Abstract
G-Protein Coupled Estrogen Receptor 1 (GPER1), also known as G-Protein Coupled Receptor 30 (GPR30) and initially considered an orphan receptor, has become one of the most important pharmacological targets in cardiovascular research. Since the gene encoding this putative receptor was cloned nearly 20 years ago, researchers have addressed its role in various aspects of physiology, including cardioprotection. Although extensive research has been carried out to understand the role of GPER1 as a pharmacological target to treat cardiovascular diseases, there are few current reviews addressing the overall cardioprotective benefits of this receptor and the signaling intermediates involved. This review considers the origins of GPER1, its cell biology, its physiological and pharmacological roles as a therapeutic target in cardiovascular disease, and what future research on GPER1 might entail. More specifically, the review focuses on GPER1 regulation of Angiotensin Type I Receptor (AT1R) and the role of estrogen receptors, epidermal growth factor receptor (EGFR) and matrix metalloproteinases (MMPs) in bringing about the cardioprotective effects of GPER1. Areas where improved knowledge of GPER1 biology is still needed to better understand the receptor’s cardioprotective effects are also discussed.
Introduction
Originally named G-Protein Coupled Receptor 30 (GPR30) and described as an orphan receptor, interest in G-Protein Coupled Estrogen Receptor 1 (GPER1) intensified after the discovery by Filardo and colleagues that estrogen is a significant GPER1 agonist (Deschamps et al., Citation2010; Filardo et al., Citation2000). Those authors observed that, although the human breast cancer cell line SKBR3 does not express the classical estrogen receptors α and β, 17 β-estradiol could nonetheless activate the ERK signaling cascade in SKBR3 cells, indicating the existence of a distinct estrogen target. GPER1 is a seven-transmembrane receptor that partners with G-proteins to function and execute its signaling pathways. The GPER1 receptor has been found at several subcellular locations, including the plasma membrane, the endoplasmic reticulum (ER), and the nucleus. One of the most significant studies to date was carried out by Revankar et al. (Citation2005), who demonstrated a link between GPER1 on the surface of the ER and rapid, non-genomic signaling involving mobilization of intracellular calcium. In addition, this research group has made other important discoveries, including generating agonists and antagonists for GPER1, which are vital tools for understanding this receptor. Our own studies confirmed that GPER1 is present on the ER of rat liver epithelial cells, and further showed that GPER1 mediates its action through regulation of ERK1/2 and involves epidermal growth factor receptor (EGFR) (Koganti et al., Citation2014).
Since it was shown previously that the estrogen metabolite 2-methoxestradiol (2ME2), a metabolite of estrogen, is cardioprotective by the inhibition of endothelin-1 synthesis, a known potent vasoconstrictor, and this inhibition is more potent than estrogen (Dubey et al., Citation2001). In that study, the authors isolated endothelial cells from porcine coronary artery and tested the ability of estrogen, 2ME2 and 2-hydroxyestradiol to inhibit endothelin-1. Interestingly, it was observed that 2ME2 is a more potent inhibitor than 2-hydroxyestradiol, and both metabolites are more potent than the parent estrogen compound. Since 2ME2 has very limited or no known binding capacity to classical estrogen receptors alpha and beta (LaVallee et al., Citation2002), this raised the profile of GPER1 as a potential receptor of this metabolite. We recently showed that (Koganti et al., Citation2014) 2ME2, the metabolite of estrogen which has previously shown to be cardioprotective (Dubey et al., Citation2001) binds to GPER1 localized on the ER with very high affinity (Kd = 10 nM), leading to activation of epidermal growth factor receptor (EGFR), activation of ERK1/2, and down-regulation of Angiotensin Type I Receptor (AT1R), thus bringing about its cardioprotective effects. Although our study established a relationship between 2ME2, AT1R and GPER1, the study was done in a rat liver epithelial cell model and therefore it will be important to replicate the findings in an animal model to confirm the details of the signaling cascade.
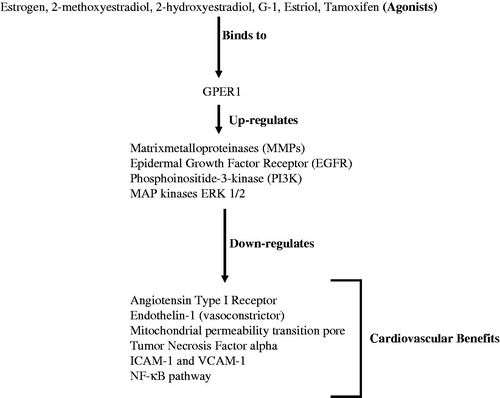
It has been shown that, following ischemia-reperfusion injury, the GPER1 agonist G-1 can protect the heart through a mechanism which involves inhibition of mitochondrial permeability transition pore opening (Bopassa et al., Citation2010). The results of that study suggest involvement of MAP kinases, ERK1/2 in this inhibition. Further study of this mechanism will need to include elucidation of the roles of various mitochondrial uncoupling proteins and their regulation by GPER1.
A recent study by Davis et al. (Citation2014) described some very interesting results regarding GPER and obesity. The authors showed that GPER1 knockout (KO) mice gained significantly more weight as compared to wild-type littermates after starting out at the same weight. Moreover, the GPER1 KO mice gained proportionally more body fat and their level of energy expenditure (measured by oxygen consumption) was lower. These findings not only suggest a role for GPER1 in energy metabolism, but also highlight GPER1 as a potential therapeutic target in cardiovascular disease and obesity. Furthermore, the study pointed out a functional parallel between GPER1 and leptin, one of the most well-known regulators of obesity. Although the study established a direct link between weight gain and absence of GPER1, there are several issues that require further investigation. For example, the study used a diet containing just 4% fat, whereas standard animal diets contain 9% fat. Could the lean diet have accentuated the difference in energy metabolism observed between GPER1 KO and wild-type mice? Also, there was no explanation as to why male GPER1 KO mice gained weight early compared to female GPER1 KO mice. A detailed study addressing these questions will only help us more understand the importance of this receptor.
GPER1 localization and its effects on cardioprotection
Numerous studies have investigated the subcellular localization of GPER1 receptor. Research conducted by Revankar et al. (Citation2005) and Koganti et al. (Citation2014) showed that GPER1 is localized to the ER. In a pioneering study by Revankar et al. (Citation2005), the authors showed that, after estrogen binding to the ER, a series of signaling cascades are activated, highlighted by activation of the phosphoinositide3-kinase (PI3K) pathway and resulting in calcium mobilization in the nucleus.
There is evidence to suggest that localization of the receptor plays a key role in determining its physiological effects. GPER is expressed in the adipocyte fraction of adipose tissue in addition to being expressed on the ER membrane and the plasma membrane (Davis et al., Citation2014; Filardo & Thomas, Citation2012). A contrasting and surprising result from two studies highlights the fact that the organelle that this receptor is located on plays a key role in determining its beneficial effects. In the studies mentioned above by Filardo and colleagues, the authors presented some interesting cases of GPER1 presence being associated with ovarian adenocarcinoma and several endometrial cancers. So, while GPER1 expression has been shown to have a cardioprotective effect, its expression may also be associated with some serious cancers. Thus, could GPER1 agonists that are developed as cardio-therapeutics also have unintended carcinogenic effects? Clearly, our understanding of GPER1 biology is still in its infancy, and much more about the receptor remains to be discovered. Even with these potential cancer-related side-effects, the cardiovascular benefits of GPER1 activity are becoming clear. For example, the fact that the anti-diabetic drug miglitol activates the phosphoinositide3-kinase (PI3K) pathway (Iwasa et al., Citation2011) supports an association between GPER1 and the PI3K pathway in having a beneficial role against diabetes.
It was shown that GPER1 induces vasorelaxation in isolated rat aortas in a dose-dependent manner (Seok et al., Citation2012). Interestingly, the vasorelaxation observed was independent of 17 β-estradiol, the known ligand of this receptor. Therefore, it seems the signaling pathways of GPER1 agonists and those of classical estrogen signaling are different, though they may converge at some point. Moreover, this result again raises the question as to what the unknown ligands of this receptor might be. Also, does GPER1 subcellular localization play a role in determining what ligands bind to this receptor? The study by Chakrabarti and Davidge (Citation2012) showed that, in human endothelial cells, GPER1 is localized predominantly in the nucleus rather than the cytoplasm, whereas Li et al. (Citation2012) reported that the receptor is present in the cytoplasm of rat aortic endothelial cells.
Chakrabarti and Davidge (Citation2012) also showed that GPER1 activation down-regulates the activity of the major pro-inflammatory marker Tumor Necrosis Factor alpha (TNF-α) and ultimately leads to reduced expression of proteins like ICAM-1 and VCAM-1 and activation of the NF-κB pathway. These pro-inflammatory markers play a key role in the development or progression of atherosclerosis (Zhang et al., Citation2014), which can lead to a number of cardiovascular diseases (Sessa et al., Citation2014). From all of the above studies, we can infer that, wherever GPER1 is localized, it fulfills an important role in reducing cardiovascular-related diseases through its own distinct pathway.
GPER1 cardioprotection and the role of epidermal growth factor receptor (EGFR) and matrix metalloproteinases (MMPs)
Activation of EGFR requires cleavage by matrix metalloproteinases (MMPs), which themselves must be activated by proteolytic cleavage. During this process there is cleavage and release of pro-HB-EGF, which is the pro-heparin-bound epidermal growth factor. This cascade leads to the activation of ERK1/2 (do Nascimento et al., Citation2009). Studies by Barchiesi et al. (Citation2010) have shown that 2ME2 induces MMP-1. This result, combined with the results of Koganti et al. (Citation2014) – that 2ME2 can bind to GPER1 leading to down-regulation of AT1R, a prime effector of hypertension and several cardiovascular diseases – establishes a link between MMP activation and induction of GPER1 to play a cardioprotective role. Although mainly MMP-1 and MMP-9 have been shown to play a role in this cascade, relationships between GPER1 and other MMPs are not well understood. Another point of interest is that up-regulation of EGFR is cancer-promoting, so how this same EGFR can be both beneficial in terms of cardiovascular protection and at the same time harmful in terms of promoting many cancers is a matter in need of investigation. Possibly, activation of GPER1 causes specific sites of EGFR to be either phosphorylated or oxidized when it is cardioprotective, and there are other sites that are active when EGFR is oncogenic. Thus, studies focusing on the finer details of the motifs of EGFR that are regulated by GPER1 when it is cardioprotective are required.
Different ligands that can bind to GPER1 and exert cardioprotection
With the increasing importance attached to GPER1, there have been quite a few studies demonstrating the different ligands that can bind to this receptor. Most of these compounds act as GPER1 agonists, triggering a series of signaling pathways to bring its effects downstream. In addition to the two endogenous human estrogens 17β-estradiol and estriol (Pupo et al., Citation2013), metabolites of estrogen, notably 2ME2, the synthetic GPER1 agonist G-1, and estrogen receptor modulators like tamoxifen bind to GPER1 with very high affinity (Prossnitz & Barton, Citation2014). Some GPER1 antagonists include synthetic compounds like G-15, G-36. A critical finding is that the classical estrogen receptor antagonists act as agonists on GPER1, an effect that will need to be fully explored in future research. If this can be elucidated and harnessed effectively, there are several ailments (e.g. cancer; as discussed above) for which over-expression of estrogens is believed to be harmful, and in which case anti-estrogen therapy combined with the advantages of GPER1 agonists could be beneficial. Clearly, a research priority will be to determine the exact regions or motifs of GPER1 to which its numerous ligands bind. This knowledge would greatly improve our understanding of the physiology and functioning of the receptor and its signaling.
Future goals
Despite its potential as a therapeutic target in cardioprotection, clinical trials for GPER1 agonists are still some years away. One of the most important aspects of GPER1 research that still needs to be validated is the relationship between this receptor and its sympathetic activity. Sympathetic nervous activity has long been considered a major component of cardiovascular physiology when it comes to determining the benefits of this receptor.
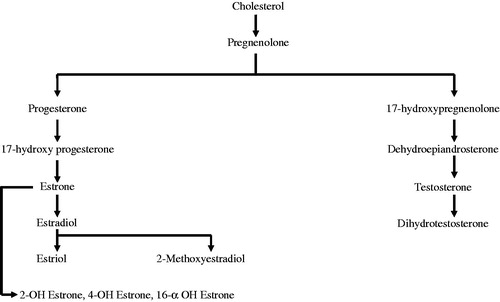
The beneficial role of GPER1 in heart failure is still not researched. Considering the cardiovascular benefits of this receptor, this is an area that needs to be explored. If we consider the synthesis of estradiol from cholesterol (), cholesterol first gives rise to pregnenolone catalyzed by desmolase. Pregnenolone can then enter two pathways, being converted either to progesterone in the presence of Δ-5-4-isomerase or to 17-hydroxy pregnenolone in the presence of cytochrome P450 enzymes. Both progesterone and 17-hydroxy pregnenolone can undergo various intermediary steps, finally forming estriol, 2ME2, 2-hydroxyestrone, 4-hydroxyestrone, 16α-hydroxyestrone or dihydrotestosterone (do Nascimento et al., Citation2009).
Only a few physiological studies have investigated the interaction of these metabolites with GPER1 and how they might play a role in providing cardiovascular benefits. For example, Barchiesi et al. (Citation2010) demonstrated that 2ME2 lowers serum cholesterol and also protects against atherosclerosis caused by cholesterol. The authors demonstrated that 2ME2 is more potent than PPAR-γ ligands like rosiglitazone at inhibiting growth of human aortic smooth muscle.
Having seen the many potential benefits of targeting GPER1 (), and at the same time looking at the areas that still need to be investigated, it is safe to say that the next decade of research on GPER1 will prove whether pharmacological targeting of the receptor can benefit human health in the long run.
Declaration of interest
The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Barchiesi F, Lucchinetti E, Zaugg M, Ogunshola OO, Wright M, Meyer M, et al. 2010. Candidate genes and mechanisms for 2-methoxyestradiol-mediated vasoprotection. Hypertension 56:964–972
- Bopassa JC, Eghbali M, Toro L, Stefani E. 2010. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 298:H16–23
- Chakrabarti S, Davidge ST. 2012. G-protein coupled receptor 30 (GPR30): A novel regulator of endothelial inflammation. PLoS One 7:e52357
- Davis KE, Carstens EJ, Irani BG, Gent LM, Hahner LM, Clegg DJ. 2014. Sexually dimorphic role of G protein-coupled estrogen receptor (GPER) in modulating energy homeostasis. Horm Behav 66:196–207
- Deschamps AM, Murphy E, Sun J. 2010. Estrogen receptor activation and cardioprotection in ischemia reperfusion injury. Trends Cardiovasc Med 20:73–78
- do Nascimento GR, Barros YV, Wells AK, Khalil RA. 2009. Research into specific modulators of vascular sex hormone receptors in the management of postmenopausal cardiovascular disease. Curr Hypertens Rev 5:283–306
- Dubey RK, Jackson EK, Keller PJ, Imthurn B, Rosselli M. 2001. Estradiol metabolites inhibit endothelin synthesis by an estrogen receptor-independent mechanism. Hypertension 37:640–644
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR Jr. 2000. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14:1649–1660
- Filardo EJ, Thomas P. 2012. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology 153:2953–2962
- Iwasa M, Yamada Y, Kobayashi H, Yasuda S, Kawamura I, Sumi S, et al. 2011. Both stimulation of GLP-1 receptors and inhibition of glycogenolysis additively contribute to a protective effect of oral miglitol against ischaemia-reperfusion injury in rabbits. Br J Pharmacol 164:119–131
- Koganti S, Snyder R, Gumaste U, Karamyan VT, Thekkumkara T. 2014. 2-methoxyestradiol binding of GPR30 down-regulates angiotensin AT(1) receptor. Eur J Pharmacol 723:131–140
- LaVallee TM, Zhan XH, Herbstritt CJ, Kough EC, Green SJ, Pribluda VS. 2002. 2-Methoxyestradiol inhibits proliferation and induces apoptosis independently of estrogen receptors alpha and beta. Cancer Res 62:3691–3697
- Li ZL, Liu JC, Liu SB, Li XQ, Yi DH, Zhao MG. 2012. Improvement of vascular function by acute and chronic treatment with the GPR30 agonist G1 in experimental diabetes mellitus. PLoS One 7:e38787
- Prossnitz ER, Barton M. 2014. Estrogen biology: new insights into GPER function and clinical opportunities. Mol Cell Endocrinol 389:71–83
- Pupo M, Vivacqua A, Perrotta I, Pisano A, Aquila S, Abonante S, et al. 2013. The nuclear localization signal is required for nuclear GPER translocation and function in breast Cancer-Associated Fibroblasts (CAFs). Mol Cell Endocrinol 376:23–32
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. 2005. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630
- Seok YM, Jang EJ, Reiser O, Hager M, Kim IK. 2012. 17beta-Estradiol induces vasorelaxation in a G-protein-coupled receptor 30-independent manner. Naunyn Schmiedebergs Arch Pharmacol 385:945–948
- Sessa R, Pietro MD, Filardo S, Turriziani O. 2014. Infectious burden and atherosclerosis: A clinical issue. World J Clin Cases 2:240–249
- Zhang Y, Yang X, Bian F, Wu P, Xing S, Xu G, et al. 2014. TNF-alpha promotes early atherosclerosis by increasing transcytosis of LDL across endothelial cells: crosstalk between NF-kappaB and PPAR-gamma. J Mol Cell Cardiol 72:85–94