yeast transformants lacking the endogenous aquaglyceroporin Fps1 (BY4742 fps1Δ) were grown at 29 °C in liquid medium containing a toxic amount of methylammonium (MA, 10 mM, pH 5.5). The major nitrogen source was 8.7 mM proline. (F) Average AUC48 h (n = 4) for the second set of rAQP1 mutants. (G) As in F, with no methylammonium. Error bars denote SD, referring to the number of yeast cultures. Please note the varying ordinate scales. Yeast growth data for Fps1, no AQP, rAQP1 and its mutant rAQP1 H180I is the same as that shown in .
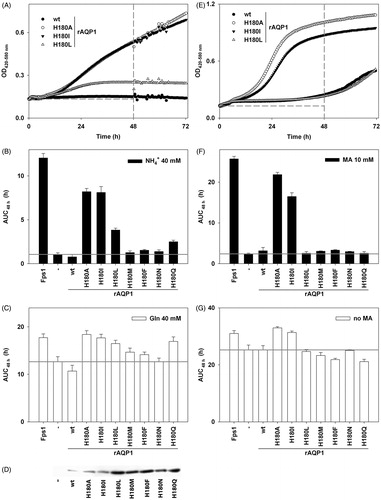
Replacing ammonium ions with glutamine, or excluding methylammonium, reduced differences in yeast growth markedly but not completely ( and ). SDS PAGE and Western blotting proved the presence of every rAQP1 mutant in the total membrane fraction of the yeast strain 31019b fps1Δ ().
Water permeability of rAQP1 is retained when Histidine 180 is mutated to the larger and lipophilic phenylalanine
All rAQP1 Histidine 180 mutants conducted water (). Keeping in mind that the data does not represent single channel water permeability, the following can be said in particular ( and ): (i) rAQP1 H180F increased the shrinkage rate of 31019b fps1Δ sphaeroplasts from 0.11–0.20 s−1, and that of BY4742 fps1Δ sphaeroplasts from 0.10–0.12 s−1. The latter was barely significant. However, rAQP1 H180F increased the osmotic water permeability coefficient of Xenopus laevis oocytes 3.6-fold (Supplementary Figure S3); (ii) Water permeability tends to decrease with increasingly large lipophilic amino acids in place of Histidine 180, leucine being a curious exception; and (iii) Shrinkage rates were consistently lower for BY4742 fps1Δ sphaeroplasts.
Figure 6. Water permeability of rAQP1 mutants as determined with yeast sphaeroplasts. (A) Sphaeroplasts were subjected to a hyperosmotic sorbitol gradient (150 mM, tonicity increase of ∼11%), and their shrinkage was followed by measuring light scattering at 20 °C. Three normalized average curves are shown (n = 8–11). The yeast strain in this example was 31019b fps1Δ. The horizontal line marks the ordinate value at which the nominal relaxation time (τ) was read. (B) Inverse nominal relaxation times (τ−1) of sphaeroplast shrinkage (n = 3–11). Data obtained with both yeast strains (31019b fps1Δ and BY4742 fps1Δ). The grey line marks the smallest value. (C) Mercuric chloride inhibition of rAQP1 mutants. BY4742 fps1Δ sphaeroplasts were incubated with 25 μM HgCl2 for at least 10 min. τ−1 ratios are shown, with values below 1 indicating inhibition (n = 3–9). Inhibition data for rAQP1 H180Q was obtained with strain 31019b fps1Δ (asterisk). Error bars denote SD, referring to the number of individually evaluated curves. No error is shown for rAQP1 and rAQP1 H180F (n = 2). Data for no AQP, rAQP1 and rAQP1 H180I is the same as that shown in . Yeast transformants were from the same frozen stocks as those used in the ammonia and methylamine assays shown in .
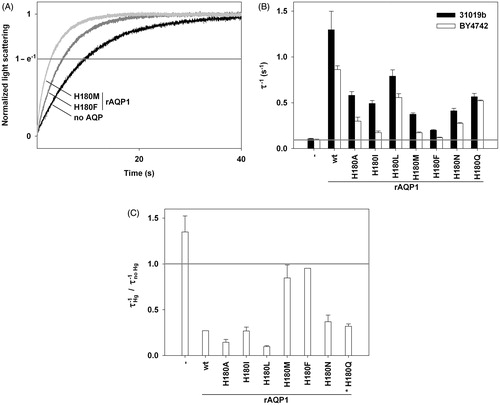
25 μM HgCl2 inhibited all rAQP1 mutants except H180M and H180F (). rAQP1 H180M was significantly inhibited in another experiment ( = 0.76 ± 0.10, Supplementary Table S1, available online). In case of rAQP1 H180F, two other experiments yielded either no significant inhibition (
= 0.86 ± 0.23) or an apparent enhancement of water permeability (
= 1.19 ± 0.07, Supplementary Table S1), the latter being similar to that seen with AQP-deficient yeast sphaeroplasts (
= 1.34 ± 0.17).
In summary, the water permeability of rAQP1 was reduced but not abolished by an exchange of Histidine 180 with amino acids ranging in size from alanine to phenylalanine. In ammonia-dependent yeast growth assays, a significant difference in phenotype was found only for rAQP1 mutants H180A, H180I and H180L, i.e., those in which Histidine 180 is replaced by an amino acid with a short and lipophilic side chain. In methylamine-dependent assays, rAQP1 H180L did not improve growth ().
Figure 7. Effect of rAQP1 His180 substitution on water, methylamine and apparent ammonia permeability, as determined by real-time light scattering measurements with yeast sphaeroplasts (water) and long-term yeast growth assays (ammonia and methylamine). The spatial orientation of the amino acids is similar to that of the histidine shown in . Images were generated from the crystal structure of bovine AQP1 following mutation of His182 to the respective amino acid using the Swiss-PDB Viewer (PDB: 1J4N).
Discussion
Our first set of rAQP1 mutants were designed to have an ar/R constriction mimicking that of hAQP8. Glycine in place of Cysteine 189 did not lead to marked changes in permeability. The cysteine residue does not overlap with the solute pathway (). Histidine in place of Phenylalanine 56 lowered water permeability. It did not increase apparent ammonia or methylamine permeability, probably because the exchange does not widen the constriction much ( and ). The largest increase in channel cross section was attained by mutating Histidine 180 to isoleucine. rAQP1 H180I increased the ammonia and methylamine tolerance of yeast. The double mutant rAQP1 F56H H180I and especially the triple mutant rAQP1 F56H H180I C189G increased the ammonia but not the methylamine tolerance of yeast. Initially, this was explained by ammonia being a smaller molecule than methylamine. As described below, the actual reason may be a different one. The double mutation F56H C189G affected neither ammonia nor methylamine permeability, further suggesting that Histidine 180 is a major barrier of the rAQP1 constriction.
The second set of rAQP1 mutants was intended to answer two questions: (i) How large can the arginine-facing amino acid residue be without reducing apparent ammonia or methylamine passage through rAQP1?; and (ii) Does it have to be lipophilic? The answer to the first question appears to be the size of the isoleucine residue. The isomeric leucine residue marks a threshold. The second question cannot be answered with confidence. Sub-angstrom differences in size between the leucine and asparagine residues may mask the contribution of polarity and hydrogen bonding. rAQP1 H180L increased the ammonia tolerance of yeast, but less than rAQP1 H180I. It did not increase their methylamine tolerance. As for the triple mutant rAQP1 F56H H180I C189G, this was initially explained by methylamine being a larger molecule than ammonia. However, we have shown previously that both assays may indicate AQP-facilitated passage of other molecules such as carbon dioxide (Krenc, Citation2012; Krenc et al., Citation2013). In addition, the ammonia-permeable hAQP8 failed to increase the ammonia tolerance of yeast (Supplementary Figure S4, online). Whatever the molecule in question, it is larger, more lipophilic and less hydrogen-bonding than water, but smaller than urea or glycerol. So we can imagine the following scenario (Hub & de Groot, Citation2008): A molecule entering the selectivity filter of rAQP1 needs to replace water. It is larger and it cannot substitute all hydrogen bonds between water and the ar/R residues, so its permeation requires more energy. Mutating the Histidine 180 residue to a shorter aliphatic one, increases the pore size and eliminates one hydrogen bond acceptor, making permeation more favorable. We have thus reduced water selectivity.
Conclusion
The rat AQP1 Histidine 180 residue is aligned so that changing it to a shorter or longer one widens or narrows the constriction accordingly. The side chains of Phenylalanine 56 and Cysteine 189 are oriented less directly towards the pore. For this reason, it seems, Histidine 180 mutations most effectively reduced water selectivity in our experiments. Choosing alkyl residues also eliminates a hydrogen bond acceptor.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was funded by the European Commission (FP7-HEALTH 201924 EDICT).
Supplementary Material available online
Supplementary Figures S1–4
Supplementary Table S1.
Supplementary Material
Download PDF (1.7 MB)Acknowledgements
A small but most useful aliquot of mercuric chloride was a gift from Patrizia Gena and Giuseppe Calamita, Bari. Human AQP8 DNA was kindly provided by Thomas P. Jahn, Copenhagen.
References
- Agemark M, Kowal J, Kukulski W, Nordén K, Gustavsson N, Johanson U, et al. 2012. Reconstitution of water channel function and 2D-crystallization of human aquaporin 8. Biochim Biophys Acta 1818:839–850
- Beitz E, Wu B, Holm LM, Schultz JE, Zeuthen T. 2006. Point mutations in the aromatic/arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons. Proc Natl Acad Sci USA 103:269–274
- Bertl A, Kaldenhoff R. 2007. Function of a separate NH3-pore in Aquaporin TIP2;2 from wheat. FEBS Lett 581:5413–5417
- Bok D, Dockstader J, Horwitz J. 1982. Immunocytochemical localization of the lens main intrinsic polypeptide (MIP26) in communicating junctions. J Cell Biol 92:213–220
- Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, Hieter P, Boeke JD. 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14:115–132
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
- Calamita G, Bishai WR, Preston GM, Guggino WB, Agre P. 1995. Molecular cloning and characterization of AqpZ, a water channel from Escherichia coli. J Biol Chem 270:29063–29066
- Coskun D, Britto DT, Li M, Becker A, Kronzucker HJ. 2013. Rapid ammonia gas transport accounts for futile transmembrane cycling under NH3/NH4+ toxicity in plant roots. Plant Physiol 163:1859–1867
- de Groot BL, Frigato T, Helms V, Grubmüller H. 2003. The mechanism of proton exclusion in the aquaporin-1 water channel. J Mol Biol 333:279–293
- de Groot BL, Grubmüller H. 2001. Water permeation across biological membranes: mechanism and dynamics of aquaporin-1 and GlpF. Science 294:2353–2357
- Dynowski M, Mayer M, Moran O, Ludewig U. 2008. Molecular determinants of ammonia and urea conductance in plant aquaporin homologs. FEBS Lett 582:2458–2462
- Fu D, Libson A, Miercke LJ, Weitzman C, Nollert P, Krucinski J, Stroud RM. 2000. Structure of a glycerol-conducting channel and the basis for its selectivity. Science 290:481–486
- Geyer RR, Musa-Aziz R, Qin X, Boron WF. 2013. Relative CO(2)/NH(3) selectivities of mammalian aquaporins 0–9. Am J Physiol Cell Physiol 304:C985–C994
- Gietz RD, Schiestl RH, Willems AR, Woods RA. 1995. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11:355–360
- Gupta AB, Verma RK, Agarwal V, Vajpai M, Bansal V, Sankararamakrishnan R. 2012. MIPModDB: a central resource for the superfamily of major intrinsic proteins. Nucleic Acids Res 40:D362–D369
- Hol WG, van Duijnen PT, Berendsen HJ. 1978. The alpha-helix dipole and the properties of proteins. Nature 273:443–446
- Holm LM, Jahn TP, Møller AL, Schjoerring JK, Ferri D, Klaerke DA, Zeuthen T. 2005. NH3 and NH4+ permeability in aquaporin-expressing Xenopus oocytes. Pflugers Arch 450:415–428
- Hub JS, de Groot BL. 2008. Mechanism of selectivity in aquaporins and aquaglyceroporins. Proc Natl Acad Sci USA 105:1198–1203
- Iacovache I, Biasini M, Kowal J, Kukulski W, Chami M, van der Goot FG, et al. 2010. The 2DX robot: a membrane protein 2D crystallization Swiss Army knife. J Struct Biol 169:370–378
- Jahn TP, Møller AL, Zeuthen T, Holm LM, Klaerke DA, Mohsin B, et al. 2004. Aquaporin homologues in plants and mammals transport ammonia. FEBS Lett 574:31–36
- Khoo KH, Culberson CH, Bates RG. 1977. Thermodynamics of the dissociation of ammonium ion in seawater from 5 to 40 °C. J Solution Chem 6:281–290
- Kosinska Eriksson U, Fischer G, Friemann R, Enkavi G, Tajkhorshid E, Neutze R. 2013. Subangstrom resolution X-ray structure details aquaporin-water interactions. Science 340:1346–1349
- Krenc D, Wu B, Beitz E. 2013. Specific aquaporins increase the ammonia tolerance of a Saccharomyces cerevisiae mep1-3 fps1 deletion strain. Mol Membr Biol 30:43–51
- Krenc D. 2012. Studies on the ammonia permeability of aquaporins. Chapter 4.3.5. Methylamine assay. University library Kiel. Available at: http://macau.uni-kiel.de/receive/dissertation_diss_00010564. [last accessed 4 July 2014]
- Lee JK, Kozono D, Remis J, Kitagawa Y, Agre P, Stroud RM. 2005. Structural basis for conductance by the archaeal aquaporin AqpM at 1.68 A. Proc Natl Acad Sci USA 102:18932–18937
- Li H, Chen H, Steinbronn C, Wu B, Beitz E, Zeuthen T, Voth GA. 2011. Enhancement of proton conductance by mutations of the selectivity filter of aquaporin-1. J Mol Biol 407:607–620
- Marini AM, Soussi-Boudekou S, Vissers S, Andre B. 1997. A family of ammonium transporters in Saccharomyces cerevisiae. Mol Cell Biol 17:4282–4293
- Mulders SM, Preston GM, Deen PM, Guggino WB, van Os CH, Agre P. 1995. Water channel properties of major intrinsic protein of lens. J Biol Chem 270:9010–9016
- Mumberg D, Müller R, Funk M. 1994. Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res 22:5767–5768
- Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y. 2000. Structural determinants of water permeation through aquaporin-1. Nature 407:599–605
- Nakhoul NL, Hering-Smith KS, Abdulnour-Nakhoul SM, Hamm LL. 2001. Transport of NH3/NH4+ in oocytes expressing aquaporin-1. Am J Physiol Renal Physiol 281:F255–263
- Nelson DD Jr, Fraser GT, Klemperer W. 1987. Does ammonia hydrogen bond? Science 238:1670–1674
- Preston GM, Carroll TP, Guggino WB, Agre P. 1992. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256:385–387
- Preston GM, Jung JS, Guggino WB, Agre P. 1993. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J Biol Chem 268:17–20
- Pugh CEM, Hirsch Quastel J. 1937. Micro-determination of ammonia in presence of aliphatic amines. Biochem J 31:282–285
- Richardson JS, Richardson DC. 1988. Amino acid preferences for specific locations at the ends of alpha helices. Science 240:1648–1652
- Ritchie RJ. 2013. The ammonia transport, retention and futile cycling problem in cyanobacteria. Microb Ecol 65:180–196
- Saparov SM, Liu K, Agre P, Pohl P. 2007. Fast and selective ammonia transport by aquaporin-8. J Biol Chem 282:5296–5301
- Savage DF, O’Connell JD III, Miercke LJ, Finer-Moore J, Stroud RM. 2010. Structural context shapes the aquaporin selectivity filter. Proc Natl Acad Sci USA 107:17164–17169
- Skyttä E, Mattila-Sandholm T. 1991. A quantitative method for assessing bacteriocins and other food antimicrobials by automated turbidometry. J Microbiol Methods 14:77–88
- Song J, Almasalmeh A, Krenc D, Beitz E. 2012. Molar concentrations of sorbitol and polyethylene glycol inhibit the Plasmodium aquaglyceroporin but not that of E. coli: involvement of the channel vestibules. Biochim Biophys Acta 1818:1218–1224
- Soria LR, Marrone J, Calamita G, Marinelli RA. 2013. Ammonia detoxification via ureagenesis in rat hepatocytes involves mitochondrial aquaporin-8 channels. Hepatology 57:2061–2071
- Soveral G, Madeira A, Loureiro-Dias MC, Moura TF. 2008. Membrane tension regulates water transport in yeast. Biochim Biophys Acta 1778:2573–2579
- Sui H, Han BG, Lee JK, Walian P, Jap BK. 2001. Structural basis of water-specific transport through the AQP1 water channel. Nature 414:872–878
- Tamás MJ, Luyten K, Sutherland FC, Hernandez A, Albertyn J, Valadi H, et al. 1999. Fps1p controls the accumulation and release of the compatible solute glycerol in yeast osmoregulation. Mol Microbiol 31:1087–1104
- von Bülow J, Müller-Lucks A, Kai L, Bernhard F, Beitz E. 2012. Functional characterization of a novel aquaporin from Dictyostelium discoideum amoebae implies a unique gating mechanism. J Biol Chem 287:7487–7494
- Weast RC. 1977. Handbook of chemistry and physics. Cleveland, OH: CRC Press
- Wree D, Wu B, Zeuthen T, Beitz E. 2011. Requirement for asparagine in the aquaporin NPA sequence signature motifs for cation exclusion. FEBS J 278:740–748
- Wu B, Beitz E. 2007. Aquaporins with selectivity for unconventional permeants. Cell Mol Life Sci 64:2413–2421
- Wu B, Steinbronn C, Alsterfjord M, Zeuthen T, Beitz E. 2009. Concerted action of two cation filters in the aquaporin water channel. EMBO J 28:2188–2194
- Zampighi GA, Kreman M, Boorer KJ, Loo DD, Bezanilla F, Chandy G, et al. 1995. A method for determining the unitary functional capacity of cloned channels and transporters expressed in Xenopus laevis oocytes. J Membr Biol 148:65–78
- Zeidel ML, Nielsen S, Smith BL, Ambudkar SV, Maunsbach AB, Agre P. 1994. Ultrastructure, pharmacologic inhibition, and transport selectivity of aquaporin channel-forming integral protein in proteoliposomes. Biochemistry 33:1606–1615
- Zeuthen T, Wu B, Pavlovic-Djuranovic S, Holm LM, Uzcategui NL, Duszenko M, et al. 2006. Ammonia permeability of the aquaglyceroporins from Plasmodium falciparum, Toxoplasma gondii and Trypansoma brucei. Mol Microbiol 61:1598–1608
- Zhu T, Yang W. 1994. Structure of the ammonia dimer studied by density functional theory. Int J Quantum Chem 49:613–623