Abstract
Cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells target infected or transformed cells with perforin-containing cytotoxic granules through immune synapses, while platelets secrete several types of granules which contents are essential for thrombosis and hemostasis. Recent work has culminated in the notion that an exocytic SNARE complex, based on a very similar set of components, is primarily responsible for exocytosis of the diverse granules in these different cell types. Granule exocytosis is, in particular, uniquely dependent on the atypical Q-SNARE syntaxin 11, its interacting partners of the Sec/Munc (SM) family, and is regulated by Rab27a. Mutations in these exocytic components underlie disease manifestations of familial hemophagocytic lymphohistiocytosis (FHL) subtypes, characterized by hyperactivation of the immune system, as well as platelet granule secretion defects. Here we discuss the key discoveries that led to the converging notion of the syntaxin 11-based exocytosis machinery for cytotoxic granules and platelet-derived granules.
Introduction
All mammalian cells have a constitutive exocytic or secretory pathway, where proteins and lipid components are transported in an anterograde direction from the endoplasmic reticulum (ER) towards the plasma membrane. However, a large number of regulated secretions also take place in specialized cell types, such as neurotransmitter and hormone-secreting neural or endocrine cells, as well as a myriad of cell types of the hematopoietic and immune system. In regulated secretion, molecules to be secreted are often packaged into secretory granules which may undergo a process of maturation, and secretion of mature granular contents is subsequently triggered by extracellular signals.
Exocytosis and endocytosis in eukaryotic cells occur via a range of vesicular transport processes involving sequential vesicle budding and fusion within intracellular compartments and at the plasma membrane (Bonifacino & Glick, Citation2004). Pioneering work by Rothman, Sudhof, Scheller and others have identified the basic molecular components required for membrane docking and fusion in the exocytic and endocytic pathways (Ferro-Novick & Brose, Citation2013; Mellman & Emr, Citation2013; Pfeffer, Citation2013). The primary mediators of membrane fusion are members of the family of soluble N-ethylmaleimide sensitive factor (NSF) attachment protein receptors (SNAREs) (Kasai et al., Citation2012). These proteins are either hydrophobic tail-anchored or lipid-anchored to transport vesicles/carriers and target membranes, with their cytoplasmic SNARE domains forming a stoichiometric complex of a 4-α-helical bundle. SNARE complex formation and action provide both the physical driving force necessary for the fusion of two negatively charged phospholipid membranes via the formation of the minimal fusion machinery known as SNAREpin (Weber et al., Citation1998), and determines the compartment specificity of vesicle-target membrane fusion (McNew et al., Citation2000).
The SNAREs could be broadly classified as vesicle (v)-SNAREs and target (t)-SNAREs based on where they primarily reside, or based on conserved structural features in the complex forming SNARE domains as Q- or R-SNAREs (Fasshauer et al., Citation1998). General sequence homology also allows their broad classification into syntaxins, VAMPs and SNAP25-like subfamilies (Bock et al., Citation2001). The syntaxins are all Q-SNAREs present primarily on target membranes, and these likely define the various membrane boundaries of vesicular traffic in the cell (Teng et al., Citation2001). Syntaxins 1, 2, 3 and 4 are plasma membrane syntaxins (Bennett et al., Citation1993), syntaxins 5 and 6 are found at the Golgi (Nichols & Pelham, Citation1998), syntaxin 16 is rather specifically trans-Golgi network (TGN) localized (Simonsen et al., Citation1998; Tang et al., Citation1998b), syntaxin 18 is endoplasmic reticulum (ER) localized (Iinuma et al., Citation2009), while syntaxins 7 and 8 are found in endosomal compartments (Prekeris et al., Citation1999). SNARE pairing is regulated by proteins of the Sec1/Munc18 (SM) family (Südhof & Rothman, Citation2009), which could either promote or inhibit trans-SNARE pairing depending on their interaction with one or more SNARE molecules (Yu et al., Citation2013). The actual membrane fusion step is preceded by events of transport vesicle/carrier targeting and docking, and all these events are regulated in one way or other by members of the large Rab family of small GTPases (Stenmark, Citation2009).
The plasma membrane fusion step in secretion has been extensively investigated and is reasonably well understood in several regulated secretion systems, at least in terms of the molecular factors involved. Synaptic vesicle exocytosis in neurons specifically involves the pairing in trans between the vesicle bound R-SNARE synaptobrevin2/VAMP2 and the Q-SNAREs syntaxin 1 and SNAP-25 on the presynaptic plasma membrane (Südhof & Rizo, Citation2011). The SM protein Munc18-1 binds syntaxin 1 in a closed configuration that could initially prevent trans-SNARE formation, but in the presence of SNAP25 and VAMP2 could instead facilitate SNAREpin formation. The isoforms of Rab3 and their effectors such as rabphilin regulates Munc18-1’s interaction with SNAREs and modulate docking and fusion (Sudhof, Citation2004). Secretory granule exocytosis in pancreatic and parotic acinar cells involves the R-SNAREs VAMP2 and VAMP8, the plasma membrane syntaxins 2, 3 and 4, as well as SNAP-23 and SNAP-29 (Messenger et al., Citation2014). Munc18-1 likewise plays a role in insulin granule exocytosis in pancreatic acinar cells (Tomas et al., Citation2008).
Cytolytic T lymphocytes (CTLs) and natural killer (NK) cells, belonging to the adaptive and innate arms of immunity respectively, are key mediators of the demise of viral-infected cells and tumor cells. Activation of the former depends on major histocompatibility complex (MHC) class 1 presentation of antigens, while the latter is triggered by ligation of specific activating receptors and is inhibited by recognition of self MHC class I molecules. Both these cell types kill diseased target cells using cytotoxic (also known as lytic) granules that are generated and targeted via regulated secretion (de Saint Basile et al., Citation2010). CTLs form a physical, albeit transient, junctional contact known as an immune synapse (Yokosuka & Saito, Citation2010) with target cells upon activation, and re-orientate their cellular microtubule structures towards the synapse. Cytotoxic granules are delivered via microtubules to the synaptic junction where they dock and fuse, releasing the pore-forming perforin which facilitate the entry of granzymes (Voskoboinik et al., Citation2015), serine proteases that induce programmed cell death, into target cells.
Platelets (thrombocytes) are enucleated blood cells responsible for hemostasis (blood clot formation in the event of blood vessel damage), as well as pathological thrombosis (localized clot formation within a blood vessel). It is activated via interaction of its surface glycoprotein receptors with exposed extracellular matrix factors of the blood vessel endothelium such as collagen and von Willebrand factor, as well as by other G-protein coupled receptor signaling downstream from soluble agonists forming at the site of the blood clot. Platelet secretion occurs via α-granules (containing the blood clotting factors and a host of other proteins), δ-granules (or dense granules, containing small molecules like ADP, ATP, Ca2+, Mg2+ and serotonin) or γ-granules (secretory lysosomes, containing several lysosomal hydrolases) (Golebiewska & Poole, Citation2015).
An interesting insight from several laboratories in the past few years has been the elucidation of the exocytic machinery responsible for the secretion of lytic granules and platelet granules. As discussed below, there appears to be a common need for a SNARE complex that centers upon the atypical Q-SNARE, syntaxin 11.
Syntaxin 11-discovery and early functional characterization
Syntaxin 11 was first reported as a member of syntaxin family of SNAREs based on homology (Prekeris et al., Citation2000; Tang et al., Citation1998a; Valdez et al., Citation1999). However, unlike the other syntaxins, it does not have an apparent C-terminal stretch of hydrophobic amino acids for tail-anchorage (Tang et al., Citation1998a). Instead, it has a cysteine-rich C-terminus, which likely contained a signal for lipid modification. Syntaxin 11 was indeed shown subsequently to be acylated, and acylation provided the SNARE molecule with a membrane anchorage that is critical for its function (Hellewell et al., Citation2014).
A yeast 2-hybrid screen quickly identified VAMP and SNAP-23 as syntaxin 11’s cognate SNARE partners, and syntaxin 11 was shown to colocalizes with the mannose 6-phosphate receptor (MPR) on late endosomes and the TGN (Valdez et al., Citation1999). Another report, however, showed that syntaxin 11, like the other known surface syntaxins 2, 3 and 4 is associated with the plasma membrane domains of polarized Madin-Darby canine kidney (MDCK) cells (Low et al., Citation2000). Interestingly, loss of cell polarity resulted in a redistribution of syntaxin 11 and other surface syntaxins to intracellular compartments that are likely of endosomal nature. These early work therefore indicated that syntaxin 11 may function in an exocytic step at the plasma membrane, and also likely to have endocytic roles. Prekeris and colleagues developed specific antibodies against syntaxin 11 and showed that the SNARE molecule, while present in varying low to moderate levels in various tissues, is specifically enriched in cells and tissues of the immune system, including thymus, spleen and lymph nodes (Prekeris et al., Citation2000). This provided the first hint that syntaxin 11 may have unique roles in immune cells or the hematopoietic lineage. Indeed, this notion was subsequently confirmed with the identification of mutations in syntaxin 11 and its interaction/functional partners in the immune disorder familial hemophagocytic lymphohistiocytosis (FHL) (Cetica et al., Citation2010; Sieni et al., Citation2012).
Syntaxin 11 and its interacting partners in familial hemophagocytic lymphohistiocytosis (FHL)
Hemophagocytic lymphohistiocytosis is a rare systemic hyperinflammatory syndrome with uncontrolled hypercytokinemia, a proliferation of histiocytes in solid organs and phagocytosis of blood cells (Chandrakasan & Filipovich, Citation2013; Mehta & Smith, Citation2013). Acquired hemophagocytic lymphohistiocytosis is usually associated with Epstein-Barr virus (EBV) infections, malignancy, or autoimmune disease. Familial hemophagocytic lymphohistiocytosis with genetic abnormalities, or FHL (Cetica et al., Citation2010; Gholam et al., Citation2011; Sieni et al., Citation2012), is an autosomal recessive, juvenile or adult (Zhang et al., Citation2011) onset disorder with five different subtypes. The locus of FHL1 has been mapped to chromosome 9q, and definitive monogenetic mutations are known for FHL2 (PRF1, encoding perforin 1), FHL3 (UNC13D, encoding MUNC13-4), FHL4 (STX11, encoding syntaxin 11) and FHL5 (STXBP2, encoding syntaxin-binding protein-2 or Munc18b/Munc18-2). All these known mutations affect aspects of cytotoxic granule function and exocytosis.
Perforin (Voskoboinik et al., Citation2015) is the pore-forming cytolytic protein in the granules of CTLs and NK cells, and FHL2 mutations linked to its encoding PRF1 gene at 9q21.3-22, 10q21-22 was the first causative gene mutation identified for FHL (Stepp et al., Citation1999). FHL3 patients have mutations mapped to chromosome 17q25 and the causative gene was subsequently identified as UNC13D, which encodes human Munc13-4, a member of the Munc13 family involve in vesicle priming (Feldmann et al., Citation2003). Human Munc13-4 localizes with cytotoxic granules at immune synapses, and its loss affected granule fusion and content release but not docking of polarized granules to the synapses. Munc13-4 interacts with the small GTPase Rab27a as an effector (Neeft et al., Citation2005), and Rab27a orthologue mutations in Ashen mice (Haddad et al., Citation2001) and human Griscelli syndrome (Ménasché et al., Citation2000) have impaired lystic granule exocytosis and haemophagocytic syndrome phenotype. Rab27a appears to have a broad range of activities, being known to be involve in the biogenesis of lysosome-like organelles such as melanosomes (Booth et al., Citation2012; Hume & Seabra, Citation2011), as well as secretory and endocytic functions in insulin secreting cells (Kimura et al., Citation2008). In CTLs, Rab27a also orchestrates a transport complex containing its effector synaptotagmin-like protein 3 (Slp3) (Fukuda, Citation2002), which links to the motor protein kinesin 1 for the transport of lytic granules to the immune synapse (Kurowska et al., Citation2012). Rab27a may thus function in multiple steps of lytic granule exocytosis via its engagement of different effectors.
With syntaxin 11 identified as the FHL4 gene in 2005, the underlying pathological mechanism of syntaxin 11 mutations in FHL4 was further revealed by two reports in 2007. Bryceson et al. (Citation2007) and Arneson et al. (Citation2007) both noted the expression of syntaxin 11 in CTLs and NK cells (Arneson et al., Citation2007; Bryceson et al., Citation2007) The former group found that NK cells from FHL4 patients fail to degranulate and cause lysis of K562 target cells like those from healthy patients (Bryceson et al., Citation2007). Interestingly, NK cell cytotoxicity could be partially restored by IL-2 treatment, which may explain the milder disease phenotype in this FLH subtype. Arnesan and colleagues found that over-expression of syntaxin 11 in isolated human NK cells increase baseline cytotoxic killing of target cells, while siRNA-mediated silencing of syntaxin 11 expression diminished NK cell cytotoxicity (Arneson et al., Citation2007). The authors also showed that silencing of syntaxin 11 did not affect proximal signaling events of cytotoxic lymphocyte activation, but rather specifically inhibited granule exocytosis, even those stimulated by pharmacologic secretagogues to bypass the upstream signaling events. Both these studies indicated a pivotal role for syntaxin 11 in a distal step of CTL/NK cytotoxic granule exocytosis, which is most likely the final fusion step with the plasma membrane.
The above notion is strengthened by recent reports presenting dual color evanescent wave imaging that indicate a multistage sequence of events in syntaxin 11-mediate granule exocytosis. Syntaxin 11 is first transported to the immune synapse through recycling endosomes (Halimani et al., Citation2014) in a VAMP8-dependent manner, and this precedes perforin-containing cytotoxic granule fusion (Marshall et al., Citation2015). These syntaxin 11 clusters at the immune synapse then serve as a t-SNARE for the plasma membrane fusion of lytic granules (Halimani et al., Citation2014; Marshall et al., Citation2015). In conjunction with the discovery of Rab27a and Munc13-4’s involvement in cytotoxic granule exocytosis, it has been proposed that he Rab27a-Munc13-4 complex might engage syntaxin11 in the process (Hong, Citation2005). Munc13-4 could promote Ca2+-dependent SNARE complex formation and stimulates SNARE-dependent liposome fusion, and may thus function as a Ca2+ sensor at rate-limiting priming steps in granule exocytosis (Boswell et al., Citation2012).
The last FHL subtype causative gene located at chromosome 19p was identified as one encoding Munc18-2 or syntaxin-binding protein-2 (STXBP2) (Côte et al., Citation2009; Zur Stadt et al., Citation2009). Lymphoblast cells from affected subjects had much reduced Munc18-2 expression, and their NK cells exhibited impaired cytotoxic granule exocytosis that could be rescued by ectopic expression of wild-type Munc18-2 (Côte et al., Citation2009). Analogous to the previously known Munc18-1 binding to neuronal syntaxin 1, Munc18-2 binds syntaxin 11. Known Munc18-2 mutation in FLH5 patients impairs its binding to syntaxin 11(Côte et al., Citation2009; Müller et al., Citation2014; Zur Stadt et al., Citation2009). Monoallelic Munc18-2 mutations that act in a dominant-negative manner were also recently reported (Spessott et al., Citation2015). These Munc18-2 mutant proteins bind and stabilize syntaxin 11, but appear to hinder membrane fusion in vitro by arresting the late steps of SNARE complex assembly.
Finding a compendium of FHL genes which functions centers round syntaxin 11 indicates that we are looking at components of the cytotoxic granule exocytic machinery that is common to both CTLs and NK cells. More recent findings have indicated that Munc18-2 mutations also cause granule mobilization and bacterial killing effects of neutrophils (Zhao et al., Citation2013). Syntaxin 6 (Martín-Martín et al., Citation2000) and the plasma membrane syntaxins 3 (Naegelen et al., Citation2015) and 4 (Mollinedo et al., Citation2006) have all been implicated in neutrophil exocytosis. However, given that Munc18-2 binds the N-terminal peptide of syntaxin 11 with a high affinity (∼20-fold higher affinity than that of syntaxin 3) (Hackmann et al., Citation2013), and that syntaxin 11 is expressed in neutrophils (Xie et al., Citation2009), the latter could also have an important role in neutrophil granule exocytosis. This was in fact confirmed recently in the examination of Stx11(-/-) mice (D’Orlando et al., Citation2013), when it was shown that while syntaxin 11 deficiency does not affect macrophage phagocytosis, cytokine secretion and mast cell activation, it affected CTL and NK cytotoxicity, as well as neutrophil degranulation.
It should be noted that syntaxin 11 provides the unique SNARE complex identity for granule exocytosis. The other Q-SNARE involve in this complex is supposedly SNAP23, which is ubiquitously expressed and has broad functions in plasma membrane fusion, including mast cell secretion which is not impaired in Stx11 knockout mice (Hepp et al., Citation2005). SNAP23 deletion, not unexpectedly, results in preimplantation embryonic lethality in mice (Suh et al., Citation2011). More intriguingly, however, is the realization that the syntaxin 11-based SNARE complex has a broader function. Recent works have also found both syntaxin 11 and Munc18-2 to be required for platelet secretion (Al Hawas et al., Citation2012; Ye et al., Citation2012), as described in the section below.
Syntaxin 11 and Munc18b/STXBP2 in platelet secretion
It was shown fairly early that enucleated platelets are endowed with regulated secretory pathway components, and platelet secretion likely uses mechanisms that are common to other secretory cell types (Lemons et al., Citation1997). SNARE proteins such as VAMP3 and VAMP8 (Polgár et al., Citation2002), as well as syntaxins 2, 3 and 4 (Chen et al., Citation2000a, Citation2000b, Lemons et al., Citation2000; Reed et al., Citation1999) have all been implicated in mediating platelet secretion, likewise the Munc18 isoforms (Houng et al., Citation2003; Schraw et al., Citation2003). However, two papers from the Whiteheart group recently showed that the platelet secretion mechanism is much closer to that found in CTL and NK cells compared to those mediating granule release in parotic and pancreatic cells. Ye et al. (Citation2012) found that platelet secretion is not impaired in platelets from mouse lacking syntaxin 2, or with conditional knockout of syntaxin 4, or even a combined deficiency in both these syntaxins. Instead, syntaxin 11, which the authors found to be abundant in human platelets is the key syntaxin molecule required for platelet secretion (Ye et al., Citation2012). Thrombin-induced secretion of radiolabelled serotonin and platelet factor 4 (PF4) were greatly diminished in platelets from a FLH4 patient compared to those from a healthy donor.
In a parallel report, Al Hawas et al. (Citation2012) showed that Munc18-2/STXBP2, which is the more abundant Munc18 isoform in human platelets, is also required for platelet secretion. Assessment of platelets from biallelic and heterozygous FHL5 patients indicated that the former exhibited a severe defect while the latter a partial defect in thrombin-induced secretion from platelet dense granules, α-granules, and lysosomes. In platelets, Munc18b mainly associates with syntaxin 11, and interestingly, both Rab27a and Munc13-4 co-immunoprecipitated with Munc18b, while Munc13-4 is also precipitated by a syntaxin 11 antibody (Al Hawas et al., Citation2012). These results strongly suggest that platelet secretion requires a set of syntaxin 11-interacting components that are similarly required for lytic granule exocytosis.
Alternatives and redundancies
That cells of the hematopoietic lineage share a highly similar set of components for regulated exocytosis that is distinctly different from those involved in neuronal and endocrine secretion, particularly in terms of the central Q-SNARE, is perhaps not surprising from an evolutionary point of view. However, is the function of syntaxin 11 limited to granule exocytosis in blood cells? The answer is almost certainly not. Syntaxin 11 has been shown to have an intracellular membrane localization pattern and appear to regulate late endosome to lysosome fusion in macrophages (Offenhäuser et al., Citation2011). On the other hand, is the syntaxin 11-based SNARE complex the only one required for granule exocytosis in CTLs, NK cells and platelets? There is some evidence that this requirement may not be as absolute as it seems, and there are certainly other SNARE-mediated events involved in triggering granule exocytosis. A recent report suggested that the endosomal syntaxin 7 is required for lytic granule release from CTLs (Pattu et al., Citation2011). The authors showed that syntaxin 7 accumulates at the immune synapses upon activation of human CTLs, and is apparently required for lytic granule accumulation and fusion. However, blocking of syntaxin 7 functions also resulted in defective T-cell receptor (TCR) accumulation in the immune synapses. It is therefore unclear at the moment if syntaxin 7 has a more upstream function in TCR recycling and immune synapse formation (or even syntaxin 11 recycling), or it also has a role in lytic granule fusion itself.
Another report has indicated that another endosomal Q-SNARE syntaxin 8 regulates platelet dense granule secretion (Golebiewska et al., Citation2015). Interestingly, syntaxin 8 forms a complex with syntaxin 11 in platelets (Golebiewska et al., Citation2015), and it was previously shown that it may also be found on lytic granules (Pattu et al., Citation2012). However, platelet from syntaxin 8-null mice has a rather specific deficiency in dense granule secretion, and not α-granule or lysosome secretion. By comparison, syntaxin 11-defective platelets from FLH4 patients exhibited inhibitions in all three types of platelet secretion. While these syntaxins may well be acting further upstream of the distal plasma membrane fusion step required for granule secretion, in the absence of syntaxin 11 these, and perhaps the other plasma membrane localized syntaxins, may be recruited to form semifunctional fusion machineries that allow some degree of granule secretion. Their exact roles, if any, in the distal exocytic step of granule fusion with the plasma membrane, require further verification.
Finally, one might ask if there is any difference between CTLs, NK cells and platelets in terms of other accessory and regulatory proteins involve in the workings of this syntaxin 11-based exocytic machinery. It is conceivable that more proximal or earlier events in granule secretion may differ between cells (and for that matter between the different granule types in platelets). While lytic granule targeting to immune synapses by CTLs and NK cells may rely more on Rab27a (Kurowska et al., Citation2012), some platelet granule secretion is likely more dependent on Rab27b (Tolmachova et al., Citation2007), which is also known to mediate mast cell (Mizuno et al., Citation2007) and neutrophil azurophilic granule exocytosis (Johnson et al., Citation2010). Tomosyn-1/Munc18-5/STXBP5, and syntaxin binding protein with an R-SNARE-like domain (Williams et al., Citation2011) was also known to modulate platelet secretion (Ye et al., Citation2014; Zhu et al., Citation2014). Tomosyn-1 is previously known to be involved in a post-vesicle docking event in synaptic vesicle and pancreatic beta-cell exocytosis (Cheviet et al., Citation2006), but is not known to be involve in lytic granule secretion.
Concluding remarks
In this short review, we have discussed a recent convergence in terms of our understanding of how granule exocytosis is executed in CTLs, NK cells, platelets and even neutrophils. In particular, an exocytic SNARE machinery consisting of the unique Q-SNAREs syntaxin 11 and a more generally deployed SNAP23, whose stability of trans-SNARE complex formation with VAMPs is regulated by Munc18b, is central to granule exocytosis in these hematopoietic cell types. This common SNARE machinery is regulated by vesicle priming components Rab27a and Munc13-4. A very simple and generalized schematic representation of this notion is now possible, and this is shown in .
Figure 1. Schematic diagram with a generalized depiction of granule secretion from CTLs, NK cells and platelets. The simplified schema outlines the key steps in granule targeting, docking, priming and fusion, and indicates the key SNAREs and SM proteins involved in the final plasma membrane fusion step that is common for granule secretion in these cell types (see text for details). ER, endoplasmic reticulum; G, granule; PM, plasma membrane.
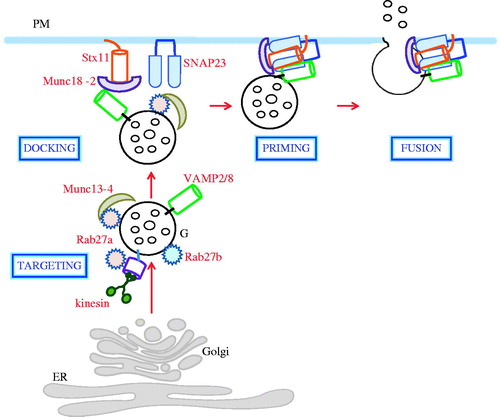
Our understanding of this machinery is still at its infancy [at least in comparison to the machinery responsible for synaptic vesicle exocytosis (Benarroch, Citation2013; Sudhof, Citation2004)]. New players that may interact with and modulating the function of syntaxin 11 and its SNARE complex are still being discovered, for example the recently reported tomosyn-1/Munc18-5/STXBP5 in platelet secretion (Ye et al., Citation2014; Zhu et al., Citation2014). Other than blood cells, syntaxin 11 is also present in appreciable quantities in other tissues, such as heart and lung (Prekeris et al., Citation2000; Tang et al., Citation1998a). The role of syntaxin 11 in other tissue types are unclear at the moment, and although there are some hints that it may be involved in polarized transport (Low et al., Citation2000), elucidation of its exact roles awaits further work.
Acknowledgements
The author is grateful to the NUS Graduate School for Integrative Sciences and Engineering for its support. He is grateful to the reviewers whose constructive comments improved the manuscript.
Declaration of interest
The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Al Hawas R, Ren Q, Ye S, Karim ZA, Filipovich AH, Whiteheart SW. 2012. Munc18b/STXBP2 is required for platelet secretion. Blood 120:2493–2500
- Arneson LN, Brickshawana A, Segovis CM, Schoon RA, Dick CJ, Leibson PJ. 2007. Cutting edge: syntaxin 11 regulates lymphocyte-mediated secretion and cytotoxicity. J Immunol 179:3397–3401
- Benarroch EE. 2013. Synaptic vesicle exocytosis: molecular mechanisms and clinical implications. Neurology 80:1981–1988
- Bennett MK, García-Arrarás JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Scheller RH. 1993. The syntaxin family of vesicular transport receptors. Cell 74:863–873
- Bock JB, Matern HT, Peden AA, Scheller RH. 2001. A genomic perspective on membrane compartment organization. Nature 409:839–841
- Bonifacino JS, Glick BS. 2004. The mechanisms of vesicle budding and fusion. Cell 116:153–166
- Booth AEG, Seabra MC, Hume AN. 2012. Rab27a and melanosomes: a model to investigate the membrane targeting of Rabs. Biochem Soc Trans 40:1383–1388
- Boswell KL, James DJ, Esquibel JM, Bruinsma S, Shirakawa R, Horiuchi H, Martin TFJ. 2012. Munc13-4 reconstitutes calcium-dependent SNARE-mediated membrane fusion. J Cell Biol 197:301–312
- Bryceson YT, Rudd E, Zheng C, Edner J, Ma D, Wood SM, et al. 2007. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood 110:1906–1915
- Cetica V, Pende D, Griffiths GM, Aricò M. 2010. Molecular basis of familial hemophagocytic lymphohistiocytosis. Haematologica 95:538–541
- Chandrakasan S, Filipovich AH. 2013. Hemophagocytic lymphohistiocytosis: advances in pathophysiology, diagnosis, and treatment. J Pediatr 163:1253–1259
- Chen D, Bernstein AM, Lemons PP, Whiteheart SW. 2000a. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 in dense core granule release. Blood 95:921–929
- Chen D, Lemons PP, Schraw T, Whiteheart SW. 2000b. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 and 4 in lysosome release. Blood 96:1782–1788
- Cheviet S, Bezzi P, Ivarsson R, Renström E, Viertl D, Kasas S, et al. 2006. Tomosyn-1 is involved in a post-docking event required for pancreatic beta-cell exocytosis. J Cell Sci 119:2912–2920
- Côte M, Ménager MM, Burgess A, Mahlaoui N, Picard C, Schaffner C, et al. 2009. Munc18-2 deficiency causes familial hemophagocytic lymphohistiocytosis type 5 and impairs cytotoxic granule exocytosis in patient NK cells. J Clin Invest 119:3765–3773
- de Saint Basile G, Ménasché G, Fischer A. 2010. Molecular mechanisms of biogenesis and exocytosis of cytotoxic granules. Nat Rev Immunol 10:568–579
- D’Orlando O, Zhao F, Kasper B, Orinska Z, Müller J, Hermans-Borgmeyer I, et al. 2013. Syntaxin 11 is required for NK and CD8+ T-cell cytotoxicity and neutrophil degranulation. Eur J Immunol 43:194–208
- Fasshauer D, Sutton RB, Brunger AT, Jahn R. 1998. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci USA 95:15781–15786
- Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, et al. 2003. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell 115:461–473
- Ferro-Novick S, Brose N. 2013. Nobel 2013 Physiology or medicine: traffic control system within cells. Nature 504:98
- Fukuda M. 2002. The C2A domain of synaptotagmin-like protein 3 (Slp3) is an atypical calcium-dependent phospholipid-binding machine: comparison with the C2A domain of synaptotagmin I. Biochem J 366:681–687
- Gholam C, Grigoriadou S, Gilmour KC, Gaspar HB. 2011. Familial haemophagocytic lymphohistiocytosis: advances in the genetic basis, diagnosis and management. Clin Exp Immunol 163:271–283
- Golebiewska EM, Harper MT, Williams CM, Savage JS, Goggs R, Fischer von Mollard G, Poole AW. 2015. Syntaxin 8 regulates platelet dense granule secretion, aggregation, and thrombus stability. J Biol Chem 290:1536–1545
- Golebiewska EM, Poole AW. 2015. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev 29:153–162
- Hackmann Y, Graham SC, Ehl S, Höning S, Lehmberg K, Aricò M, et al. 2013. Syntaxin binding mechanism and disease-causing mutations in Munc18-2. Proc Natl Acad Sci USA 110:E4482–4491
- Haddad EK, Wu X, Hammer JA, Henkart PA. 2001. Defective granule exocytosis in Rab27a-deficient lymphocytes from Ashen mice. J Cell Biol 152:835–842
- Halimani M, Pattu V, Marshall MR, Chang HF, Matti U, Jung M, et al. 2014. Syntaxin11 serves as a t-SNARE for the fusion of lytic granules in human cytotoxic T lymphocytes. Eur J Immunol 44:573–584
- Hellewell AL, Foresti O, Gover N, Porter MY, Hewitt EW. 2014. Analysis of familial hemophagocytic lymphohistiocytosis type 4 (FHL-4) mutant proteins reveals that S-acylation is required for the function of syntaxin 11 in natural killer cells. PLoS One 9:e98900
- Hepp R, Puri N, Hohenstein AC, Crawford GL, Whiteheart SW, Roche PA. 2005. Phosphorylation of SNAP-23 regulates exocytosis from mast cells. J Biol Chem 280:6610–6620
- Hong W. 2005. Cytotoxic T lymphocyte exocytosis: bring on the SNAREs!. Trends Cell Biol 15:644–650
- Houng A, Polgar J, Reed GL. 2003. Munc18-syntaxin complexes and exocytosis in human platelets. J Biol Chem 278:19627–19633
- Hume AN, Seabra MC. 2011. Melanosomes on the move: a model to understand organelle dynamics. Biochem Soc Trans 39:1191–1196
- Iinuma T, Aoki T, Arasaki K, Hirose H, Yamamoto A, Samata R, et al. 2009. Role of syntaxin 18 in the organization of endoplasmic reticulum subdomains. J Cell Sci 122:1680–1690
- Johnson JL, Brzezinska AA, Tolmachova T, Munafo DB, Ellis BA, Seabra MC, et al. 2010. Rab27a and Rab27b regulate neutrophil azurophilic granule exocytosis and NADPH oxidase activity by independent mechanisms. Traffic 11:533–547
- Kasai H, Takahashi N, Tokumaru H. 2012. Distinct initial SNARE configurations underlying the diversity of exocytosis. Physiol Rev 92:1915–1964
- Kimura T, Kaneko Y, Yamada S, Ishihara H, Senda T, Iwamatsu A, Niki I. 2008. The GDP-dependent Rab27a effector coronin 3 controls endocytosis of secretory membrane in insulin-secreting cell lines. J Cell Sci 121:3092–3098
- Kurowska M, Goudin N, Nehme NT, Court M, Garin J, Fischer A, et al. 2012. Terminal transport of lytic granules to the immune synapse is mediated by the kinesin-1/Slp3/Rab27a complex. Blood 119:3879–3889
- Lemons PP, Chen D, Bernstein AM, Bennett MK, Whiteheart SW. 1997. Regulated secretion in platelets: identification of elements of the platelet exocytosis machinery. Blood 90:1490–1500
- Lemons PP, Chen D, Whiteheart SW. 2000. Molecular mechanisms of platelet exocytosis: requirements for alpha-granule release. Biochem Biophys Res Commun 267:875–880
- Low SH, Miura M, Roche PA, Valdez AC, Mostov KE, Weimbs T. 2000. Intracellular redirection of plasma membrane trafficking after loss of epithelial cell polarity. Mol Biol Cell 11:3045–3060
- Marshall MR, Pattu V, Halimani M, Maier-Peuschel M, Müller ML, Becherer U, et al. 2015. VAMP8-dependent fusion of recycling endosomes with the plasma membrane facilitates T lymphocyte cytotoxicity. J Cell Biol 210:135–151
- Martín-Martín B, Nabokina SM, Blasi J, Lazo PA, Mollinedo F. 2000. Involvement of SNAP-23 and syntaxin 6 in human neutrophil exocytosis. Blood 96:2574–2583
- McNew JA, Parlati F, Fukuda R, Johnston RJ, Paz K, Paumet F, et al. 2000. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature 407:153–159
- Mehta RS, Smith RE. 2013. Hemophagocytic lymphohistiocytosis (HLH): a review of literature. Med Oncol 30:740
- Mellman I, Emr SD. 2013. A Nobel Prize for membrane traffic: vesicles find their journey’s end. J Cell Biol 203:559–561
- Ménasché G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, et al. 2000. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet 25:173–176
- Messenger SW, Falkowski MA, Groblewski GE. 2014. Ca2+-regulated secretory granule exocytosis in pancreatic and parotid acinar cells. Cell Calcium 55:369–375
- Mizuno K, Tolmachova T, Ushakov DS, Romao M, Abrink M, Ferenczi MA, et al. 2007. Rab27b regulates mast cell granule dynamics and secretion. Traffic 8:883–892
- Mollinedo F, Calafat J, Janssen H, Martín-Martín B, Canchado J, Nabokina SM, Gajate C. 2006. Combinatorial SNARE complexes modulate the secretion of cytoplasmic granules in human neutrophils. J Immunol 177:2831–2841
- Müller ML, Chiang SCC, Meeths M, Tesi B, Entesarian M, Nilsson D, Wood SM, Nordenskjöld M, Henter JI, Naqvi A, Bryceson YT. 2014. An N-terminal missense mutation in STX11 causative of FHL4 abrogates syntaxin-11 binding to Munc18-2. Front Immunol 4:515
- Naegelen I, Plançon S, Nicot N, Kaoma T, Muller A, Vallar L, Tschirhart EJ, Bréchard S. 2015. An essential role of syntaxin 3 protein for granule exocytosis and secretion of IL-1α, IL-1β, IL-12b, and CCL4 from differentiated HL-60 cells. J Leukoc Biol 97:557–571
- Neeft M, Wieffer M, de Jong AS, Negroiu G, Metz CHG, van Loon A, et al. 2005. Munc13-4 is an effector of rab27a and controls secretion of lysosomes in hematopoietic cells. Mol Biol Cell 16:731–741
- Nichols BJ, Pelham HR. 1998. SNAREs and membrane fusion in the Golgi apparatus. Biochim Biophys Acta 1404:9–31
- Offenhäuser C, Lei N, Roy S, Collins BM, Stow JL, Murray RZ. 2011. Syntaxin 11 binds Vti1b and regulates late endosome to lysosome fusion in macrophages. Traffic 12:762–773
- Pattu V, Qu B, Marshall M, Becherer U, Junker C, Matti U, et al. 2011. Syntaxin7 is required for lytic granule release from cytotoxic T lymphocytes. Traffic 12:890–901
- Pattu V, Qu B, Schwarz EC, Strauss B, Weins L, Bhat SS, et al. 2012. SNARE protein expression and localization in human cytotoxic T lymphocytes. Eur J Immunol 42:470–475
- Pfeffer SR. 2013. A prize for membrane magic. Cell 155:1203–1206
- Polgár J, Chung SH, Reed GL. 2002. Vesicle-associated membrane protein 3 (VAMP-3) and VAMP-8 are present in human platelets and are required for granule secretion. Blood 100:1081–1083
- Prekeris R, Klumperman J, Scheller RH. 2000. Syntaxin 11 is an atypical SNARE abundant in the immune system. Eur J Cell Biol 79:771–780
- Prekeris R, Yang B, Oorschot V, Klumperman J, Scheller RH. 1999. Differential roles of syntaxin 7 and syntaxin 8 in endosomal trafficking. Mol Biol Cell 10:3891–3908
- Reed GL, Houng AK, Fitzgerald ML. 1999. Human platelets contain SNARE proteins and a Sec1p homologue that interacts with syntaxin 4 and is phosphorylated after thrombin activation: implications for platelet secretion. Blood 93:2617–2626
- Schraw TD, Lemons PP, Dean WL, Whiteheart SW. 2003. A role for Sec1/Munc18 proteins in platelet exocytosis. Biochem J 374:207–217
- Sieni E, Cetica V, Mastrodicasa E, Pende D, Moretta L, Griffiths G, Aricò M. 2012. Familial hemophagocytic lymphohistiocytosis: a model for understanding the human machinery of cellular cytotoxicity. Cell Mol Life Sci 69:29–40
- Simonsen A, Bremnes B, Rønning E, Aasland R, Stenmark H. 1998. Syntaxin-16, a putative Golgi t-SNARE. Eur J Cell Biol 75:223–231
- Spessott WA, Sanmillan ML, McCormick ME, Patel N, Villanueva J, Zhang K, et al. 2015. Hemophagocytic lymphohistiocytosis caused by dominant-negative mutations in STXBP2 that inhibit SNARE-mediated membrane fusion. Blood 125:1566–1577
- Stenmark H. 2009. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10:513–525
- Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, Mathew PA, et al. 1999. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science 286:1957–1959
- Sudhof TC. 2004. The synaptic vesicle cycle. Annu Rev Neurosci 27:509–547
- Südhof TC, Rizo J. 2011. Synaptic vesicle exocytosis. Cold Spring Harbor Perspect Biol 3:a005637
- Südhof TC, Rothman JE. 2009. Membrane fusion: grappling with SNARE and SM proteins. Science 323:474–477
- Suh YH, Yoshimoto-Furusawa A, Weih KA, Tessarollo L, Roche KW, Mackem S, Roche PA. 2011. Deletion of SNAP-23 results in pre-implantation embryonic lethality in mice. PLoS One 6:e18444
- Tang BL, Low DY, Hong W. 1998a. Syntaxin 11: a member of the syntaxin family without a carboxyl terminal transmembrane domain. Biochem Biophys Res Commun 245:627–632
- Tang BL, Low DY, Lee SS, Tan AE, Hong W. 1998b. Molecular cloning and localization of human syntaxin 16, a member of the syntaxin family of SNARE proteins. Biochem Biophys Res Commun 242:673–679
- Teng FY, Wang Y, Tang BL. 2001. The syntaxins. Genome Biol 2:REVIEWS3012
- Tolmachova T, Abrink M, Futter CE, Authi KS, Seabra MC. 2007. Rab27b regulates number and secretion of platelet dense granules. Proc Natl Acad Sci USA 104:5872–5877
- Tomas A, Meda P, Regazzi R, Pessin JE, Halban PA. 2008. Munc 18-1 and granuphilin collaborate during insulin granule exocytosis. Traffic 9:813–832
- Valdez AC, Cabaniols JP, Brown MJ, Roche PA. 1999. Syntaxin 11 is associated with SNAP-23 on late endosomes and the trans-Golgi network. J Cell Sci 112(Pt 6):845–854
- Voskoboinik I, Whisstock JC, Trapani JA. 2015. Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol 15:388–400
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, et al. 1998. SNAREpins: minimal machinery for membrane fusion. Cell 92:759–772
- Williams AL, Bielopolski N, Meroz D, Lam AD, Passmore DR, Ben-Tal N, et al. 2011. Structural and functional analysis of tomosyn identifies domains important in exocytotic regulation. J Biol Chem 286:14542–14553
- Xie LX, de la Iglesia-Vicente J, Fang YX, Mollinedo F. 2009. Expression and subcellular localization of syntaxin 11 in human neutrophils. Inflamm Res 58:407–412
- Ye S, Huang Y, Joshi S, Zhang J, Yang F, Zhang G, et al. 2014. Platelet secretion and hemostasis require syntaxin-binding protein STXBP5. J Clin Invest 124:4517–4528
- Ye S, Karim ZA, Al Hawas R, Pessin JE, Filipovich AH, Whiteheart SW. 2012. Syntaxin-11, but not syntaxin-2 or syntaxin-4, is required for platelet secretion. Blood 120:2484–2492
- Yokosuka T, Saito T. 2010. The immunological synapse, TCR microclusters, and T cell activation. Curr Top Microbiol Immunol 340:81–107
- Yu H, Rathore SS, Lopez JA, Davis EM, James DE, Martin JL, Shen J. 2013. Comparative studies of Munc18c and Munc18-1 reveal conserved and divergent mechanisms of Sec1/Munc18 proteins. Proc Natl Acad Sci USA 110:E3271–3280
- Zhang K, Jordan MB, Marsh RA, Johnson JA, Kissell D, Meller J, et al. 2011. Hypomorphic mutations in PRF1, MUNC13-4, and STXBP2 are associated with adult-onset familial HLH. Blood 118:5794–5798
- Zhao XW, Gazendam RP, Drewniak A, van Houdt M, Tool ATJ, van Hamme JL, et al. 2013. Defects in neutrophil granule mobilization and bactericidal activity in familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) syndrome caused by STXBP2/Munc18-2 mutations. Blood 122:109–111
- Zhu Q, Yamakuchi M, Ture S, de la Luz Garcia-Hernandez M, Ko KA, Modjeski KL, et al. 2014. Syntaxin-binding protein STXBP5 inhibits endothelial exocytosis and promotes platelet secretion. J Clin Invest 124:4503–4516
- Zur Stadt U, Rohr J, Seifert W, Koch F, Grieve S, Pagel J, et al. 2009. Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in Munc18-2 and impaired binding to syntaxin 11. Am J Hum Genet 85:482–492
