Abstract
“Perinatal programming” is a phenomenon describing how early life environmental conditions can produce long-term physiological alterations that either enhance or inhibit adaptive functioning. Previously, we have demonstrated that neonatal exposure to lipopolysaccharide (LPS) predisposes to anxiety-like behaviour in later life, which was associated with changes to the neuroendocrine response to stress. Given the known interactions between the neuroendocrine and neuroimmune systems, here we investigated whether neonatal exposure to a bacterial mimetic alters neuroimmune responses to acute stress in adulthood. Male and female Wistar rats were administered LPS (0.05 mg/kg, i.p.), or saline vehicle (equivolume) on days 3 and 5 post-partum. One group of rats was euthanised following early life treatment to assess immediate hypothalamic–pituitary–adrenal axis and central cytokine responses to treatment. A second group was assessed in adulthood (85 days) following exposure to either a “stress” (30-min restraint) or “no stress” condition. Blood was collected from all rats at baseline, 30, 60 and 90 min after “stress”, “no stress” treatment to assess peripheral corticosterone responses, and brains were collected 180 min following baseline to assess hippocampal content of interleukin-1β (IL-1β), tumour necrosis factor-α (TNFα) and IL-6 protein. Radioimmunoassay revealed that neonatal LPS treatment resulted in a prolonged corticosterone response to stress in adulthood compared to controls (p < 0.05). Enzyme-linked-immunosorbent assays revealed no group differences in hippocampal IL-6 content. However, brain IL-1β and TNFα protein concentrations were significantly greater in rats neonatally exposed to LPS and then exposed to stress in adulthood when compared to all other groups (p < 0.05). These findings suggest that early life bacterial toxin exposure results in a prolonged neuroendocrine response to acute stress in adulthood, which may be a consequence of increased release of IL-1β and TNFα in the brain.
Introduction
Recent investigations have demonstrated a close interaction between the neuroendocrine and neuroimmune systems, whereby bidirectional communication between these systems is evidently integral in the regulation of endocrine (Zhu et al. Citation1999), pain (Moldofsky Citation1995; Kostarczyk Citation2000), metabolic (Nilsson et al. Citation2002; Owens et al. 2006) and immune (Li et al. Citation2004, Cutolo et al. Citation2006) functioning. In addition to these essential processes, the response to stress also involves complex neuroimmune–neuroendocrine interactions (Sternberg and Licinio Citation2006). While the impact of stress on immune outcomes has been well characterised (Webster Marketon and Glaser Citation2008), the ability for the immune system to modulate stress responsivity is less well understood.
Provocative findings in the recent animal literature have pointed towards potential cytokine-specific regulation of the hypothalamic–pituitary–adrenal (HPA) axis response to stress with interleukin -1β (IL-1β), tumour necrosis factor-α (TNFα), and IL-6 being putative primary candidates. Regulation of HPA activity by these cytokines has been demonstrated by their ability to increase plasma adrenocorticotropic hormone and corticosterone concentrations, a process initiated by stimulation of corticosterone-releasing hormone release from the hypothalamus. Furthermore, these effects can be dissociated from pyrogenic actions of cytokines (Turnbull and Rivier Citation1995).
Examination of central cytokine upregulation (e.g. IL-1β mRNA or protein) has also been reported in rodents in response to inescapable foot shock in HPA-related regions including the hypothalamus, hippocampus and pituitary (Nguyen et al. Citation1998, Citation2000; O'Connor et al. Citation2003). Intraperitoneal administration of bacterial endotoxin (lipopolysaccharide, LPS) to adult rodents has similarly demonstrated increases in hippocampal IL-1β in a dose-dependent fashion (Nguyen et al. Citation1998). Inconsistency, however, has been observed in relation to central TNFα and IL-6 following stress with mixed findings resulting in greater uncertainty as to the actions of these cytokines (Takao et al. Citation1997; O'Connor et al. Citation2003). Changes in central and peripheral cytokine abundance have been, however, consistently demonstrated to coincide with behavioural changes and neuroendocrine perturbations (Nguyen et al. Citation1998, Citation2000; Song et al. Citation2003).
The nature of these interactions between the endocrine and immune systems is determined early in life. It is well established that the ratio of T helper 1 (Th1) to 2 (Th2) immune cells is altered when there is exposure to high levels of corticosterone early in life (Knackstedt et al. Citation2005; Coe and Lubach Citation2006). Likewise, it is believed that factors involved in determining the functional tone of the HPA axis [i.e. the number of glucocorticoid receptors (GRs)] and onset of expression of GRs and mineralocorticoid receptors are determined early in life by exposure to specific cytokines (Reul et al. Citation1994; CitationShanks and Lightman 2001). An appropriately balanced ratio of Th1 to Th2 immune cells is important in preventing polarisation away from the normal range of immune responses to infection in adults (Prescott and Dunstan Citation2006). Glucocorticoids play a role in setting the balance of these cells and thus contribute to the functional tone of the immune system by altering the hormonal milieu during early life. It is less clear how the immune system could developmentally alter the HPA axis given the generally restricted access of immune cells to the central nervous system.
The developing HPA axis is highly susceptible to early life environmental perturbations (CitationShanks et al. 2000), and peripheral immunomodulators have been shown to be associated with central upregulation of cytokines (Bilbo et al. Citation2005, Citation2007). In the present study, therefore, we investigated whether neonatal LPS exposure could alter central immune functioning in an HPA axis-related region of the brain following exposure to a psychological stressor in adulthood, thus revealing a neuroimmune role in perinatal programming of the HPA axis.
We have previously demonstrated both behavioural (Walker et al. Citation2004a, Citation2008, Citation2009) and neuroendocrine (Hodgson et al. Citation2001; Hodgson and Knott Citation2002; Walker et al. Citation2008, Citation2009) perturbations in adulthood following exposure of rats to 0.05 mg/kg bacterial endotoxin (LPS) on days 3 and 5 of post-natal life. Importantly, our findings have demonstrated that although neonatal exposure to LPS alone can be sufficient in producing later life alterations to physiology and behaviour (Walker et al. Citation2004a), such alterations are more consistently observable, and indeed amplified, when animals are exposed to a subsequent stressor in later life (Walker et al. Citation2009). These findings are consistent with the double-hit hypothesis of psychopathology (Maynard et al. Citation2001). The double-hit hypothesis posits that a genetic or environmental insult during early life disrupts neural development establishing a susceptibility to a “second hit” in later life. In line with this hypothesis, we examined whether exposure of rats to LPS during the neonatal period, when combined with acute restraint stress in adulthood, coincided with changes in hippocampal IL-1β, IL-6 and TNFα protein regulation in both early and later life.
Materials and methods
Animals
Thirty-one experimentally naïve female Wistar rats obtained from the University of Newcastle animal house were mated in the University of Newcastle Psychology vivarium resulting in a total of 316 (146 males and 170 females) offspring, which were used in this study. Pregnant females were caged separately, and at birth [postnatal day (PND) 1], litters were randomly allocated into either LPS or saline control conditions. No significant difference in litter size was observed between litters allocated to LPS (M = 13.25 pups, SD = .25) or saline (M = 12.71 pups, SD = 3.35). On PND 3 and PND 5, rats were briefly removed from their home cages, weighed and administered either LPS or saline. To determine whether neonatal LPS administration was effective in activating the neonatal HPA axis, a subgroup of rats (61 males and 68 females) derived from 14 litters was killed either on PND 3 or PND 5 following neonatal treatment to assess corticosterone responses. The remaining litters were left with their dams until weaning (PND 22), at which point rats were segregated into same-sex paired housing (41.5 × 28.0 × 22.0 cm cages; Mascot Wire Works, Sydney, Australia). With the exception of a weekly weighing, rats were left undisturbed from weaning until behavioural testing in adulthood (PND 85). Rats were maintained in a temperature-(21 ± 1°C) and humidity (34 ± 2)-controlled environment, under a 12-h light-12-h dark schedule (lights on 06:00 h). Food (Rat and Mouse Pellets, Glen Forest, Western Australia) and water were available ad libitum. All experimentation occurred in accordance with the 2004 National Health and Medical Research Council Australian code of practice for the care and use of animals for scientific practice.
Neonatal treatments
On PND 3 and PND 5, rats were removed from their home cage, placed in an incubator maintained at 34°C to control for loss of body temperature, and administered either 0.05 mg/kg LPS [Salmonella enterica, serotype Enteritidis (Sigma-Aldrich Chemical Co., St Louis, MO, USA, dissolved in 20-μl sterile pyrogen-free saline] or an equivolume of non-pyrogenic 0.9% saline (Livingstone International, Australia) via intraperitoneal microinjection. All injections were administered between 09:00 and 10:00 h, and litters were immediately returned to their housing following drug administration. This model has been employed previously in this (Walker et al. Citation2004a, Citation2006, Citation2008, Citation2009) and other laboratories (CitationShanks et al. 1995; Breivik et al. Citation2002; Nilsson et al. Citation2002), with the dosage and timing of exposure having been shown to produce a rapid, sustained febrile response, but no mortality.
Neonatal blood and hippocampal collection
A subgroup of rats was killed during neonatal life in order to assess serum corticosterone and hippocampal cytokine responses to treatment. Four hours following injection on PND 5, trunk blood or brains were collected. Collection of trunk blood occurred via rapid decapitation without anaesthesia (LPS: males n = 9, females n = 10; saline: males n = 14, females n = 18), and brains were removed following a 20-μl i.p. injection of Lethabarb (Virbac, Pty. Ltd, Milperra, Australia) and 0.9% saline cardiac perfusion (LPS: males n = 12, females n = 16; saline: males n = 13, females n = 16). Brains were rapidly dissected and the hippocampus was separated and snap frozen in liquid nitrogen. Tissue was stored at − 80°C until processed.
Estrous cycle in females
Estrous cyclicity for females was monitored in adulthood using a Rat Vaginal Impedance Checker (Muromachi Kikai, Tokyo, Osaka) according to the manufacturer's instructions. Briefly, the impedance probe was sterilised with diluted ethyl alcohol and inserted into the vagina. The electrical impedance of the epithelial cell layer of vaginal mucosa was measured at a frequency of 1 kHz. Cyclicity was monitored over several days to determine peaks and troughs in the readout, thus providing an accurate profile of each female's cycle. Females were tested only in the diestrous phase to limit the possibility of a ceiling effect as it is known that basal corticosterone concentrations are significantly greater during proestrus (Mitsushima et al. Citation2003).
Stress protocol
In adulthood, rats were randomly allocated into either a “stress”, or “no stress” condition such that there were four groups for each sex: (1) rats exposed to neonatal LPS and stress in adulthood (nLPS/stress; males n = 10, females n = 9), (2) rats exposed to neonatal LPS and no stress in adulthood (nLPS/no stress; males n = 8, females n = 9), (3) rats exposed to neonatal 0.9% saline and stress in adulthood (nSAL/stress; males n = 9, females n = 8) and (4) those exposed to neonatal 0.9% saline and no stress in adulthood (nSAL/no stress; males n = 8, females n = 7). These allocations were made across litters to avoid nesting effects. Rats allocated to the stress condition underwent 30 min of acute stress exposure starting on PND 85. This consisted of one 30-min session of restraint stress. The restraint apparatus was constructed using soft wire mesh (25.0 × 20.0 cm) folded around the rat and secured using butterfly clips to restrict ambulatory movement. We have previously successfully employed a wire mesh restraining device in this manner (Walker et al. Citation2009). Rats in the “no stress” condition remained undisturbed except for blood collection at equivalent times to those rats in the “stress” condition.
Resistance to restraint
Computer-automated tracking software (Motion Mensura Pty Ltd, Newcastle, Australia) was employed to measure the amount of activity during restraint, described in Walker et al. (Citation2009). Briefly, the actual dimensions of the apparatus were calibrated to pixel dimensions by the computer, which monitored changes in pixel brightness intensity as the basis for measuring the centre of gravity of the rats’ movements. Movements in the centre of gravity were reconverted back to actual distance, expressed in millimetres. Testing threshold for detection examines only the significant movement, thus providing an accurate measure of time spent in movement. This was used to provide a behavioural indication of group differences in response to restraint.
Blood sampling and radioimmunoassay procedures
Rats allocated for assessment of HPA axis activation following neonatal treatment were rapidly decapitated, without anaesthetic, 4 h after injection on PND 5, and trunk blood was collected into EDTA-coated tubes. Blood was collected from adult males via the saphenous vein at baseline, immediately following completion of exposure to restraint (30 min), and at 60 and 90 min following baseline. For rats not exposed to restraint stress, blood was collected at identical time points. Blood was collected by briefly ( < 30 s) restraining the rat and puncturing the saphenous vein using a sterile 21 g needle and collecting ∼250μl of blood into EDTA-coated tubes (Livingstone International, Australia). Rats were habituated to the procedure by brief exposure to handling and restraint prior to collection. All blood samples were centrifuged at 1000g for 20 min at 4°C, and plasma was stored at − 20°C until assayed. Plasma corticosterone concentrations were assessed using a rat corticosterone 125I radioimmunoassay kit (MP Biomedicals, CA, USA). The recovery of free corticosterone was 100%, with a mean inter- and intra-assay variability of 4.4 and 6.5%, respectively.
Tissue processing and cytokine analysis
Tissue processing and cytokine analysis were adapted from Nguyen et al. (Citation1998). To ensure that any IL-1β, TNFα or IL-6 measured was from hippocampal tissue and not from blood, all rats were deeply anaesthetised via i.p. injection with 1 ml of lethabarb (Virbac, Pty. Ltd, Milperra, Australia) and cardiac perfused with 0.9% pyrogen-free saline. Brains were quickly removed and whole hippocampi were dissected and snap frozen in liquid nitrogen. Samples were stored at − 80°C until processing, at which time each tissue was added to 0.25–0.3 ml of Dulbeccoi's Iscoves culture medium (Sigma-Aldrich, Sydney, Australia) containing 10% cocktail protease inhibitor (Sigma-Aldrich, Sydney, Australia). Total protein was mechanically dissociated from tissue using an ultrasonic cell disruptor (Polytron P2100; Kinematica AG, Switzerland) for 10 s at the highest setting. Sonicated samples were then centrifuged at 15 000g at 4°C for 10 min. The supernatant was removed and stored at − 20°C until enzyme-linked-immunosorbent assays (ELISAs) were performed for IL-1β, TNFα and IL-6. Bradford protein assays (Bradford reagent obtained from Sigma-Aldrich, Sydney, Australia) were performed to determine total protein concentration in sonicated samples.
IL-1β, TNFα and IL-6 concentrations were assessed using a Quantikine Rat IL-1β, Quantikine Rat TNFα Immunoassay and Quantikine Rat IL-6 kit, respectively (R&D Systems, USA). ELISAs were performed according to kit instructions. The mean recovery of IL-1β in cell culture was reported to be 100% with a mean inter- and intra-assay variability of 4.73 and 5.53%, respectively. The mean recovery of TNFα in cell culture was reported to be 101% with a mean inter- and intra-assay variability of 9.36 and 3.13% respectively. The mean recovery of IL-6 in cell culture was reported to be 103% with a mean inter- and intra-assay variability of 8.36 and 7.36%, respectively.
Data analysis
Data were analysed using the Statistical Package for the Social Sciences for Windows, Version 17. Analyses of covariance (ANCOVA), treating litter size and male to female litter ratio as the covariates, were conducted for all analyses. Litter effects were controlled for by nesting litter into treatment for each ANCOVA model. Planned comparisons between experimental conditions were performed using Bonferroni's α correction to p < 0.05 and t-test analyses where significant interactions were observed; t-tests were corrected for multiple comparisons to p < 0.05.
Results
Developmental weight gain
Neonatal weight gain
A significant interaction between neonatal treatment and sex was observed for neonatal weight gain, F(1, 81) = 8.3, p < 0.001. Planned comparisons revealed that females given LPS gained significantly less weight between PND 3 and 5 (M = 2.33 g, SEM = 0.1, n = 35) compared to females given saline (M = 2.82 g, SEM = 0.15, n = 23), t0.5(56) = 2.16, p < 0.05. Although a similar trend was replicated in the males, it failed to reach statistical significance. Litter size and male to female ratio were not significant covariates.
Weight gain from weaning to adulthood
A significant sex × age interaction, F(2.47, 377.40) = 353.38, p < 0.001 was observed for weight gain from weaning (PND 22) to adulthood (PND 85). Planned comparisons revealed that weight differences between the sexes occurred at adolescence (PND 36), whereby males were significantly heavier than females (p < 0.05, for all). Weight gain differed between neonatal treatment groups for male rats between both weaning (PND 22) and adolescence [PND 50; F(1, 127) = 33.28, p < 0.001], as well as adolescence and adulthood [PND 85; F(1, 153) = 4.30, p < 0.05] with LPS-treated males gaining significantly less weight during each period (M = 204.5 g, SEM = 2.23, n = 40; and M = 148.3 g, SEM = 4.93, n = 40, respectively) compared to saline-treated males (M = 212.7 g, SEM = 3.0, n = 32; and M = 171.2 g, SEM = 4.6, n = 32, respectively), p < 0.05 for all comparisons. No difference in weight gain was observed between neonatal treatment groups for females. Litter size and male to female ratio were not significant covariates.
Neonatal corticosterone response to treatment
Assessment of corticosterone responses four hours following neonatal treatment on day 5 yielded a significant effect of neonatal treatment, F(1, 42) = 4.92, p < 0.05. Rats neonatally treated with LPS displayed significantly greater plasma corticosterone concentrations (M = 24.8 ng/ml, SEM = 1.22, n = 19) compared to saline-treated controls (M = 21.3 ng/ml, SEM = 1.03, n = 27). Litter size and male to female ratio were not significant covariates.
Activity during restraint in adulthood
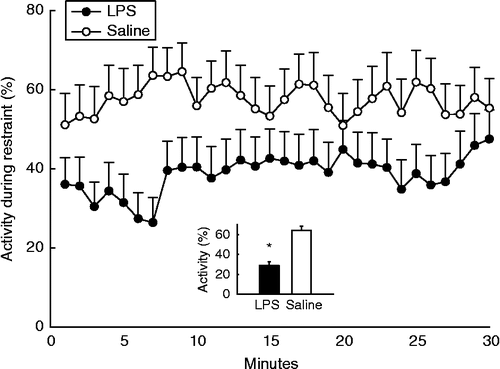
Rats treated neonatally with LPS spent a significantly lower percentage of time (M = 28.8%, SEM = 4.03, n = 29) resisting restraint in adulthood compared to their saline-treated counterparts (M = 64.1%, SEM = 4.28, n = 25), F(1, 43) = 36.29, p < 0.001; ). No sex differences were observed, and litter size and male to female ratio were not significant covariates.
Figure 1. Effect of neonatal LPS exposure on the mean time per minute (%; ± SEM) spent as adults resisting acute restraint. Filled circles represent rats neonatally challenged with LPS (n = 29) and open circles represent rats neonatally challenged with saline (n = 25). A repeated measures nested ANCOVA revealed a significant main effect of neonatal treatment across each minute spent in restraint, indicated by the inset graph which represents the mean ± SEM overall time spent resisting restraint, *p < 0.05.
Adult corticosterone response
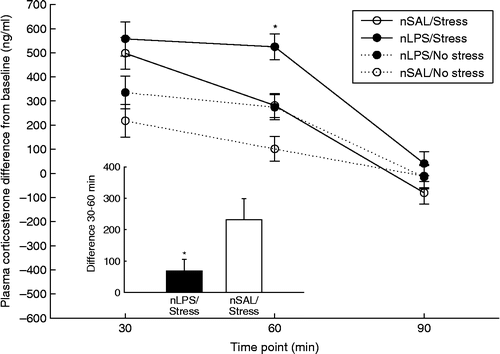
There was a significant interaction between neonatal treatment and adult stress for the difference in corticosterone concentrations from baseline to 30, 60 and 90 min in adulthood [F(2, 80) = 1.93, p < 0.05; . LPS- and saline-treated rats exposed to restraint stress in adulthood demonstrated significantly greater peak corticosterone responses at 30 min compared to their no-stress counterparts: nLPS/stress vs. nLPS/no stress [t0.5(34) = 5.77, p < 0.05]; nSAL/stress vs. nSAL/no stress (t0.5(30) = 6.88, p < 0.05). Planned comparisons revealed no differences between neonatal treatment groups exposed to stress in adulthood at 30 min, but the corticosterone response from baseline was significantly greater for LPS-treated rats exposed to restraint stress in adulthood at 60 min compared to all other groups: nLPS/stress vs. nSAL/stress [t0.5(34) = 6.31, p < 0.05]; nLPS/stress vs. nLPS/no stress [t0.5(34) = 6.50, p < 0.05]; nLPS/stress vs. nSAL/no stress [t0.5(32) = 10.99, p < 0.05]. Furthermore, the decline from peak corticosterone levels at 30–60 min was significant for saline-treated rats exposed to stress in adulthood [t0.5(16) = 5.31, p < 0.05], but LPS-treated rats exposed to stress in adulthood did not exhibit significant attenuation of the stress response at this time point. Plasma corticosterone concentrations returned to baseline by 90 min in all groups.
Figure 2. Effect of neonatal LPS exposure on plasma corticosterone concentration changes from baseline (ng/ ml; mean ± SEM) in response to acute restraint stress. Filled circles represent adult rats neonatally challenged with LPS (nLPS) and exposed to either stress (n = 19) or no stress (n = 17) in adulthood, and open circles represent adult rats neonatally challenged with saline (nSAL) and exposed to either stress (n = 16) or no stress (n = 15) in adulthood. A repeated measures nested ANCOVA revealed nLPS-treated rats to have significantly greater plasma corticosterone concentrations at 60 min following baseline when exposed to restraint stress in adulthood compared to all other groups represented by *p < 0.05. The inset graph represents the change in plasma corticosterone concentration between 30 and 60 min for rats neonatally challenged with LPS or saline and exposed to restraint stress in adulthood as measured by Bonferroni post-hoc comparisons and t-test adjustments for multiple comparisons, *p < 0.05.
Finally, a significant effect of sex across time was observed for corticosterone differences from baseline [F(2, 80) = 68.70, p < 0.001]; the difference from baseline for corticosterone concentrations in males remained significantly greater (M = 129.7 ng/ml, SEM = 32.72, n = 36) than females at 90 min [M = − 157.2 ng/ml, SEM = 35.47, n = 32), t0.5(66) = 2.67, p < 0.05]. Litter size and male to female ratio were not significant covariates.
Hippocampal cytokine content in adulthood
Hippocampal IL-1β
With regard to adult hippocampal IL-1β content, a significant neonatal treatment × adult stress × sex interaction was observed, F(1,43) = 2.03, p < 0.05 (). Planned comparisons revealed that both male and female rats treated with LPS during neonatal life combined with restraint stress in adulthood exhibited significantly higher hippocampal IL-1β/ total protein ratios compared to all other groups: male nLPS/stress vs. male nSAL/stress [t0.5(27) = 2.92, p < 0.05]; male nLPS/stress vs. male nLPS/no stress [t0.5(21) = 3.22, p < 0.05]; male nLPS/stress vs. male nSAL/no stress [t0.5(18) = 5.50, p < 0.05]; female nLPS/stress vs. female nSAL/stress [t0.5(14) = 6.67, p < 0.05]; female nLPS/stress vs. female nLPS/no stress [t0.5(19) = 4.66, p < 0.05]; female nLPS/stress vs. female nSAL/no stress [t0.5(13) = 4.62, p < 0.05].
Figure 3. A–D represent significant effects of neonatal LPS exposure combined with acute restraint stress in adulthood on hippocampal cytokine contents (Mean ± SEM). A & B represent IL-1β/total protein ratios for males and females respectively. Filled bars represent rats neonatally challenged with LPS and exposed to either restraint stress (males n = 14, females n = 9) or no stress (males n = 9, females n = 12) in adulthood, and open bars represent rats neonatally challenged with saline and exposed to either restraint stress (males n = 9, females n = 7) or no stress (males n = 6, females n = 6) in adulthood. A between-subjects nested ANCOVA revealed male and female rats neonatally treated with LPS exhibited significantly greater hippocampal IL-1β content in reponse to restraint stress in adulthood compared to all other groups, *p < 0.05. C & D represent TNFα/total protein ratios for males and females respectively. Filled bars represent rats neonatally challenged with LPS exposed to either restraint stress (males n = 9, females n = 11) or no stress (males n = 9, females n = 16) in adulthood, and open bars represent rats neonatally challenged with saline and exposed to either restraint stress (males n = 14, females n = 6) or no stress (males n = 6, females n = 7) in adulthood. A between-subjects nested ANCOVA revealed male rats neonatally treated with LPS exhibited significantly greater hippocampal TNFα content in response to restraint stress in adulthood compared to all other groups, *p < 0.05.
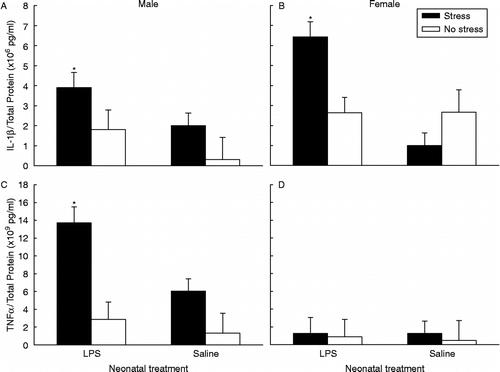
The only difference between the sexes existed for LPS-treated females and LPS-treated males exposed to stress in adulthood, whereby females exhibited significantly greater IL-1β/ total protein ratios compared to males, t0.5(21) = 3.87, p < 0.05. Litter size and male to female ratio were not significant covariates.
Hippocampal TNFα
A significant neonatal treatment × adult stress × sex interaction was observed [F(1,43) = 2.91, p < 0.01] for adult hippocampal TNFα protein levels after the significant covariates of litter size [F(1,68) = 10.62, p < 0.01] and male to female ratio [F(1,68) = 4.11, p < 0.05] were accounted for (). Planned comparisons revealed that males treated with LPS in neonatal life combined with restraint stress in adulthood exhibited significantly higher hippocampal TNFα/total protein ratios compared to all other groups: male nLPS/stress vs. male nSAL/stress (t0.5(21) = 3.67, p < 0.05); male nLPS/stress vs. male nLPS/no stress [t0.5(16) = 5.21, p < 0.05]; male nLPS/stress vs. male nSAL/no stress [t0.5(13) = 5.94, p < 0.05]. TNFα protein ratios of females remained at baseline levels for all groups.
Hippocampal IL-6
As for IL-1β and TNFα, assessment of adult hippocampal IL-6 was conducted using a 2 neonatal treatment × 2 sex × 2 adult stress ANCOVA with litter size and male to female ratio used as covariates. No differences were observed in hippocampal IL-6 concentrations (data not shown). Litter size and male to female ratio were not significant covariates.
Discussion
The present study demonstrates that neonatal LPS treatment alters hippocampal concentrations of proinflammatory cytokines IL-1β and TNFα, as well as HPA axis functioning as determined by plasma levels of corticosterone in adulthood. That is, known cytokine activation during periods of restraint stress in adulthood appears to be differentially expressed in the hippocampus following early life LPS challenge.
Developmental weight gain following neonatal LPS exposure
Weight gain was monitored to determine if any spurious effects observed were attributable to growth disturbances. LPS-treated females gained significantly less weight compared to their saline-treated counterparts following LPS exposure on PND 3, consistent with previous findings in our laboratory (Walker et al. Citation2004b). This effect was reflected in the males but failed to reach statistical significance until weaning, at which point males treated with LPS during neonatal life gained significantly less weight throughout adolescence and adulthood compared to saline-treated males. Weight gain differences between neonatal treatment groups in females, however, did not differ in later life as the early post-natal life effects of LPS exposure on growth persisted between treatment groups throughout development.
Neonatal corticosterone response to LPS
LPS treatment significantly increased circulating corticosterone concentrations in the neonate following exposure on PNDs 3 and 5, consistent with previous findings (Walker et al. Citation2004a, Citation2009). This demonstrates LPS to be an immunological stressor capable of activating the HPA axis in the neonate during the stress hyporesponsive period (SHRP). The SHRP, which occurs following birth and is characterised by a rapid reduction in corticosterone levels during early neonatal life, is considered to facilitate the development of neural structures that are sensitive to glucocorticoids (Arborelius et al. Citation1999), and likely explains the relatively feeble corticosterone response of the rats observed during this period.
Corticosterone response to acute psychological stress in adulthood
Assessment of plasma corticosterone concentration differences from baseline revealed a significant interaction between neonatal and adult treatment across time, whereby neonatal LPS exposure prolonged the peak corticosterone secretion in response to restraint stress in adulthood compared to all other groups. These findings are consistent with the “double hit hypothesis”, whereby the impact of the environmental immunological stress, in this case LPS exposure, was amplified by the subsequent later life restraint stress such that recovery from the stress response did not occur until 90 min following baseline. However, cessation of the stress response was evident in saline-treated rats exposed to stress in adulthood a full 30 min earlier. This finding may represent a fundamental difference in the recovery of the HPA axis response to stress in these rats, and is reflective of previous studies demonstrating neonatal LPS exposure to produce long-term alterations in HPA axis functioning (CitationShanks et al. 1995, Citation2000; Hodgson and Knott Citation2002; Walker et al. Citation2008).
Importantly, comparison of males and females indicated that the prolonged sustainment of peak corticosterone concentrations in neonatally LPS-treated rats compared to saline-treated controls may be more pronounced for males as they took significantly longer to return to baseline concentrations than females. Sex differences in relation to HPA axis responsivity to a variety of stimuli are not uncommon in both the animal (Abelson, et al. Citation2005) and human literature (Hardie et al. Citation2002).
Hippocampal cytokine content
The immediate effect of neonatal treatment on protein expression of hippocampal cytokines IL-1β, TNFα and IL-6 was assessed. However, levels of these cytokines were below the minimum detectability of the ELISA. In adulthood, no differences in IL-6 content were observed between neonatal or adult treatment conditions. This is consistent with previous reports of no change in central IL-6 mRNA or protein levels following psychological stress in adulthood (O'Connor et al. Citation2003). The present results indicate that early life LPS exposure does not amplify central IL-6 responses to acute stress in adulthood.
Notably, a significant interaction between sex, neonatal, and adult treatment was observed for both hippocampal IL-1β and hippocampal TNFα protein contents. Both males and females neonatally treated with LPS demonstrated significantly greater IL-1β/ total protein ratios in response to an emotional stressor in adulthood compared to all other treatment groups. As with the corticosterone responses of these animals, these findings are consistent with the “double hit hypothesis”, whereby the cytokine levels were amplified following the combination of both neonatal and adult stress. Interestingly, stress in adulthood alone did not produce an increase in hippocampal IL-1β protein content, as has been previously reported (Nguyen et al. Citation1998; Citation2000; O'Connor et al. Citation2003). This difference is likely attributable to differences in the nature of the stressor used.
Amplification following only combined neonatal LPS exposure and restraint stress in adulthood was also observed for hippocampal TNFα content. However, only males that were neonatally exposed to LPS demonstrated significantly greater TNFα/total protein ratios in response to stress in adulthood. Unlike for IL-1β, no difference was observed among female treatment conditions. Such immune-related sexual dimorphism has been widely demonstrated among a variety of measures (Tenk et al. Citation2008). Furthermore, this is one of the few studies to report a significant change in central TNFα protein concentrations in response to a psychological stressor. Madrigal et al. (Citation2002) previously reported an increase in cortical TNFα release, but other studies have not observed significant change in brain TNFα protein or mRNA levels in response to psychological stress (Plata-Salaman et al. Citation2000; O'Connor et al. Citation2003).
Sexually dimorphic effects
Both males and females exposed to neonatal LPS and adulthood restraint stress exhibited increased hippocampal IL-1β and prolonged peak corticosterone responses to stress in adulthood. However, hippocampal TNFα changes differed depending on sex. The combined insults of neonatal LPS exposure and adult restraint stress elevated TNFα levels in the males, but not in females. This finding may be closely associated with the sex differences in corticosterone secretion observed, which indicated more sustained corticosterone secretion in males following restraint stress. If hippocampal TNFα responses followed that of the corticosterone responses, then in females there might have been a transient increase in TNFα content in these rats that subsided before brains were collected for analysis. Differences in gonadal hormones between males and females may be largely responsible for the sexually dimorphic effects observed in regard to stress responsivity (Young et al. Citation2001), and estradiol has been suggested to mediate many of the effects of stress (MacNiven et al. Citation1990). The possibility of corticosterone or oestrogen effects on hippocampal TNFα responses to stress was not further investigated in the present study.
Conclusion
The impact of neonatal bacterial endotoxin exposure on later life stress responsivity demonstrated marked alterations for both peripheral corticosterone and central cytokine production. Neonatal LPS exposure resulted in prolonged corticosterone secretion, and likely delayed negative-feedback, in response to acute stress in adulthood. Furthermore, the associated change in IL-1β and TNFα, but not IL-6 for rats exposed to LPS in early life indicates the potential for central cytokine-specific upregulation in modulating such endocrine perturbations. The increase in hippocampal IL-1β and TNFα content was evident only when the double-hit model was employed (i.e. neonatal LPS exposure and acute restraint stress in adulthood), suggestive of the cumulative effects of stressors throughout the lifespan. Given the known roles of proinflammatory cytokines in regulating HPA axis activity (CitationTurnbull and Rivier 1995), it is possible that the functional alterations observed in hippocampal IL-1β and TNFα content may exert long-lasting changes in neuroendocrine regulation. However, whether the mechanistic action is a result of peripheral cytokine trafficking to the brain, or centrally derived “neurotransmitter-like” actions remain unknown in this model.
Acknowledgements
We sincerely acknowledge Eleanor Huber and all conjoint BSAF staff for their assistance in maintaining animal requirements.
Declaration of interest The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abelson KS, Adem B, Royo F, Carlsson HE, Hau J. 2005. High plasma corticosterone levels persist during frequent automatic blood sampling in rats. In Vivo. 19:815–819.
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. 1999. The role of corticotrophin releasing factor in depression and anxiety disorders. J Endocrinol. 160:1–12.
- Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF. 2005. Neontal infection induces memory impairments following an immune challenge in adulthood. BehavNeurosci. 119:293–301.
- Bilbo SD, Newsum NJ, Sprunger DB, Watkins LR, Rudy JW, Maier SF. 2007. Differential effects of neonatal handling on early life infection-induced alterations in cognition in adulthood. Brain Behav Immun. 21:332–342.
- Breivik T, Stephan M, Brabant GE, Straub RH, Pabst R, von Horsten S. 2002. Postnatal lipopolysaccharide-induced illness predisposes to periodontal disease in adulthood. Brain Behav Immun. 16:421–438.
- Coe CL, Lubach GR. 2006. Prenatal influences on immunity and the developmental trajectory of infant primates. In: Hodgson DM, Coe CL. editors. Perinatal programming: Early life determinates of adult health & disease. London: Taylor & Francis. 132–142.
- Cutolo M, Sulli A, Pizzorni C, Secchi ME, Soldano S, Seriolo B, Straub RH, Otsa K, Maestroni GJ. 2006. Circadian rhythms: Glucocorticoids and arthritis. Ann NY Acad Sci. 1069:289–299.
- Hardie TL, Moss HB, Vanyukov MM, Yao JK, Kirillovac GP. 2002. Does adverse family environment or sex matter in the salivary cortisol responses to anticipatory stress?. Psychiatry Res. 112:121–131.
- Hodgson DM, Knott B. 2002. Potentiation of tumor metastasis in adulthood by neonatal endotoxin exposure: Sex differences. Psychoneuroendocrinology. 27:791–804.
- Hodgson DM, Knott B, Walker FR. 2001. Neonatal endotoxin exposure influences HPA responsivity and impairs tumor immunity in Fischer 344 rats in adulthood. Pediatric Research. 50:750–755.
- Knackstedt MK, Hamelmann E, Arck PC. 2005. Mothers in stress: Consequences for the offspring. Am J Reprod Immunol. 54:63–69.
- Kostarczyk E. 2000. Immunomodulation of pain. Postepy Hig Med Dosw. 54:445–466.
- Li S, Lu A, Li B, Wang Y. 2004. Circadian rhythms on hypothalamic-pituitary-adrenal axis hormones and cytokines of collagen induced arthritis in rats. J Autoimmun. 22:277–285.
- MacNiven E, de Catanzaro D, Goodison T. 1990. Enhanced maternal estrogen: Possible mediator of stress induced pregnancy blocks. Neuroendocrinol Lett. 12:300.
- Madrigal JLM, Hurtado O, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, Bosca L, Leza JC. 2002. The increase in TNF-α levels is implicated in NF-κB activation and inducible nitric oxide synthase expression in brain cortex after immobilisation stress. Neuropsychopharmacology. 26:155–163.
- Maynard TM, Sikich L, Lieberman JA, Lamantia AS. 2001. Neural development, cell-cell signalling, and the “two-hit” hypothesis of schizophrenia. Schizophr Bull. 27:457–476.
- Mitsushima D, Masuda J, Kimura F. 2003. Sex differences in the stress-induced release of acetycholine in the hippocampus and corticosterone from the adrenal cortex in rats. Neuroendocrinology. 78:234–240.
- Moldofsky H. 1995. Sleep, neuroimmune and neuroendocrine functions in fibromyalgia and chronic fatigue syndrome. Adv Neuroimmunol. 5:39–56.
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins M, Watkins LR, Maier SF. 1998. Exposure to acute stress induces brain interleukin-1β protein in the rat. J Neurosci. 18:2239–2246.
- Nguyen KT, Deak T, Will MJ, Hansen MK, Hunsaker BN, Fleshner M, Watkins LR, Maier SF. 2000. Timecourse and corticosterone sensitivity of the brain, pituitary, and serum interleukin-1β protein response to acute stress. Brain Res. 859:193–201.
- Nilsson C, Jennische E, Hoi-Por H, Eriksson E, Bjorntorp P, Homang A. 2002. Postnatal endotoxin exposure results in increased insulin sensitivity and altered activity of neuroendocrine axes in adult female rats. Eur J Endocrinol. 146:251–260.
- O'Connor KA, Johnson JD, Hansen MK, Wiesler Frank JL, Maksimova E, Watkins LR, Maier SF. 2003. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res. 991:123–132.
- Owens JA, Gatford KL, De Blasio MJ, Kind KL, Robinson JS. 2006. Perinatal programming of metabolic homeostasis. In: Hodgson DM, Coe CL. editors. Perinatal programming: Early life determinates of adult health & disease. London: Taylor & Francis. 97–115.
- Plata-Salaman CR, Ilyin SE, Turrin NP, Gayle D, Flynn MC, Bedard T, Merali Z, Anisman H. 2000. Neither acute nor chronic exposure to a naturalistic (predator) stressor influences the interleukin-1beta system, tumor necrosis factor-alpha, transforming growth factor-beta1, and neuropeptide mRNAs in specific brain regions. Brain Res Bull. 51:187–193.
- Prescott SL, Dunstan J. 2006. Role of prenatal events in the development of allergic disease. In: Hodgson DM, Coe CL. editors. Perinatal programming: Early life determinates of adult health and disease. London: Taylor & Francis. 144–154.
- Reul JHM, Stec I, Wiegers GJ, Labeur MS, Linthorst ACE, Arzt E, Holsboer F. 1994. Prenatal immune challenge alters the hypothalamic–pituitary-adrenocortical axis in adult rats. J Clin Invest. 93:2600–2607.
- Shanks N, Lightman SL. 2001. The maternal-neonatal neuro-immune interface: Are there long-term implications for inflammatory or stress-related disease. J Clin Invest. 108:1567–1573.
- Shanks N, Larocque S, Meaney MJ. 1995. Neronatal endotoxin alters the development of the hypothalamic-pituitary-adrenal axis: Early illness and later responsivity to stress. J Neurosci. 15:376–364.
- Shanks N, Windle RJ, Perks PA, Harbuz MS, Jessop DS, Ingram CD, Lightman SL. 2000. Early-life exposure to endotoxin alters hypothalamic–pituitary-adrenal function and predisposition to inflammation. Proc Natl Acad Sci, USA. 97:5645–5650.
- Song C, Phillips AG, Leonard BE. 2003. Interleukin-1 beta enhances conditioned fear memory but impairs spatial learning in rats: Possible involvement of glucocorticoid and monoamine function in the amygdala and hippocampus. Eur J Neurosci. 18:1–6.
- Sternberg EM, Licinio J. 2006. Overview of neuroimmune stress interactions implications for susceptibility to inflammatory disease. Ann NY Acad Sci. 771:364–371.
- Takao T, Nanamiya W, Takemura T, Nishiyama M, Asaba K, Makino S, Hashimoto K, De Souza EB. 1997. Endotoxin induced increases in rat plasma pituitary-adrenocortical hormones are better reflected by alterations in tumor necrosis factor α than interleukin-1ß. Life Sci. 17:263–268.
- Tenk CM, Kavaliers M, Ossenkopp KP. 2008. Sexually dimorphic effects of neonatal immune system activation with lipopolysaccharide on the behavioural response to a homotypic adult immune challenge. Int J Dev Neurosci. 26:331–338.
- Turnbull AV, Rivier C. 1995. Regulation of the HPA axis by cytokines. Brain, Behav, Immun. 9:253–275.
- Walker FR, March J, Hodgson DM. Endotoxin exposure in early life alters the development of anxiety-like behaviour in the Fischer 344 rat. Behav Brain Res. 2004a; 154:63–69.
- Walker FR, Brogan A, Smith R, Shastry S. A profile of the immediate endocrine, metabolic and behavioural responses following a dual exposure to endotoxin in early life. Physiol, Behav. 2004b; 83:495–504.
- Walker FR, Owens J, Ali S, Hodgson DM. 2006. Individual differences in glucose homeostasis: Do our early life interactions with bacteria matter?. Brain, Behav, Immun. 20:401–409.
- Walker FR, Knott B, Hodgson DM. 2008. Neonatal endotoxin exposure modifies the acoustic startle response and circulating levels of corticosterone in the adult rat but only following acute stress. J Psychiatr Res. 42:1094–1103.
- Walker AK, Nakamura T, Byrne RJ, Naicker S, Tynan RJ, Hunter M, Hodgson DM. 2009. Neonatal lipopolysaccharide and adult stress exposure predisposes rats to anxiety-like behaviour and blunted corticosterone responses: Implications for the double-hit hypothesis. Psychoneuroendocrinology. 34:1515–1525.
- Webster Marketon JI, Glaser R. 2008. Stress hormones and immune function. Cell Immunity. 252:16–26.
- Young EA, Altemus M, Parkison V, Shastry S. 2001. Effects of estrogen antagonists and agonists on the ACTH response to restraint stress in female rats. Neuropsychopharmacology. 25:881–891.
- Zhu C, Liu Q, Wei Y, Ma C, Hao J, Yan P. 1999. Coexistence of immune-neuro-endocrine substances in the rat central neurons. J Tongji Med Univ. 19:81–85.