Abstract
Sonic hedgehog (Shh) is a morphogen active during vertebrate development and tissue homeostasis in adulthood. Dysregulation of the Shh signalling pathway is known to incite carcinogenesis. Due to the highly lipophilic nature of this protein imparted by two post-translational modifications, Shh’s method of transit through the aqueous extracellular milieu has been a long-standing conundrum, prompting the proposition of numerous hypotheses to explain the manner of its displacement from the surface of the producing cell. Detection of high molecular-weight complexes of Shh in the intercellular environment has indicated that the protein achieves this by accumulating into multimeric structures prior to release from producing cells. The mechanism of assembly of the multimers, however, has hitherto remained mysterious and contentious. Here, with the aid of high-resolution optical imaging and post-translational modification mutants of Shh, we show that the C-terminal cholesterol and the N-terminal palmitate adducts contribute to the assembly of large multimers and regulate their shape. Moreover, we show that small Shh multimers are produced in the absence of any lipid modifications. Based on an assessment of the distribution of various dimensional characteristics of individual Shh clusters, in parallel with deductions about the kinetics of release of the protein from the producing cells, we conclude that multimerization is driven by self-assembly underpinned by the law of mass action. We speculate that the lipid modifications augment the size of the multimolecular complexes through prolonging their association with the exoplasmic membrane.
Introduction
The vertebrate Hedgehog (Hh) family is a group of closely related, secreted signalling proteins comprising three homologues: Sonic hedgehog (Shh), Desert hedgehog (Dhh) and Indian hedgehog (Ihh). Their partially overlapping roles encompass developmental patterning of the organism’s body plan, regeneration of certain epithelial tissues (e.g. taste buds, gut, hair follicles) and healing of injuries. These important processes are orchestrated by a tightly regulated Hh concentration gradient composed of multimeric, soluble forms of Hh designated for long-distance signalling (Zeng et al., Citation2001).
Aberrant upregulation of Shh activity, on the other hand, can lead to cancer (Gupta et al., Citation2010). Interfering with the protein-protein interactions involved in assembling the multimers could provide a means for effective therapy of such cancers (Ivanov et al., Citation2013). Thus, unravelling the mechanism of multimerization could provide useful clues for devising appropriate strategies of interference.
From a biochemical standpoint, the Hh family is unusual. Following biosynthesis, the Hh preprotein becomes covalently modified with a C-terminal cholesterol moiety (Porter et al., Citation1996) and an N-terminal palmitate (Pepinsky et al., Citation1998). Interestingly, Hh is currently the only known protein family that is covalently modified by cholesterol (Resh, Citation2013). Furthermore, rather than being linked to the N-terminus with conventional thioester linkage, palmitic acid is unusually attached via an amide bond, which is a particularly stable form of covalent attachment (Buglino & Resh, Citation2008). In the next step of the protein’s life cycle, the post-translationally modified protein is externalized to the outer membrane leaflet with the aid of Dispatched (Disp), a 12-pass transmembrane protein with a sterol-sensing domain. At this stage, how Hh is capable of disengaging from the cell membrane in spite of its two hydrophobic anchors has puzzled the research community for over a decade. Multiple models attempting to explain the solubilization of Hh have accrued, including: homomultimers (Zeng et al., Citation2001), lipoprotein particle carriers (Panáková et al., Citation2005), exovesicles (Tanaka et al., Citation2005) and Hh/Heparan sulphate proteoglycan (HSPG) heteromultimers (Farshi et al., Citation2011). However, pinpointing categorically the organization and method of assembly remains outstanding. Previous findings have relied on either interpretation of developmental phenotypes or use of conventional biochemical techniques, such as fractionation assays, gel filtration and co-immunoprecipitation. However, these have produced inconclusive evidence which has not permitted to arbitrate the role and function of the lipid modifications, or the putative involvement of auxiliary molecules such as HSPGs.
Some lines of evidence point towards HSPGs as a means of stabilization of the multimeric complexes during their intercellular transit. In Drosophila, with the aid of diffraction-limited confocal microscopy, Hh and glypican HSPGs have been reported to colocalize in lipid rafts, membrane areas with high abundance of embedded cholesterol (Rietveld et al., Citation1999). However, this type of imaging technique has insufficient resolution to state confidently whether the molecules were indeed close enough to be interacting.
The cholesterol moiety has been shown to have a regulatory influence over Shh diffusion and be equally important for long-range, as well as short-range signalling (Resh, Citation2013). N-palmitoylation, on the other hand, is thought to enhance the potency of the Hh signal (Kohtz et al., Citation2001). The impact of each lipid modification on the ability of Hh to multimerize has been investigated previously in numerous studies. Directly conflicting as well as more compatible reports are available on the role of palmitate and cholesterol in multimerization. Publications in support of the conjecture that multimerization can proceed without palmitate or cholesterol include Ohlig et al. (Citation2011) and Feng et al. (Citation2004). Contrastingly, Goetz et al. (Citation2006) and Chen et al. (Citation2004) claim that palmitate is absolutely required for multimerization. In view of this dichotomy, the problem at hand clearly necessitates a different investigative toolkit, and an interdisciplinary approach.
Here we employ deconvolution-enhanced confocal microscopy to study Shh below the optical diffraction limit. We survey the dimensional and morphological distribution of extracellular wild-type Shh and variants of Shh lacking either or both lipid modifications, in order to determine the manner in which each lipid modification individually affects Shh multimolecular output. We find that both the acyl group and the sterol on their own are compatible with producing the full range of multimer sizes seen with normal, dually lipidated Shh. In addition, we provide evidence that the acyl group favours multimeric architecture with relatively symmetrical shapes. Judging by the profile of the size distribution spectra, we speculate that the multimerization of Shh is a spontaneous self-assembly process underpinned by the law of mass action, albeit one which may involve other molecular species.
Methods
Cell culture
Except during experiments, when cells were transferred to either six- or 24-well plates, COS-7 cells were cultured in 175-cm2 uncoated Nunc™ flasks in Dulbecco’s modified Eagle’s growth medium (DMEM), supplemented with 10% foetal calf serum (FCS) and 1% penicillin streptomycin antibiotics, and maintained at 37 °C and 5% CO2. Cells were routinely passaged upon reaching ∼80% confluence with trypsin-EDTA (0.05% w/v and 0.02% w/v, respectively; PAA laboratories).
Immunoblotting
COS-7 cells were seeded in a six-well plate at a density of 75 000/well, incubated at 37 °C overnight, then transfected with pCMV6-XL5 expression vectors encoding wild-type (WT) human Shh (henceforth Shh-WT), Shh-C24S (unpalmitoylated), Shh-N (uncholesterylated), Shh-NC24S (unpalmitoylated and uncholesterylated), or Shh::EGFP. Cells were left to express the plasmids over a 24-h period at 37 °C. Cells were then lysed by adding 100 μl lysis buffer per well (1% Triton X-100 (BDH Chemicals Ltd, Poole, UK), 0.1% sodium dodecyl sulphate (SDS; Merck Chemicals Ltd, Nottingham, UK), 1× cOmplete mini protease inhibitor tablet (Roche Diagnostics Ltd, Burgess Hill, UK) made up in 10 ml phosphate-buffered saline (PBS), pH 7.4) and passed through a 0.5-mm syringe needle three times to shear genomic DNA. At least 0.5 million cells expressing each type of plasmid were harvested. Loading buffer (4 parts NuPAGE® LDS to 1 part β-mercaptoethanol) was added to each sample at 1/5 of the final volume; the protein concentration and total volume of each sample were kept the same by adjusting with lysis buffer when required. The proteins were separated according to molecular weight by SDS-PAGE in a gel matrix composed of 4% stacking gel and 15% resolving gel, then immobilized by semi-dry transfer on to a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA). The membrane was incubated overnight in blocking buffer (5% w/v Marvel dried skimmed milk powder, 0.02% NaN3 in PBS, filtered) at 4 °C, then on the following day incubated with anti-Shh rabbit polyclonal (H-160 sc-9024, #K2211, Santa Cruz Biotechnology) diluted 1:200 in blocking solution for 2 h at room temperature. The primary antibodies were detected with secondary horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (#4050-05, Southern Biotech), diluted 1:20,000 in PBST (0.05% Tween® 20 in PBS v/v) during an incubation period of 50 min. Following extensive washing in PBST, immunocomplexes bound to the protein bands were visualized using enhanced chemiluminescence (ECL) Plus Western Blotting Detection Reagent (GE Healthcare, Amersham, UK) on an Ettan DIGE Imager (GE Healthcare, Amersham, UK).
Immunostaining and fluorescence microscopy
COS-7 cells were seeded in 24-well plates on No. 1.5, Ø 13-mm coverslips at a density of 60 000 cells/well. Cells were then incubated at 37 °C overnight and transfected with plasmids encoding Shh-WT, Shh-C24S, Shh-N or Shh-NC24S. Proteins were expressed for 24 h, whereupon cells were fixed with 3% paraformaldehyde (PFA) in 1 × PBS for 5 min. Subsequently, the sample was incubated in blocking buffer (5% BSA in PBS) for 1 h. Extracellular Shh was visualized using either anti-Shh H-160 or rabbit monoclonal anti-Shh (C9C5, #2207, Cell Signaling Technology) at a concentration of 1:200, detected with Alexa Fluor® 488-conjugated goat anti-rabbit IgG H + L or anti-mouse IgG1 secondary antibodies. Subsequently, coverslips were mounted on glass slides using ProLong® Gold antifade agent (Invitrogen). The cells were imaged with a 488-nm spectral line from a 25-mW argon laser, operated at 1% of its maximum power, on a confocal microscope (Zeiss LSM 780) equipped with a 63×, 1.4 NA plan apochromat oil-immersion objective lens and operated by ZEN software. Using an inbuilt grating, detection of fluorescence was restricted to a pass band of 490–534 nm and detected using an array of GaAsP detectors. The pinhole was adjusted to 1 Airy unit (49.35 μm). The sampling distances in x, y and z were set to satisfy the Nyquist criterion, as determined using the SVI online calculator tool at http://www.svi.nl/NyquistCalculator. A 1.6× zoom was applied, and the pixel size was set to 41 nm. For each field of view, depending on the local height of the cells, up to 120 slices were obtained at 128-nm vertical intervals. This corresponds to a scanned volume of 84.0 × 84.0 × 15.4 μm inside the sample. Images of a 2048 × 2048 pixel format were acquired at 763 lines per sec, and were line-averaged four times.
Deconvolution of confocal optical sections
A z-stack of green PS-Speck™ (Invitrogen, Carlsbad, CA) sub-resolution fluorescent microspheres (175 ± 5-nm) was deconvolved via the Classical Maximum Likelihood Estimation (CMLE) algorithm within Huygens Essential (Scientific Volume Imaging, Hilversum, The Netherlands). Deconvolution was carried out once using the empirical microscope PSF, obtained through deconvolving the size and shape of the microspheres from their images, and once with a PSF calculated from electromagnetic diffraction theory. The deconvolution result with the theoretical PSF successfully resolved a small aggregate of microspheres. Conversely, this was not the case with the empirical PSF, which rendered them as a large monolithic object. We interpreted this as an indication that the theoretical PSF was more appropriate in this case; hence, we proceeded to deconvolve all of the biological data with this PSF.
Quantitation of the physical characteristics of Shh clusters
Cell-associated and dispersed cluster populations were determined based on differential interference contrast (DIC) images of the cells gathered in parallel with the fluorescence images. Clusters found within areas spanned by the cells were considered to be membrane bound, whereas those residing in regions free of cell coverage were assumed to be dispersed Shh forming the Shh gradient. Supplementary Figure 1 (available online) shows the process of allotting separate areas for quantitating the cell-associated and dispersed Shh fractions. Quantitation of the cluster volume, surface area, cross-sectional area in the x-y plane, 2D and 3D shape factors was carried out using Volocity software (PerkinElmer, Waltham, MA). The greater of the two base signals detected in either negatively stained or in mock-transfected positively stained specimens was set as the minimum signal threshold of the software object-searching algorithm. A total of 122 Shh-WT-, 130 Shh-C24S-, 163 Shh-N- and 87 Shh-NC24S-transfected whole or partial cells were analyzed.
Results
Herein, we use the terms “complex”, “cluster” and “multimer” interchangeably to refer to high molecular weight aggregates of Shh. COS-7 cells were transfected with expression vectors containing Shh-WT, a mutant (Shh-N) missing the C-terminal domain absolutely required for cholesterylation (Porter et al., Citation1996), Shh-C24S with a C24S point mutation precluding palmitoylation (Pepinsky et al., Citation1998), or Shh-NC24S containing both the C24S mutation and C-terminal truncation that results in a completely unlipidated Shh protein (Supplementary Figure 2). The protein expression levels of the plasmids were first assayed using immunoblotting (Supplementary Figure 3) in order to ascertain their comparability. Indeed, band intensity quantitation of the blot showed that the 20–25 kDa fraction, which is expected to emerge on the extracellular surface, was expressed to a similar extent across the four variants: 1.00: 0.86: 0.91: 0.95, lanes 4–7, respectively. The cells used in the blot were taken from the same population of cells used for the subsequent imaging (Supplementary Figure 4).
Cells expressing each of the constructs were analyzed with confocal microscopy, whose resolution was enhanced to well below the diffraction limit post-acquisition through deconvolution (Supplementary Figure 5). Cells were formaldehyde-fixed, but not permeabilized, so that only cell-surface Shh would be observed. During the imaging it was noticed that, in addition to the punctate staining on the cell membranes, similar staining was evident in the intercellular space. We interpreted this as released Shh that had subsequently adsorbed to the coverslips. The cell-associated and dispersed Shh populations were analyzed independently of each other, in order to identify any potential differences between the populations, indicating that the clusters undergo rearrangements after leaving the cell surface.
Using Volocity, the clusters were identified and queried for volume, surface area, and 3D shape factor, as well as cross-sectional area and 2D shape factor in the plane of imaging. Measurements in the plane of imaging were carried out in order to remove any potential distortion effects caused by the inherent differential resolution in the transverse and axial directions. Comparing the volume with the surface area profile (or the surface area with the cross-sectional profile) provides an indication of whether there was a size-dependent shift in the shape of the clusters. Differences between the two- and three-dimensional shape factors, on the other hand, would flag asymmetries and consequently reveal the orientation of the clusters in respect to the plane of the plasma membrane.
The shape factor is a numerical measure of structural symmetry. The formulae for calculating the shape factors of the objects, in 2D and 3D, are:
where A is the cross-sectional area of a cluster, P is its perimeter, V is the volume of the cluster and S its surface area. Thus, objects with perfect symmetry (e.g. a circle in the 2D case and a sphere in the 3D case) are assigned a value of 1, whereas objects with lower symmetries (e.g. elliptical or filiform objects) correspond to smaller values.
Using the full width at half maximum (FWHM) as a measure of resolution whilst comparing the size of the PSFs of 20-nm nanodiamonds prior to and post deconvolution, we established that the deconvolution software afforded a 1.6× resolution enhancement in the x-y plane and nearly 2.1× in the z. We note, however, that the FWHM is by no means a precise figure of merit for resolution, and that the actual resolution attained may be even higher.
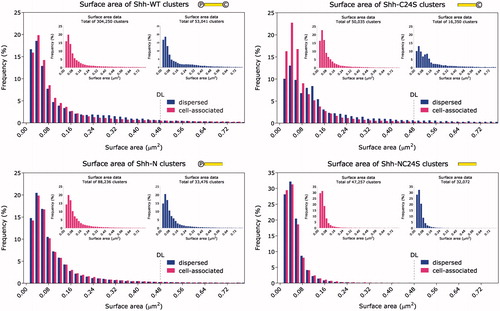
Shh-WT produced a far greater total number of clusters than any of the other protein varieties, suggesting that double lipidation is by far the most efficient for assembly of clusters. The cluster volume data for all Shh variants, plotted as histogram distributions of frequency of occurrence against cluster volume, are reproduced in . Similarly, the surface area quantitation is displayed in . From the Shh-NC24S graphs, it is clear that Shh is capable of forming small oligomers without the aid of its lipid adducts. Moreover, comparing these graphs with those of Shh-C24S provides evidence that palmitate is directly involved in large-scale multimerization of Shh. A modest shift towards increased cluster volume and surface area, at the expense of more compact clusters, is evident upon comparing the dispersed Shh-WT clusters with their cell-associated counterparts. Far more pronounced, however, is the same trend for unpalmitoylated Shh-C24S clusters. The Shh-C24S mutant appears to release larger clusters preferentially to smaller ones; this may be due to altered kinetics of assembly of the clusters owing to the cholesterol moiety acting on its own. Observing that the uncholesterylated (Shh-N) and totally unlipidated (Shh-NC24S) mutants do not show any significant differences between Shh populations on and off the cell, it appears that the cholesterol moiety is responsible for driving the size increase. While the acyl group clearly augments the size of the complexes, it can be seen that it moderates somewhat the shift in balance from small to large multimer sizes that cholesterol favours. This leads to the idea that perhaps palmitate and cholesterol strike a careful balance in order to produce a unique multimer output profile.
Figure 1. (a) Frequency distribution histogram of cluster volume. Inset: cell-associated and dispersed clusters plotted separately. The distributions exhibit a monotonically decreasing profile. Note that the unlipidated mutant produces higher amounts of small clusters, as well as smaller clusters on average relative to the other variants. ![]()
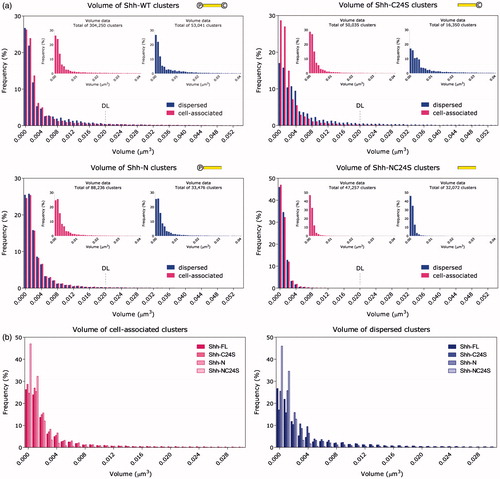
Figure 2. Frequency distribution histogram of cluster surface area. Inset: cell-associated and dispersed clusters plotted separately. As in , all Shh variants, except for Shh-C24S, exhibit similar cluster size distributions on and off the cell. The Shh-C24S mutant appears to release larger clusters preferentially. ![]()
Considering the conclusions thus far and with support from the fact that, unlike the other three Shh variants, the majority of Shh-NC24S was found scattered outside the cells and cell-bound Shh-NC24S was extremely scarce, we posit that the role of the lipid modifications is to anchor Shh to the membrane and prolong its association with the cell surface. This, in turn, could allow more time during which the nucleated oligomers could be built upon by an incremental, time-dependent process resulting in larger structures before eventually releasing them from the cell surface.
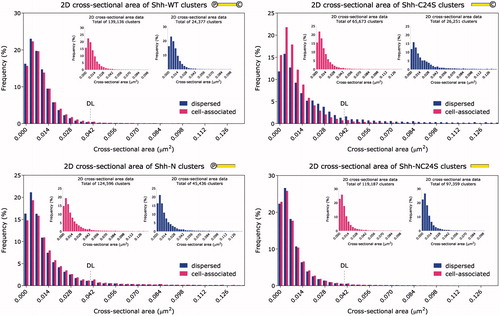
The cluster cross-sectional area frequency distribution is shown in . In contrast to the volume and surface area measurements in and , where the distributions for Shh-WT and Shh-NC24S appear considerably different, shows that the unlipidated mutant exhibits as large cross-sectional areas as the wild type. The orientation of the area measurement (the x-y plane) is approximately parallel to the cell membrane; thus, one possible way to reconcile these data would be to suggest that the multimers grow vertically from the cell surface.
Figure 3. Frequency distribution histogram of cluster cross-sectional area. Inset: cell-associated and dispersed clusters plotted separately. The cross-sectional area was measured in the sample plane (x–y). An upward shift in the cluster cross-section median is observed for dispersed compared to the cell-associated Shh-C24S clusters. ![]()
displays the 3D shape factor of the clusters. Interestingly, all four graphs are dominated by a spike at ∼0.7, indicating high abundance of clusters of a slightly oblong shape. Given that the voxels making up the image data were oblong themselves (due to poorer resolution in the z direction) and whose shape factor was of a very similar value, we were concerned that this may be a manifestation of a systematic error corrupting the measurements. In order to determine whether this was the case, scatter plots of the dependence of the 3D shape factor on the volume and surface area of the clusters were drawn. If there had been a systematic error it would affect small clusters more than large ones. However, Supplementary Figure 6 shows that the data refute this theory; the smallest clusters, in fact, tend towards perfect symmetry. We conclude, therefore, that the 3D measurements are unspoiled by directional resolution differences. We believe that the resolution heterogeneity may have been mitigated to some extent by the anisotropic gain in resolution after deconvolution, which enhances resolution in the z direction to a greater extent than in the x–y plane.
Figure 4. Frequency distribution histogram of the 3D shape factor. Inset: cell-associated and dispersed clusters plotted separately. Compared to the wild type, the Shh-C24S mutant exhibits a broader range of cluster shapes. Conversely, the Shh-NC24S mutant appears to favour an elliptical cluster shape factor of 0.7. ![]()
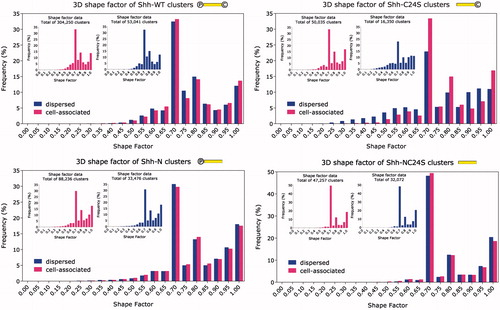
Overall, it appears that comparatively high symmetries (0.7 and above) are favoured by Shh multimers. Small shape factors corresponding to string-like formations and/or complicated shapes, on the other hand, appear to be rare. In fact, the scatter plots in Supplementary Figure 6 indicate that the irregularity of the cluster shapes increases with size. In the same vein, both 3D and 2D shape factor figures ( and ) insinuate that, besides counterbalancing cholesterol’s tendency to skew the multimerization process in favour of releasing larger multimeric structures, palmitate may possess the additional role of maintaining the globularity of the clusters, and possibly preventing “chain-multimerization”. Exercising strict control over the multimers’ compactness may, for example, be necessary to ensure their unimpeded transit through the intercellular environment and/or efficient interaction with the receptor Patched (Ptc).
Figure 5. Frequency distribution histogram of the 2D Shape factor. Inset: cell-associated and dispersed clusters plotted separately. The 2D shape factor was measured in the sample plane (x–y) where resolution is isotropic. A broader shape factor range compared to the rest of the Shh variants is evident for Shh-C24S, and to a lesser extent Shh-N. Clearly, slightly oblong clusters (shape factor 0.8) are favoured by all Shh variants. ![]()
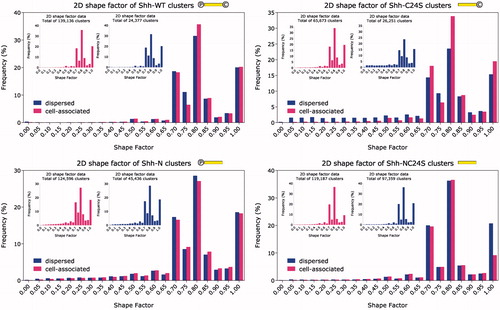
Ostensibly, all Shh variants, bar the Shh-C24S mutant, retain their physical characteristics after leaving the producing cell’s surface. However, analyzing the data with a non-parametric, two-tailed Kolmogorov–Smirnov test established that the cell-tethered and extracellular portions of Shh followed different distributions. With the exception of the Shh-N mutant which scored p = 0.0028 for the 3D shape factor, Shh-WT with p = 0.1963 for the 2D shape factor and Shh-NC24S with p = 0.0077 for the 2D shape factor as well, the p value in all other cases was exclusively below 1 × 10−4 (). Two inferences may be drawn from the fact that the cell-bound and extracellular populations differ from each other: (1) The rate of detachment of the multimers is not precisely proportional to their abundance on the cell surface, and (2) these Shh variants tend to interact with additional multimerization-mediating molecules after leaving the cell surface.
Table 1. Summary of p values and fit parameters. The p values, which were calculated using the two-tailed Kolmogorov-Smirnov test, assess whether the cell-associated and dispersed clusters of each Shh variant are identical in size and shape.
Lastly, to assess the degree of reliability of the data, we carried out the same quantitation exercise with a different primary antibody only on the Shh-WT variant (Supplementary Figure 7). The extensive agreement between the two sets of data confirmed the specificity of the antibodies and the robustness of our data.
The law of mass action and the Smoluchowski equation of aggregation kinetics
The curiously consistent tapering statistical size distributions of the clusters compelled us to investigate the possibility that the multimerization process is spontaneously driven by Shh itself. The driving forces are expected to be intermolecular Shh interactions involving ionic bonds, hydrogen bonds or van der Waals weak electrostatic forces. The intuitive notion that increasing the concentration of molecules increases the likelihood that they will collide together and bind is encapsulated in the law of mass action (Waage & Guldberg, Citation1986). Building further on this law, Smoluchowski’s rate equation (Smoluchowski, Citation1916) with an added “source” [penultimate term on the right-hand side of Equation (Equation1(1) )] to represent Shh emerging at the extracellular membrane, and a “sink” (last term) to represent the flux of clusters away from the cell surface (Connaughton et al., Citation2004), describes the rate of irreversible aggregation of a single molecular species:
(1)
where ck is the concentration of clusters of size k, and Kij is the aggregation rate constant for the association between clusters of size i and j. The first term on the right-hand side describes the increase in k-mer concentration owing to coalescence of an i-mer and a j-mer. Conversely, the second term accounts for the disappearance of k-mers upon their coalescence with other clusters (Kang & Redner, Citation1984). J0 represents the flux of monomers with mass m0 arriving at the cell surface, and J (ck) accounts for the departure of clusters of arbitrary mass, M, from the cell surface.
The addition of the source and sink terms enables reaching a time-independent steady state after a long time, known as a “stationary solution”. Solving this kinetic equation analytically should then yield the expected distribution function of the cluster sizes. Assuming a lack of allostery, that the monomers have an overall neutral electric charge at physiological pH (the isoelectric point of mature Shh is 7.83, from SwissProt http://web.expasy.org/compute_pi), and that Kij is invariant, the stationary solution gives a distribution function with a monotonically decreasing profile (Connaughton et al., Citation2004):
(2)
where α is a constant depending on the influx of monomers, the diffusion constant, probability of binding, and the mass of the monomers, and is a scaling factor connecting the k-mer concentration to the relative abundance of multimers of the corresponding size. β is then the constant of proportionality relating the volume of the individual k-mers to their corresponding molecular count, k.
Here we have made the reasonable assumption that, if the multimers contain a single molecular species, their volume will be commensurate with the total number of incorporated monomers. Fitting this function to our cluster volume measurements, therefore, could present a means to enumerate the Shh monomers incorporated into the clusters. This method should yield an accurate figure, provided that the structures are homomultimeric; otherwise, it would at least serve to ascertain an upper limit on the number of Shh molecules per multimer.
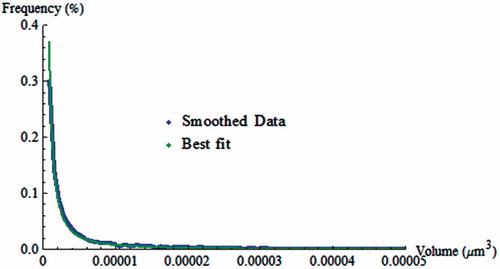
To obtain an accurate fit of our cluster volume data with Equation (Equation2(2) ), rather than using the effectively subsampled histograms above, it is necessary to find the best estimate of the underlying distribution function using a smoothing procedure. The latter is detailed in section C. 1 of the Appendix. The smoothed estimate, fitted with non-linear least squares to the theoretical model in Equation (Equation2
(2) ), is shown in . The best-fit parameters were: α = 5.725 and β = 8.076 × 106, with an R2 value of 0.99. Performing a Kolmogorov-Smirnov test returned a p value of 0.5, indicating that the likelihood that the data obeyed the theoretical model was moderate. Using the parameter values obtained through the best fit we found that 90% of the clusters were expected to contain no more than 32 monomers.
Discussion and conclusions
This research study accomplishes a dimensional and morphological characterization of Shh multimers with sub-diffraction-limit resolution. Parameters such as volume, surface area, cross-sectional area and both planar and 3D shape factor were determined for wild-type, as well as mutant Shh missing either or both lipid moieties. Complexes associated with the membrane were surveyed separately from those found in the vicinity of the cells, as they were presumed to be at different stages of the signalling cascade.
Even without super-resolution imaging, we show that highly useful information can be obtained using the less technically challenging technique of deconvolution-enhanced confocal imaging. Post-processing the confocal micrographs with the deconvolution software resulted in the ability to discriminate particles down to <112 nm in the plane of imaging and <220 nm axially. Although the resolution achieved in this work is well below the diffraction limit, it is not sufficient to distinguish individual Shh proteins [∼4 nm from crystallographic measurements (Pepinsky et al., Citation2000)]. It should therefore be borne in mind that the data may harbour a certain degree of resolution-related artefacts that may lead to misinterpreting the underlying structure. For instance, the irregular Shh complex shapes indicated by the low shape factor may in fact be densely situated monomers. Both large and small clusters can succumb to this artefact, since comparatively large clusters may also lie very close by each other. Not to be overlooked is also the unavoidable systematic error introduced by the size of the labelling immunocomplexes that causes consistent size over-estimation by approximately 30 nm at each cluster boundary. This bias, however, should not affect the overall shape of the distributions of the measured parameters.
In this study we confirm that unlipidated Shh forms small oligomeric structures first reported by Vyas et al. (Citation2008). Unequivocal evidence was revealed in respect to the role of Shh’s post-translational lipid modifications, ascertaining both cholesterol’s and palmitate’s non-essential involvement in multimerization. These results are in harmony with findings in Ohlig et al. (Citation2011) and Feng et al. (Citation2004), and contrary to reports in Goetz et al. (Citation2006) and Chen et al. (Citation2004).
In addition, we find that palmitate appears to be responsible for multimer shape regularization. Comparing the volume with the cross-sectional area of the clusters suggests that oligomers grow in a direction normal to the surface. This theory, however, does not seem to tally with the 3D shape factor distribution (), which would be expected to shift to the left for the Shh-NC24S mutant, since thin, flat structures deviate further from a sphere than structures with more balanced dimensions. More information needs to be collected to understand and reconcile these data.
The Shh-C24S mutant exhibits an interesting clear trend: The cell-surface cluster population contains a higher ratio of small to large clusters compared to the dispersed cluster population. This trend appears consistently across all of the size measurements and seems correlated with the decrease in symmetry of the dispersed clusters. It is plausible that, upon escaping the extracellular membrane leaflet, the absence of palmitate permits adjacent clusters to coalesce without rearranging into a more compact structure, thus resulting in shapes of lower symmetry.
Somewhat surprisingly, the effects of cholesterol and palmitate emerge not as amplification, but as moderation of the cluster sizes. The moieties appear to act in a synergistic manner towards sculpting a size distribution with a well-defined, precise profile. Why there should be a redundancy in the function of the lipids is not clear at present; however, this may be important for regulating the potency of the physiological signal relayed by Shh upon binding to Ptc.
Monotonically diminishing size distributions, exhibited by wild-type Shh and mutants alike, resemble those expected of a self-organizing process (Connaughton et al., Citation2004). Employing different statistical tools to assess the fit of the theoretical curve of self-assembly to our cluster volume data led to somewhat inconclusive results; the R2 goodness-of-fit parameter ascertained an excellent fit, whereas the Kolmogorov–Smirnov test indicated a mediocre fit. The source of low confidence may lie in the unsuppressed fluctuations in the smoothed curve, or skewing of the curve due to the resolution-limited measurements. As a result, the claim of multimerization through spontaneous self-organization remains to be further tested in a separate study, which would need to address experimentally (preferably with super-resolution imaging) whether other molecular species are definitively incorporated in the multimers, or whether Shh is the sole component.
Notwithstanding, this fit permits determination of the monomer count within individual clusters. Assuming that the nature of the clusters is homomultimeric, we estimate that 90% of the cluster population contains up to 32 monomers per cluster. The accuracy of this figure is subject to distortions in the data due to the inherent resolution limit, stochasticity related to sample size, as well as possible variability in the manner of packing of the molecules within the multimers. We believe ours to be the first study to apply this mathematical framework to a biological molecule. We also expect that the mutant cluster volume spectra would be obtainable through the time-dependent version of the solution proposed by Connaughton et al. which is provided in Equation 31 of ref. (Connaughton et al., Citation2004).
It is thought that, as a prerequisite for multimerization, cholesterol promotes electrostatic interactions between Shh monomers by concentrating and immobilizing them into membrane microdomains (Chen et al., Citation2004; Dierker et al., Citation2009; Ryan & Chiang, Citation2012). Taking into account that a much larger proportion of the unlipidated mutant, compared to the other variants, is released into the extracellular environment than is retained on the surface, we propose that the fatty acid and sterol serve, at least in part, to anchor Shh to the cell surface, increase its local concentration, and stimulate multimer assembly through the law of mass action.
In our proposed model, Shh can drive its own multimerization and does not require the involvement of any other molecules. However, at this stage we cannot rule out input from other molecular species – for example, HSPGs. Future colocalization studies of Shh and HSPGs with super-resolution or Förster resonance energy transfer (FRET) may be illuminating in this respect. Furthermore, the sterol-modified Shh protein is known to be released on the extracellular surface by means of Disp (Burke et al., Citation1999) whereas cholesterol-deficient Shh is capable of escaping into the extracellular milieu independently of Disp (Li et al., Citation2006). The difference in release mechanisms implies differing local concentrations, and may require to be taken into account in our model.
Supplementary material available online
Supplementary Figures 1-7
Supplemental Material
Download PDF (708.3 KB)Acknowledgements
We would like to thank Dr Birgit Leitinger for the COS-7 cell line, Dr Marta Świerczyńska and Dr Suzanne Eaton for plasmids encoding wild-type and mutant Shh DNA, Prof. Andrew McMahon for the Shh::EGFP fusion construct, Mr Hugo Sinclair for his loan of nanodiamonds, Dr Ed Cohen for recommendations regarding statistical methods, Prof. Miguel Rubí for helpful discussions on self-assembly of particles, and Dr Débora Andrade for critical reading of the manuscript. Microscopy was performed in the Facility for Imaging by Light Microscopy (FILM) at Imperial College London.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
MVK was supported by a PhD studentship from the Imperial College London Institute of Chemical Biology EPSRC Centre for Doctoral Training (grant EP/F500416/1).
References
- Buglino JA, Resh MD. 2008. Hhat is a palmitoylacyltransferase with specificity for N-palmitoylation of Sonic Hedgehog. J Biol Chem 283:22076–22088
- Burke R, Nellen D, Bellotto M, Hafen E, Senti KA, Dickson BJ, Basler K. 1999. Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell 99:803–815
- Chen MH, Li Y-J, Kawakami T, Xu S-M, Chuang P-T. 2004. Palmitoylation is required for the production of a soluble multimeric Hedgehog protein complex and long-range signaling in vertebrates. Genes Dev 18:641–659
- Connaughton C, Rajesh R, Zaboronski O. 2004. Stationary Kolmogorov solutions of the Smoluchowski aggregation equation with a source term. Phys Rev E 69:061114
- Dierker T, Dreier R, Migone M, Hamer S, Grobe K. 2009. Heparan sulfate and transglutaminase activity are required for the formation of covalently cross-linked hedgehog oligomers. J Biol Chem 284:32562–32571
- Farshi P, Ohlig S, Pickhinke U, Höing S, Jochmann K, Lawrence R, et al. 2011. Dual roles of the Cardin-Weintraub motif in multimeric Sonic hedgehog. J Biol Chem 286:23608–23619
- Feng J, White B, Tyurina OV, Guner B, Larson T, Lee HY, et al. 2004. Synergistic and antagonistic roles of the Sonic hedgehog N- and C-terminal lipids. Development 131:4357–4370
- Goetz JA, Singh S, Suber LM, Kull FJ, Robbins DJ. 2006. A highly conserved amino-terminal region of sonic hedgehog is required for the formation of its freely diffusible multimeric form. J Biol Chem 281:4087–4093
- Gupta S, Takebe N, Lorusso P. 2010. Targeting the Hedgehog pathway in cancer. Ther Adv Med Oncol 2:237–250
- Ivanov AA, Khuri FR, Fu H. 2013. Targeting protein-protein interactions as an anticancer strategy. Trends Pharmacol Sci 34:393–400
- Kang K, Redner S. 1984. Fluctuation effects in Smoluchowski reaction kinetics. Phys Rev A 30:2833–2836
- Kohtz JD, Lee HY, Gaiano N, Segal J, Ng E, Larson T, et al. 2001. N-terminal fatty-acylation of sonic hedgehog enhances the induction of rodent ventral forebrain neurons. Development 128:2351–2363
- Li Y, Zhang H, Litingtung Y, Chiang C. 2006. Cholesterol modification restricts the spread of Shh gradient in the limb bud. Proc Natl Acad Sci USA 103:6548–6553
- Ohlig S, Farshi P, Pickhinke U, van den Boom J, Höing S, Jakuschev S, et al. 2011. Sonic hedgehog shedding results in functional activation of the solubilized protein. Dev Cell 20:764–774
- Panáková D, Sprong H, Marois E, Thiele C, Eaton S. 2005. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature 435:58–65
- Pepinsky RB, Rayhorn P, Day ES, Dergay A, Williams KP, Galdes A, et al. 2000. Mapping sonic hedgehog-receptor interactions by steric interference. J Biol Chem 275:10995–11001
- Pepinsky RB, Zeng C, Wen D, Rayhorn P, Baker DP, Williams KP, et al. 1998. Identification of a palmitic acid-modified form of human Sonic hedgehog. J Biol Chem 273:14037–14045
- Porter JA, Young KE, Beachy PA. 1996. Cholesterol modification of hedgehog signaling proteins in animal development. Science 274:255–259
- Resh MD. 2013. Covalent lipid modifications of proteins. Curr Biol 23:R431–R435
- Rietveld A, Neutz S, Simons K, Eaton S. 1999. Association of sterol- and glycosylphosphatidylinositol-linked proteins with Drosophila raft lipid microdomains. J Biol Chem 274:12049–12054
- Ryan KE, Chiang C. 2012. Hedgehog secretion and signal transduction in vertebrates. J Biol Chem 287:17905–17913
- Smoluchowski M. 1916. Drei Vorträge über Diffusion, Brownsche Molekularbewegung und Koagulation von Kolloidteilchen [Three discourses on diffusion, Brownian motion and coagulation of colloidal particles]. Physik Z 17:557–571, 585–599
- Tanaka Y, Okada Y, Hirokawa N. 2005. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature 435:172–177
- Vyas N, Goswami D, Manonmani A, Sharma P, Ranganath HA, VijayRaghavan K, et al. (2008). Nanoscale organization of hedgehog is essential for long-range signaling. Cell 133:1214–1227
- Waage P, Guldberg CM. 1986. Studies concerning affinity. J Chem Educ 63:1044–1047
- Zeng X, Goetz JA, Suber LM, Scott WJ, Schreiner CM, Robbins DJ. 2001. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature 411:716–720
Appendix
C. 1 Data Smoothing
A smoothing procedure based on the statistical method of kernel density estimation (KDE) was carried out on the data to eliminate the distorting edge effects of the histograms. In brief, KDE represents each data point (i.e. individual cluster volume) by a kernel (typically a Gaussian function) whose integral is unity. The kernels are then summed, and the envelope formed constitutes the function estimate. Data were smoothed using either kernel density estimation with a fixed kernel bandwidth (FKDE) through its implementation in the ksdensity function in MATLAB (The MathWorks, Natick, MA), or using custom-written code in Mathematica (Wolfram, Champaign, IL) with an adaptive kernel bandwidth (AKDE). The difference between FKDE and AKDE is that in the former, the kernel bandwidth is kept fixed for all data points, whereas in the latter, the width of each kernel is scaled inversely with the local data point density:
where N is the total number of data points, and h(xn) is a variable multiplicative factor controlling the fixed kernel bandwidth, σ, depending on the sum of the distances between a sample point, xn, and its nearest neighbours.
The methods returned very similar results. Nevertheless, we selected our custom-written AKDE algorithm for subsequent fitting, because we believed that it estimated small clusters better than the FKDE.